Low Sex Hormone-Binding Globulin Levels are Associated with Development of Metabolic Syndrome in PCOS Patients
Xiaoli Chen1, Bin Hong2, Jia Huang1, Dongzi Yang1 and Lin Li1*
1Department of Reproductive Medicine Center, Sun Yat-Sen Memorial Hospital of Sun Yat-Sen University China
2Department of Children Health Care, Maternal and Children Health Hospital of Yuexiu District, China
Submission: March 16, 2020; Published: April 20, 2020
*Corresponding author: Lin Li, Department of Reproductive Medicine Center, Sun Yat-Sen Memorial Hospital, Sun Yat-Sen University. No.107, Yanjiang Road, Guangzhou, 510120, China
How to cite this article: Xiaoli C, Bin H, Jia H, Dongzi Y, Lin L. Low Sex Hormone-Binding Globulin Levels are Associated with Development of Metabolic Syndrome in PCOS Patients. J Gynecol Women’s Health. 2020: 18(5): 555996. DOI: 10.19080/JGWH.2020.18.555996
Abstract
This study was designed to [1] This study was performed to determine whether serum SHBG(Sex hormone-binding globulin) is a reliable predictor of metabolic syndrome in participants with PCOS(Polycystic ovary syndrome [2]. The study was to investigate the possible correlations between SHBG and PCOS-related metabolic disturbances. The study was a clinical retrospective study. PCOS women (n=1410) were selected. Age, BMI (body mass index) and SHBG were significantly and independently associated with metabolic syndrome in women, but SHBG had stronger relationships with the metabolic syndrome when compared to age. Decreased SHBG can discriminate with high sensitivity and specificity between women with and without MS. Low SHBG level(≤38.53nmol/L) proved to be an important element determining constellation of MS (metabolic syndrome) components. These findings support examination of interventions that raise circulating SHBG as a possible early strategy for preventing the development of the metabolic syndrome in PCOS women.
Keywords:PCOS; Sex hormone-binding globulin (SHBG); Metabolic syndrome
Introduction
Polycystic Ovary Syndrome (PCOS) is one of the most common endocrinopathy of reproductive-aged women, affecting 4%-8% of this population [1]. PCOS is increasingly recognized as a variant of the metabolic syndrome in women with the characteristic features of insulin resistance, central obesity, impaired glucose metabolism, dyslipidemia and hypertension [2]. Sex Hormone-Binding Globulin (SHBG) is a blood transport protein for testosterone and estradiol [3]. SHBG is derived primarily from the liver [4] and has been noted to be lower in women with PCOS compared with those without PCOS. This study was performed to determine whether serum SHBG is a reliable predictor of metabolic syndrome in participants with PCOS because SHBG levels require a single random blood draw and are easy to obtain.
Materials and Methods
Subjects
We studied 1410 PCOS women, recruited from the gynecological outpatient department of Memorial Hospital of Sun Yat-Sen University from June 2005 to December 2016. The studies were approved by the institutional review board of the hospital. The diagnosis of PCOS was based on the revised criteria of the European Society of Human Reproduction and Embryology/American Society for Reproductive Medicine in 2003 (Rotterdam PCOS Consensus Workshop Group, 2004). The diagnosis of PCOS was made by the presence of two of the following three criteria
a. oligomenorrhea and/or anovulation (eight or fewer menstrual cycles in a year or menstrual cycles more than 35 days in length).
b. clinical and/or biochemical signs of hyperandrogenism.
c. polycystic ovaries (presence of 12 or more follicles in each ovary measuring 2–9 mm in diameter and/or increased ovarian volume>10 ml) and exclusion of other etiologies (e.g. congenital adrenal hyperplasia, androgen-secreting tumors and Cushing’s syndrome).
Any medications known to affect sex hormone or carbohydrate metabolism were discontinued for at least a month before the study (except oral contraceptives, which were stopped 3 months before study entry).
All subjects did not use any hypolipidemic or Blood Pressure (BP)-lowering drugs. All women were in good health.
Oral Glucose Tolerance Test
After a 12-hour overnight fast, insulin and glucose levels were obtained. This was followed by the consumption of a 75-g glucose load, with blood draws for insulin and glucose levels at 1- and 2-hours post-glucose load.
