Three-Dimensional and Two-Dimensional Ultrasound in Assessment of Antral Follicle Count Among Infertile Females
Hossam M Abd-Elnaby*
Department of Obstetrics and Gynecology, Zagazig University Hospital, Egypt
Submission: January 02, 2019 ;Published: January 10, 2020
*Corresponding author: Hossam M Abd-Elnaby, Department of Obstetrics and Gynecology, Zagazig University Hospital, Egypt
How to cite this article: Hossam M Abd-Elnaby. Three-Dimensional and Two-Dimensional Ultrasound in Assessment of Antral Follicle Count Among 002 Infertile Females. J Gynecol Women’s Health. 2020: 18(1): 555978. DOI: 10.19080/JGWH.2020.18.555978
Abstract
Background: In the last decades 2D ultrasound had been used broadly to assess, treat and follow up infertile women. Usually transvaginal is the method of choice to study ovaries (ovarian dimensions and total ovarian volume, antral follicle count and size) and uterus (uterine position, size, myometrium, uterine cavity, endometrium and cervix uteri). Although 3D ultrasound recently shows a very good reliability of offline antral follicle counts versus real-time 2D ultrasound, 3D ultrasound is still less commonly used to monitor both of ovarian reserve and ovarian response to ovulation induction
Objective: To assess the role of 3D ultrasound imaging in evaluation of antral follicle and ovarian reserve in comparison to 2D ultrasound imagining in infertile women.
Methods: A Prospective observational study included 50 infertile women aged between (18-37) years undergone 2D and 3D transvaginal ultrasound to assess antral follicle count; the number and size of every follicle was reported. The outcome measures included in this study were: antral follicle count (AFC), small (2-6mm) AFC, larger (7-9mm) AFC, total ovarian volume (OV) and time of examination by both techniques
Result: The Mean ± SD for total AFC were 19.89 ± 10.33 and 17.16 ± 9.71 for 2D and 3D measurements respectively and the mean for larger (7-9 mm) AFC were 1.68 ± 0.93 and 1.79 ± 1.43 for 2D and 3D measurements respectively with no significant difference. While the Mean ± SD for small (2-6mm) Antral Follicle Count (AFC) were 14.16 ± 10.58 and 11.3 ± 8.62 for 2D and 3D measurements respectively with 2D significantly higher than 3D measurements. The Mean ± SD for ovarian volume (OV) were 5.7 ± 2.4and 5.6 ± 2.3 for 2D and 3D measurements respectively and the difference between the mean of OV of 2D measurements and 3D measurements was statistically insignificant.
Conclusion: 3D ultrasound is superior to 2D ultrasound in assessment of ovary, but 2D ultrasound is a reliable choice when 3D ultrasound is unavailable
Keywords: Emergency room; Patient’s laboratory; Arterial duplex; Neurology; Magnetic Resonance; Cerebro Spinal; Serology returned; Physical therapy; Tabes dorsalis
Introduction
Throughout the woman reproductive lifespan, consumption of antral follicles causes drop of the reserve of resting follicles which negatively affects ovarian reserve and conducts ovarian agenting. Ovarian reserve is defined by assessment of the quantity and the quality of the remaining primordial follicular pool within both ovaries at a given time [1]. Ovarian reserve reflects the fertility potential and impacts the possibility of conception, either spontaneously or in conjunction with assisted reproductive technologies. However, neither ovarian ageing nor reduced ovarian reserves are currently listed as a cause of sub-fertility [2].
In the last decades 2D ultrasound had been used broadly to assess, treat and follow up infertile women. Usually transvaginal is the method of choice to study ovaries (ovarian dimensions and total ovarian volume, antral follicle count and size) and uterus (uterine position, size, myometrium, uterine cavity, endometrium and cervix uteri). Although 3D ultrasound recently shows a very good reliability of offline antral follicle counts versus real-time 2D ultrasound, 3D ultrasound is still less commonly used to monitor both of ovarian reserve and ovarian response to ovulation induction [3].
Conventional transvaginal real-time 2D ultrasound technique may be time-consuming as it requires the operator to rotate the probe gradually to scan each ovary, identifying each follicle and measuring its dimensions. Some women feel discomfort with this technique, too. In contrast, 3D ultrasound study saves time and is more comfortable to the patients. It requires capturing each ovary only a single clear sonographic view. The assessment of the count and the dimensions of the follicle is performed offline later [4-5].