Parameters of Insulin Resistance
The fasting glucose-to-insulin (GI) ratios and the homeostasis model assessment of insulin status (HOMA) were analyzed. Fasting G: I was calculated with glucose expressed as milligrams per decilitre and insulin expressed as microunits per millilitre, as previously described [5]. HOMA was calculated according to the formula [plasma glucose (mmol/l) × insulin (μU/ml)]/22.5 [6-7].The presence of acanthosis nigricans was also used as an individual predictor for the presence of severe insulin resistance [8] and identifies those individuals who have the highest insulin concentrations as well as the most severe insulin resistance [9]. Patients were defined as having Metabolic Syndrome (MS), based on Adult Treatment Panel III criteria for MS (Expert Panel on Detection, 2001). Diagnosis was made when three or more of the following conditions were determined:
a. obesity=waist circumference (WC) >80 cm in women
b. hypertriglyceridemia=triglycerides (TG) ≥1.7 mmol/l;
c. low high-density lipoprotein-cholesterol (HDL-C) = HDL-C<1.3 mmol/l in women;
d. hypertension =known hypertensive or BP ≥130/85 mmHg.
e. dysglycemia =fasting plasma glucose (PG)≥6.1 mmol/l or known to have diabetes.
f. The study was approved by the institutional review board of the Sun Yat-Sen Memorial Hospital of Sun Yat-Sen University
Statistical Analysis
Data were analysed with the statistical package Stata, version 13.0 (StataCorp, College Station, Texas, USA 2007). Participants were divided into two groups according to whether they met the criteria for metabolic syndrome. Continuous variables were evaluated for normal distribution using the Kolmogorov-Smirnov test. Results are reported as mean value± standard Error (SE) of the mean. Statistical analysis of differences between two groups were assessed using independent Student’s t-tests. Binary logistic regression analyses were undertaken to explore hormonal associations with the metabolic syndrome and its components, and to obtain a graphical representation of the predicted probabilities of metabolic syndrome for a given hormone level. ROC curves were determined by calculating the sensitivity and specificity for every value observed and plotting sensitivity against 1-specificity. The best possible prediction method would yield a graph comprising a point in the upper left corner of the ROC space, i.e.100% sensitivity (all true positives are found) and 100% specificity (no false positives are found). The Area Under the Curve (AUC), which can be calculated using the trapezoidal rule, characterizes ROC curves and reflects maximal sensitivity and specificity. A perfect test has an AUC of 1.0, while a non-discriminating test has an area of 0.5. Youden index (sensitivity+specificity-1) was calculated to determine the best compromise between sensitivity and specificity. When the value was closer to 1, it was indicating the greater diagnostic value. Values of p<0.05 were considered significant.
Results
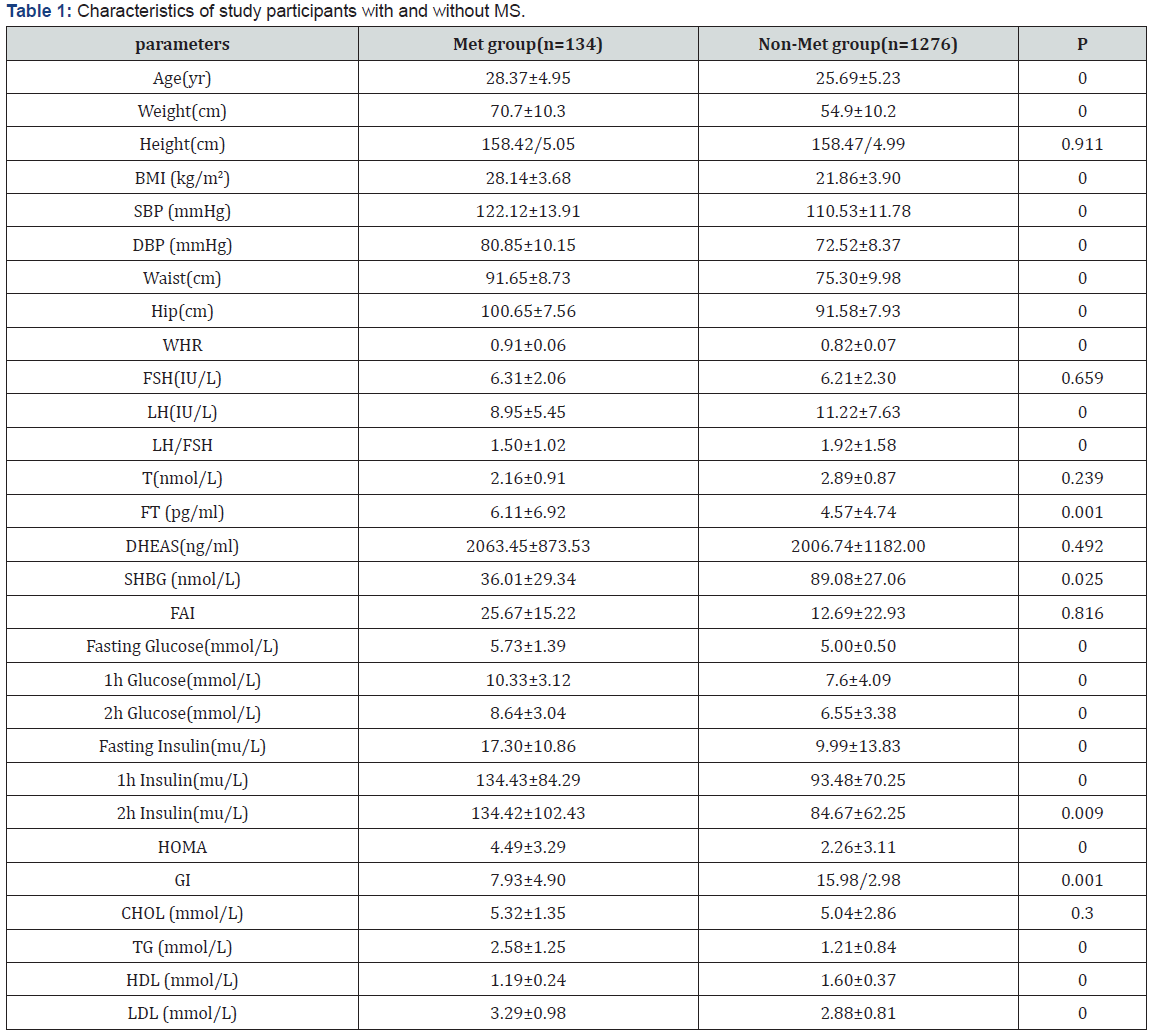
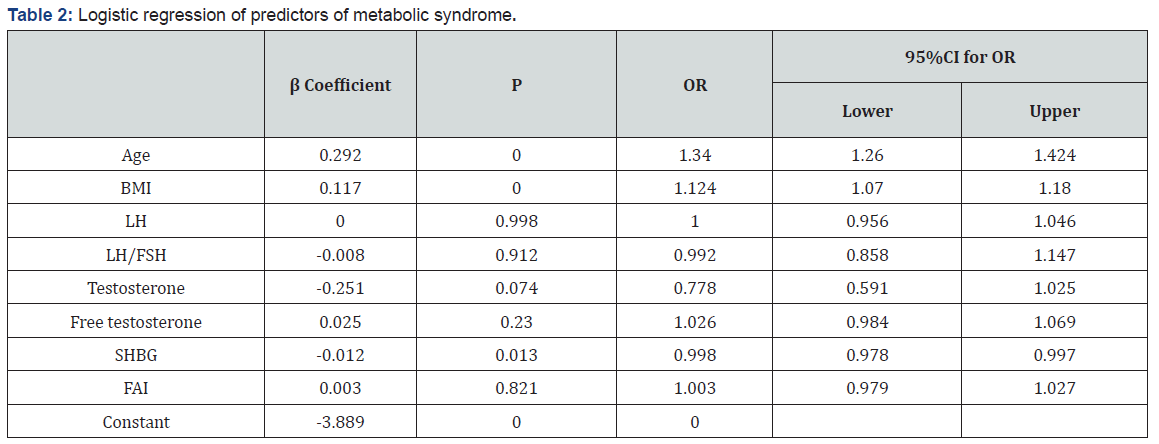
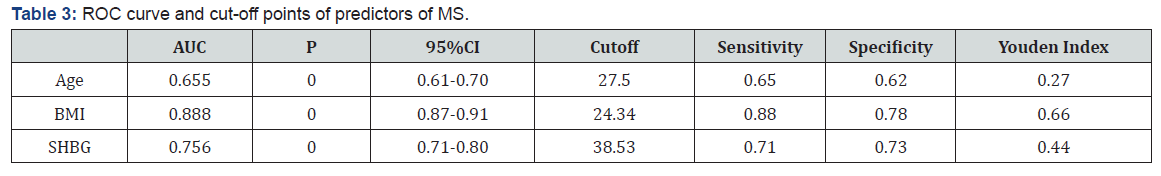