Mercé et al found in their study an excellent intraobserver and interobserver reproducibility of the ovarian volume, follicle counts, and 3D power Doppler angiographic indices. The reliability had not been affected by the ovarian functional stage. 3D ultrasound and power Doppler angiography enhance the evaluation of ovarian parameters, and their reliability offers a modification in the current clinical routine of ultrasound examination [6].
Aim of the Study
The aim of the study is to assess the role of 3D ultrasound imaging in evaluation of antral follicle and ovarian reserve in comparison to 2D ultrasound imagining in infertile women.
Patients and Methods
This study was an observational prospective study. It was done in the department of Obstetrics and Gynecology of Zagazig University Hospital, where fifty infertile women were recruited from September 2018 to June 2019. This study was approved by the local ethical committee of research department. It was done according to Helsinki declaration for research in human being. Each participated woman gave us an informed written consent
Criteria of inclusion
50 women with infertility (primary or secondary) of age (18- 37) years were recruited from the infertility outpatient clinic. Each woman in this study had a normal hystrosalpingogram HSG study (to prove patent normal tubes, normal uterine cavity and normal cervix), and with normal husband’s semen analysis to confirm male fertility [7].
Exclusion criteria
patient refusal, age younger than 18 years or older than 37 years, women undergone any ovarian surgery (ovarian cystectomy, ovarian drilling and unilateral oophorectomy), tubal factors of infertility, uterine/cervical factors of infertility, pituitary/ hypothalamic factors of infertility and male factor infertility
Procedure
Women with reproductive failure were visited the infertility clinic were subjected to: history taking age, residence, occupation, special habits, duration of marriage, manifestation of ovulation, history of previous medical or hormonal treatment and previous gynecological or other surgical procedures. Detailed menstrualhistory (age of menarche, last menstrual period, menstrual pattern). Detailed obstetric history (parity, mode of delivery, miscarriages, history of D&C following abortions, last delivery, last abortion, history of postpartum hemorrhage, history of puerperal sepsis). Examination: patients were subjected to general examination as regards; Height, body built secondary sexual characters, hair distribution, chest, heart and abdominal examination [8].
Local examination
including PV, bimanual examination, speculum examination and per-rectal examination to detect the development of external genitalia, congenital anomalies. Investigation: Semen analysis was done to the partner to exclude male factor of infertility Complete hormonal profile; FSH, LH, TSH and Prolactin. Routine abdominal 2D ultrasound to examine cervix, bladder, tubes and uterus to detect any pathology or anomalies.
All patients underwent transvaginal ultrasonography using GE Voluson PRO (5 to 10 MHZ Trans-vaginal probe) to assess the ovarian volume, follicles count, and follicular volume. 2D Transvaginal ultrasound: All Patients were scheduled according to their Menstrual cycle calculation to be examined during the early follicular phase, day3 in the menstrual cycle. The patients were instructed to evacuate the bladder before the procedure, patient lied at lithotomy position. The probe was covered with a thin layer of gel and loaded in a disposable glove and inserted slowly into the vagina. The ovaries were identified and then measured in three planes. Volumes were calculated by the formula based on a prolate ellipsoid after studying the maximum longitudinal, anteroposterior, and transverse diameters [9].
Each ovary was scanned in both longitudinal and coronal planes, to identify which offers the best image. After selecting the best image, the ovary was centered on the screen. Then, the ultrasound machine was adjusted to set gain, depth, magnifications to optimize image quality and to maximize the contrast between the follicular fluid and the ovarian stroma. While scanning the ovary, it should take at least 50% of the image of along the screen’s largest axis.