Characteristics of study participants with and without metabolic syndrome (Table 1). The characteristics of the 1410 subjects, stratified as to their metabolic syndrome status, are shown in Table 1. The prevalence of metabolic syndrome 9.5%. Women with metabolic syndrome had higher age, BMI, SBP, DBP, waist, hip, WHR, FT, fasting glucose, 1h glucose, 2h glucose, fasting insulin, 1h insulin, 2h insulin, TG and LDL than those without metabolic syndrome. While women with metabolic syndrome had lower LH, LH/FSH, SHBG, GI and HDL than those without metabolic syndrome. The difference in FSH, T, DHEAS and CHOL between two groups of women were not statistically significant. Logistic regression of predictors of metabolic syndrome (Table 2) Logistic regression was performed separately to find the predictors of MS in PCOS women. Among several covariates, age (OR=1.34, p=0.000) BMI (OR= 1.124, p=0.000) and SHBG (OR= 0.998, p=0.013) were significant predictor of MS. ROC curve and cut-off points of predictors of MS (Table 3 and Figures 1,2 & 3). In ROC analyses of the ability of age, BMI and SHBG to detect metabolic syndrome (Table 3), the area under the curve (AUCROC) was 0.655 (95% CI, 0.61-0.70) for age, 0.888 (95% CI, 0.87-0.91) for BMI and 0.756 (95% CI, 0.71–0.80) for SHBG. A decision threshold of age greater than 27.5yrs resulted in a sensitivity of 65% and a specificity of 62%. A threshold of BMI greater than 24.34kg/m2 resulted in a sensitivity of 88% and a specificity of 78%. A threshold of SHBG lower than 38.53nmol/L resulted in a sensitivity of 71% and a specificity of 73% (Figures 1, 2 & 3).
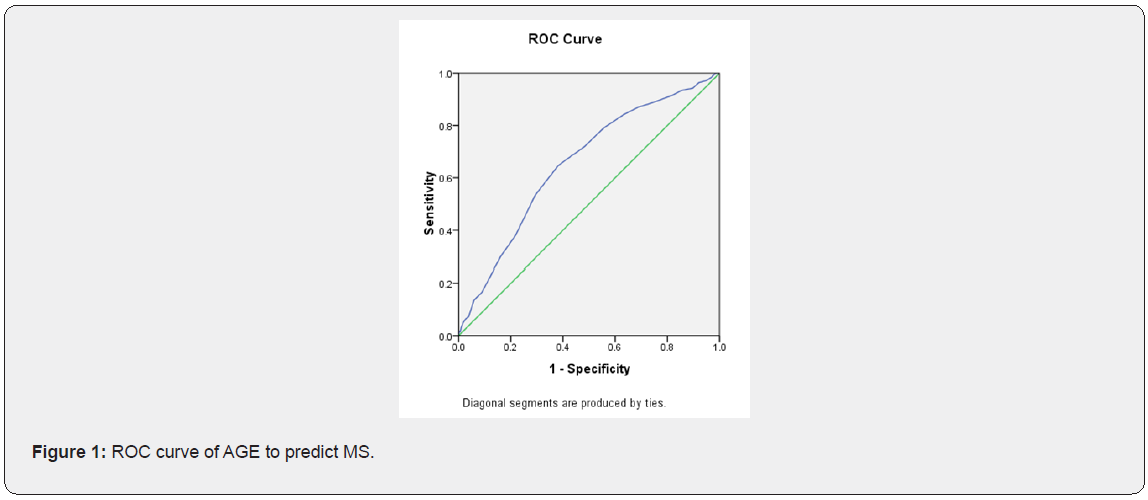
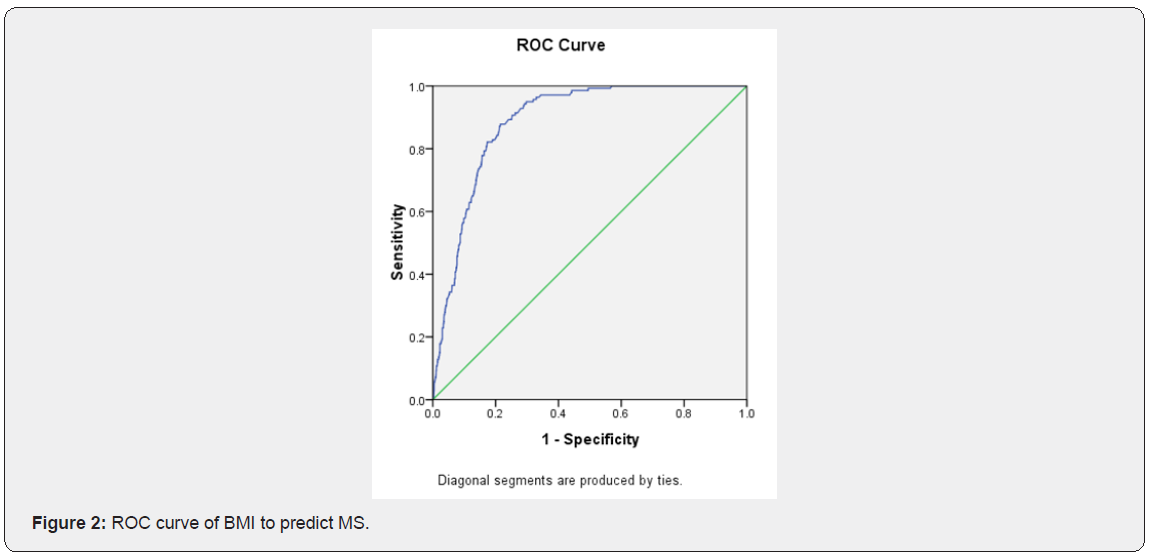
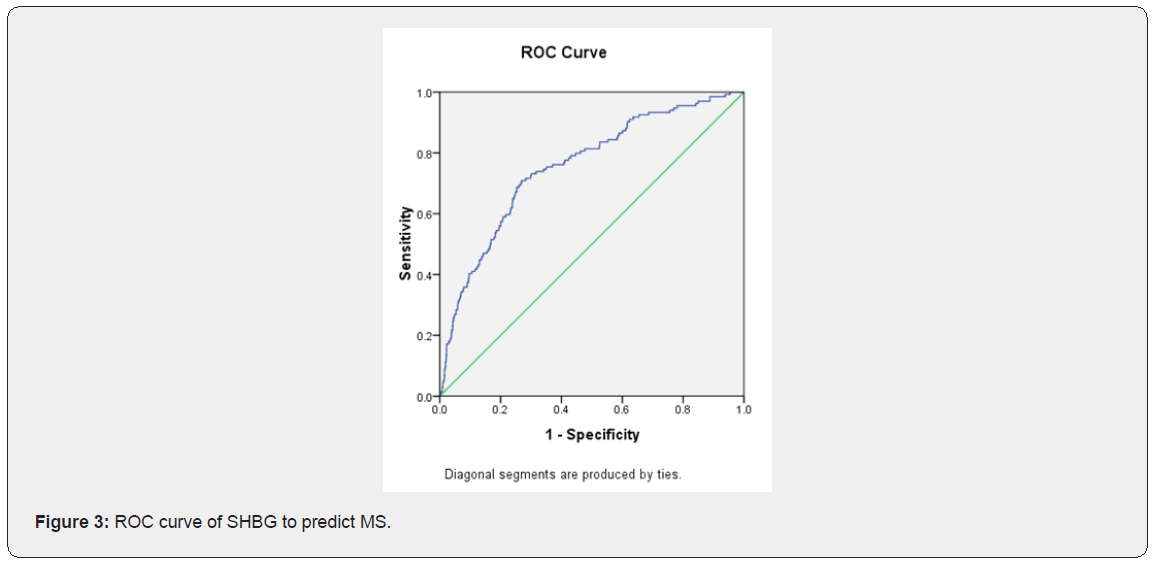
Discussion