We included in the count all follicular structures 2-10mm in diameter identified when scanning from one ovarian margin to the other. Follicular size was calculated by the internal diameter of the sonolucent area when it was doubtful whether a follicle lies within the (2-10) range. Only one measurement was taken for round follicles. For oval follicles, the mean of greatest diameter and greatest diameter perpendicular to it was calculated. We subtracted the number of follicles measuring < 2mm or > 10mm from the total number of identifiable follicles. The process was repeated in the other scanning plane. Then, the other ovary was scanned in a similar pattern and reported separately
3D ultrasound trans-vaginal examination
After 2D ultrasound, select (new patient) button to start exam for the patient & to save her ID & images captured. The ovarian maximal diameter was visualized by manipulating the probe. Then, the probe was held to stabilize the captured image. The 3D facility was selected and the whole ovary was included by adjusting the truncated sector. Conducting 3D rotational measurements of ovarian volume by rotation steps of 30º in both A-plane (longitudinal) and C-plane (coronal) was done using VOCAL, which allows inclusion of the whole ovary. The longitudinal image (plane A) represents the original data with all other images (transverse and coronal) obtained using the reconstructed data. Subsequently, an automated mechanical scan of this region of interest was performed by the slow scan mode and the 3D data were saved on the hard disk of the machine. We used the automatic volume calculation program (SonoAVC), which automatically reveals fluid-filled areas within the region of interest, to report the number, mean diameter and volume of each follicle. SonoAVC gives each follicle a specific color to identify it from others, and automatically takes its measures. Then, it records mean diameter (relaxed sphere diameter), maximum dimensions (x-y-z diameters) and volume.
We recorded the time taken for the whole process to the nearest second. The clock was started as soon as the baseline scan had been finished and the decision was made to evaluate the antral follicle population. The transducer was positioned to visualize a longitudinal uterine section. The measurement methods were done in a random order proceeding with either 2D or 3D ultrasound once the clock had been started. The 2D ultrasound technique included the time taken to locate both ovaries, count all the antral follicles, and measure and record their individual sizes. The 3D ultrasound technique also started by manipulating the probe centrally to show a longitudinal uterine. It included the time needed to locate each ovary, acquire the 3D ultrasound datasets, apply SonoAVC, necessary post-processing and recording the results of this analysis in terms of the total antral follicle number and the relative sizes of the follicles. The time consumed to assess the pelvis and exclude pathologies in each subject was not included in the time taken for both methods
Statistical Analysis
Data were collected, revised and entered to the Statistical Package for Social Science (SPSS) version 23. The quantitative data were presented as mean, standard deviations (SD) and ranges when their distribution found parametric and median with inter-quartile range (IQR) when their distribution found nonparametric. The comparison between 2D and 3D results was done by using Paired t-test. The confidence interval was set to 95% and the margin of error accepted was set to 5%. So, the p-value was considered significant at the level of < 0.05.
Result
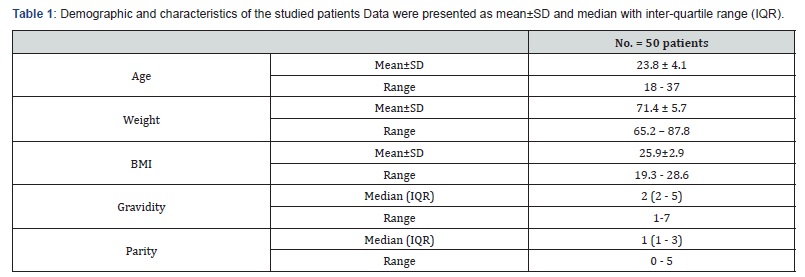
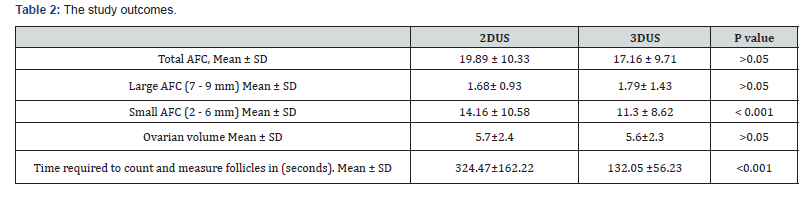
Regarding the patient characteristics; Age distribution the Mean ± SD for age were 23.8 ± 4.1. The Mean ± SD of Body mass index were 25.9±2.9 with no significance statistically (Table 1). Table 2 shows that the mean ± SD for total AFC were 19.89 ± 10.33 and 17.16 ± 9.71 for 2D and 3D measurements respectively and the mean for larger (7-9mm) AFC were 1.68 ± 0.93 and 1.79 ± 1.43for 2D and 3D measurements respectively with no significant difference. While the Mean ± SD for small (2-6 mm) AFC were 14.16 ± 10.58 and 11.3 ± 8.62 for 2D and 3D measurements respectively with 2D significantly higher than the3D measurements. The Mean ± SD for ovarian volume (OV) were 5.7 ± 2.4 and 5.6 ± 2.3 for 2D and 3D measurements respectively and the difference between the mean of OV of 2D measurements and 3Dmeasurements was statistically insignificant. The Mean ± SD for time of examination by 2D and 3D were 324.47 ± 162.22 and 132.05 ± 56.23 for 2D and 3D respectively and the difference between the mean of time of 2D examination was significantly higher than time of 3D examination.
There was no statistically significant difference regarding total AFC when counted by both techniques (P > 0.05). There was no statistically significant difference regarding large AFC (7-9 mm) when counted by both techniques (P>0.05). The mean of the small AFC (2-6mm) measured by 2D ultrasound was significantly higher than when measured by 3D ultrasound (P <0.001). There was no statistically significant difference regarding ovarian volume measured by both techniques (P > 0.05). The mean time taken for the automated analysis of the 3D ultrasound dataset with Sono AVC was significantly less than that required for the 2D ultrasound (P < 0.001).
Discussion
In the present study, comparing between 2D and 3D (sono AVC™ program) measurements regarding total AFC revealed that the Mean ± SD for total AFC were 19.89 ± 10.33 and 17.16 ± 9.71 for 2D and 3D measurements respectively and there were no significant difference between 2D and 3D measurements (P value > 0.05). This agrees with a study carried out by Moawad et al. [10] They assessed ovarian follicle counts by transvaginal ultrasound, as a part of the management of infertility workup, in fifty randomly selected women. The sonographers were oriented with the software of the Viewpoint obstetrics and gynecology reporting/ imaging archiving system and the VOCAL imaging program. They found stored 3D ultrasound data were like those obtained by 2D real-time ultrasound in assessment of follicle counts [10].
In our study, comparing between 2D and 3D measurements regarding small (2-6 mm) AFC revealed that the Mean ± SD for small AFC were 14.16 ± 10.58 and 11.3 ± 8.62for 2D and 3D measurements respectively and the mean small AFC of 3D measurements was significantly higher than the 2D measurements(P value <0.001). While larger (7-9mm) AFC - as shown in table (3) -revealed that the Mean ± SD for Large AFC were 1.68 ± 0.93 and 1.79 ± 1.43for 2D and 3D measurements respectively which show no significant difference (P value >0.05). These findings were like findings of study done by Deb et al. [9] on 24 women. All women included in this study were ultrasound scanned by a single sonographer and the results were recorded by another observer. They reported that 3D ultrasound examination offers quicker measurement of antra follicle size than 2D ultrasound examination [9].
Both 2D and 3D ultrasound have least agreement with antra follicles measurement 3.0-4.99 mm. Inability to confirm the accuracy of these measurements is an obvious limitation of our present study. However, histological examination suggests that the automated measurements are more valid than those reported by 2D ultrasound. Weenen et al. [11] compared between findings of ovarian assessment by ultrasound scan versus histological study in 12 regularly menstruating women undergone oophorectomy for prophylaxis against increased risk of ovarian cancer or for management of endometriosis. They measured follicles by ultrasound in two perpendicular planes, and the mean measurement taken as the diameter of the follicle. Then, they compared these findings with those reported histologically. They found that for counting and measuring small follicles, 3D is more accurate and reliable [11].
Evaluation of the biological and clinical importance of quantifying antral follicle size and count requires further researches and investigations. In the present study; comparing between 2D and 3D measurements concerning ovarian volume (OV) - as shown in table- revealed that the Mean ± SD for OV were 5.7 ± 2.4and 5.6 ± 2.3 for 2D and 3D measurements respectively and the difference between the mean of OV of 2D measurements and 3D measurements was statistically insignificant (P value >0.05). These findings are like findings of the study done by Brett et al, where two observers calculated ovarian volume, in 49 women, using both 2D ultrasound (prolate ellipsoid formula) and 3D ultrasound [virtual organ computer-aided analysis (VOCAL)] with rotation steps of 30° (3D-30). They concluded that using 3D ultrasound does not significantly increase the accuracy of volumetric measurements over 2D ultrasound [7].