We found that age, BMI and SHBG were significantly and independently associated with metabolic syndrome in women, but SHBG had stronger relationships with the metabolic syndrome when compared to age. Low SHBG level(≤38.53nmol/L) proved to be an important element determining constellation of MS components. Similar observations were made in men [10]and postmenopausal women [11]. Only few studies were for middle age PCOS women. Our results, showing a stronger association with metabolic syndrome for SHBG than for age, suggest that SHBG may be a more sensitive predictor for MS. The control of plasma SHBG concentration, considered initially to be under the direct regulation of sex hormone levels [12], is considered today to be under multifactorial regulation. Indeed, the basal production of SHBG seems to be steroid independent and more related to general metabolic factors and nutritional status [13-15]. Such a strategy may enable us to identify and prevent MS at an earlier stage. Decreased SHBG can discriminate with high sensitivity and specificity between women with and without MS. The significance of this finding for routine clinical work remains to be shown, but it may serve as a valuable adjunct to the diagnostic criteria in scientific studies on this heterogeneous syndrome. The finding that serum SHBG levels could be used to screen for metabolic syndrome would be convenient because a single accurate predictor of metabolic syndrome would permit early intervention and could be used to monitor response to drugs to improve metabolic disorders. In conclusion, lower SHBG(≤38.53nmol/L) is more strongly associated with metabolic syndrome in women with PCOS. These findings support examination of interventions that raise circulating SHBG as a possible strategy for preventing the development of the metabolic syndrome in PCOS women.
Funding
Funded by Guangzhou science and technology program. No. 2016201604030008; Funded by Guangdong science and technology program. No.2016A020216011.
Funded by Special fund for construction of Guangdong Administration of traditional Chinese Medicine.No.20174002
References
- Carmina E,Lobo RA (1999) Polycystic ovary syndrome (PCOS): arguably the most common endocrinopathy is associated with significant morbidity in women. J Clin Endocrinol Metab 84(6):1897-1899.
- Hopkinson ZE, Sattar N, Fleming R,Greer IA (1998) Polycystic ovarian syndrome: the metabolic syndrome comes to gynaecology. BMJ 317:329-332.
- Petra PH (1991) The plasma sex steroid binding protein (SBP or SHBG). A critical review of recent developments on the structure, molecular biology and function. J Steroid Biochem Mol Biol 40(4-6):735-753.
- Kahn SM, Hryb DJ, Nakhla AM, Romas NA, Rosner W (2002) Sex hormone-binding globulin is synthesized in target cells. J Endocrinol 175(1):113-120.
- Legro RS, Finegood D,Dunaif A (1998) A fasting glucose to insulin ratio is a useful measure of insulin sensitivity in women with polycystic ovary syndrome. J Clin Endocrinol Metab 83(8):2694-2698.
- Matthews DR, Hosker JP, Rudenski AS, Naylor BA, Treacher DF,et al.(1985) Homeostasis model assessment: insulin resistance andβ-cell function from fasting plasma glucose and insulin concentrations in man. Diabetologia 28:412-419.
- Radziuk J (2000) Insulin sensitivity and its measurement: structural commonalities among the methods. J Clin Endocrinol Metab 85(12):4426-4433.
- Stoddart ML, Blevins KS, Lee ET, Wang W, Blackett PR,et al. (2002) Association of acanthosis nigricans with hyperinsulinemia compared with other selected risk factors for type 2 diabetes in Cherokee Indians.The Cherokee Diabetes StudyDiabetes Care 25(6):1009 -1014.
- Stuart CA, Driscoll MS, Lundquist KF, Gilkison CR, Shaheb S, et al.(1998) Acanthosisf nigricans. J Basic Clin PhysiolPharmacol 9(2-4): 407-418.
- Chubb SA, Hyde Z, Almeida OP, Flicker L, Norman PE(2008)Lower sex hormone-binding globulin is more strongly associated withmetabolic syndrome than lower total testosterone in older men: the Health in Men Study. Eur J Endocrinol 158(6):785-792
- Korhonen S, Hippelainen M, Vanhala M, Heinonen S, Niskanen L (2003) The androgenic sex hormone profile is an essential feature of metabolic syndrome in premenopausal women: a controlled community-based study. Fertil Steril 79(6):1327-1334.
- Anderson DC (1974) Sex hormone-binding globulin. Clinical Endocrinology 3:69-96.
- Schoultz B,CarlstroÈm K (1989) On the regulation of sex hormone-binding globulin: a challenge of an old dogma and outlines of an alternative mechanism. Journal of Steroid Biochemistry and Molecular Biology 32: 327-334.
- Toscano V, Balducci R, Bianchi P, Guglielmi R, Mangiantini A,et al. (1992) Steroidal and non-steroidal factors in plasma sex hormone-binding globulin regulation. Journal of Steroid Biochemistry and Molecular Biology 43(5):431-437.
- Pugeat M, Crave JC, Elmidani M, Nicolas MH, Garoscio-Cholet M, et al. (1991) Pathophysiology of sex hormone-binding globulin(SHBG): relation to insulin. Journal of Steroid Biochemistry and Molecular Biology 40(4-6):841-849.






