In the present study; comparing between 2D and 3D measurements regarding the time of ultrasound examination revealed that the Mean±SD were 324.47±162.22 and 132.05±56.23 for 2D and 3D examination respectively and the difference between the mean of time of 2D examination was significantly higher than time of 3D examination (P value <0.05). When follicle size was assessed, overall time required for analysis was significantly less with 3D ultrasound examination than it was with 2D ultrasound examination. This agrees with the study done by Deb et al [9].
Recommendations
3D ultrasound examination as an imaging modality can be used as a complementary method for other endocrine markers for ovarian reserve assessment. It is excellent in evaluating the ovaries, calculating ovarian volume and direct quantitative estimation of antral follicle count. Using 3D software including sono AVC™ and VOCAL™ programs can increase accuracy and efficacy of ovarian evaluation. Patient examination with 3D ultrasound is less time consuming and less patient discomfort. Rather than small AFC count, the data acquired by both techniques are accepted. 3D ultrasound is superior to 2D ultrasound in ovarian assessment, but 2D ultrasound is reliable in case 3D is not available as in some developing countries.
References
- Broekmans F, Kwee J, Hendriks D, Mol BW, Lambalk CB et al. (2006) A systematic review of tests predicting ovarian reserve and IVF outcome. Human reproduction update 12(6): 685-718.
- Bukman A, Heineman M (2001) Ovarian reserve testing and the use of prognostic models in patients with subfertility. Human reproduction update 7(6): 581-590.
- Scheffer G, Broekmans F, Bancsi L, Habbema JD, Looman CW, et al. (2002) Quantitative transvaginal two‐and three‐dimensional sonography of the ovaries: reproducibility of antral follicle counts. Ultrasound Obstet Gynecol 20(3): 270-275.
- Benacerraf BR, Benson CB, Abuhamad AZ, Copel JA, Abramowicz JS, et al. (2005) Three‐and4‐dimensional ultrasound in obstetrics and gynecology: proceedings of the American Institute of Ultrasound in Medicine Consensus Conference. J Ultrasound Med 24(12): 1587-1597.
- Jayaprakasan K, Walker K, Clewes J, Johnson IR, Raine-Fenning NJ, et al. (2007) The interobserver reliability of off‐line antral follicle counts made from stored three‐dimensional ultrasound data: a comparative study of different measurement techniques. Ultrasound in Obstetrics and Gynecology 29(3): 335-341.
- Mercé LT, Gómez B, Engels V, Bau S, Bajo JM, et al. (2005) Intraobserver and interobserver reproducibility of ovarian volume, antral follicle count, and vascularity indices obtained with transvaginal 3-dimensional ultrasonography, power Doppler angiography, and the virtual organ computer- aided analysis imaging program. J Ultrasound Med 24(9): 1279-1287.
- Brett S, Bee N, Wallace WH, Rajkhowa M, Kelsey TW, et al. (2009) Individual ovarian volumes obtained from 2-dimensional and 3-dimensional ultrasound lack precision. Reprod Biomed Online 18(3): 348-351.
- Neto C, Ludwin A, Borrell A, Benacerraf B, Dewailly D, et al. (2018) Counting ovarian antral follicles by ultrasound: a practical guide. Ultrasound Obstet Gynecol 51(1): 10-20.
- Deb S, Campbell BK, Clewes JS, Raine‐Fenning NJ (2010) Quantitative analysis of antral follicle number and size: a comparison of two‐dimensional and automated three‐dimensional ultrasound techniques. Ultrasound Obstet Gynecol 35(3): 354-360.
- Moawad NS, Gibbons H, Liu J, Lazebnik N (2009) Comparison of 3‐and 2‐Dimensional Sonographic Techniques for Counting Ovarian Follicles. J Ultrasound Med 28(10): 1281-1288.
- Weenen C, Laven JS, Bergh VAR, Cranfield M, Groome NP, et al. (2004) Anti‐Müllerian hormone expression pattern in the human ovary: potential implications for initial and cyclic follicle recruitment. Mol Hum Reprod 10(2): 77-83.






























