DNA Fragmentation Influence on ICSI Aspects
Ayman El-Dorf1* and Mohamed Moustafa El Zayat2
1Department of Obstetrics and Gynecology, Tanta University, Egypt
2Department of Genetic Engineering and Biotechnology, Royal Fertility Center, Egypt
Submission: September 13, 2019;Published: October 11, 2019
*Corresponding author: Ayman El-Dorf, Department of Obstetrics and Gynecology, Tanta University, Egypt
Ayman El-Dorf, Mohamed Moustafa El Zayat. DNA Fragmentation Influence on ICSI Aspects. J Gynecol Women’s Health. 2019:16(5): 555950. DOI: 10.19080/JGWH.2019.16.555950
Abstract
Background: Research efforts all over the globe are gaining interest in the sperm DNA fragmentation rates as a rate limiting step in ICSI management cycles success. ICSI management cycles select the sperm according to its morphological appearance could select a sperm that have damaged chromatin casing epigenetic and genetic issue within the developing embryo.
Aim: To evaluate and assess embryonic development and quality levels, besides the genetic expression of apoptosis-related genes and microRNAs in embryo in accordance to sperm DNA fragmentation categorical level.
Methodology: A prospective clinical research trail on which semen and germinal Vesicle (GV) oocytes were obtained after written consent (60 ICSI management cycles) from whom at least two and maximum of 15% of punctured oocytes were in the GV stage after ovarian stimulation. Inclusive research criteria were male factor and unexplained infertility issues; and exclusive research criteria was female factor infertility. Long protocol was implemented for ovarian stimulation in all study subjects. cases age range was 25 till 45 years in men and women recruited. Duration of abstinence was from 3 to 5 days research groups were categorized according to sperm DNA fragmentation SDF <30% and SDF> 30% research groups.
Result: statistical correlation SDF% with fertilization rate, cleavage rate, embryo quality score and morula formation rate have shown statistical significance concerning fertilization rate, embryo quality score (p values <0.001) and no statistical significance as regards Cleavage and Morula formation rates (p values =0.315, 0.198 consecutively).
Conclusion: High sperm DNA fragmentation is directly correlated to reduced fertilization rates and embryo quality scores. However, the current research study results require verification by multicentric fashion research efforts in a larger sample sizes with consideration of racial and ethnic differences that could affect the results.
Keywords: ICSI; DNA fragmentation; Fertilization; Ethnic differences; Sperm; Epigenetic abnormalities; Pro-apoptotic microRNAs; Germinal Vesicle; Punctured oocytes
Introduction
Half of the developing embryo genetic makeup is derived from the sperm DNA. sperm genetic makeup integrity is a cornerstone factor that could affect the normal cellular and molecular levels of embryonic course of development .furthermore it could be responsible for the rates of successful implantation [1-3].
Research efforts all over the globe are gaining interest in the sperm DNA fragmentation rates as a rate limiting step in ICSI management cycles success .Genetic makeup of the sperm is affected by various factors that could be influenced by toxic exposure from reactive oxygen species ven at epigenetic levels and proper full capacity of gene function are affected due to exposure of genetic material to toxic agents [4-6].
ICSI management cycles select the sperm according to its morphological appearance could select a sperm that have damaged chromatin casing epigenetic and genetic issue within the developing embryo [7,8]. Research debate exists around the fertilization and embryonic rates among cases that have high sperm DNA fragmentation rates .that denotes that the molecular integrity of sperm DNA affects the success of all phases of embryonic and fetal development even if conception occurs and continues till full term there is a clinical risk of epigenetic abnormalities or deficits .in an interesting manner molecular and cellular research efforts have revealed that Sperm doesn’t have the capability to repair DNA fragmentation issues that could occur [9,10].
On the other hand, it was previously reported by embryonic and genetic research efforts that after sperm injection and entrance within the oocyte, molecular and genetic repair tools within matured oocytes could eliminate some of repairable DNA damage of sperm and consequently, embryonic development occurs in a normal manner. If the oocyte wasn’t capable of sperm DNA damage repair a cellular process occurs called apoptosis that is considered a form of natural selection and elimination of damaged biological cells [11,12].
The micro RNA levels are raised in cases of apoptosis that is correlated and linked to reduced embryonic survival rate all those molecular and cellular events occur due to raised expression patterns of apoptosis related genes that as a normal consequence raise the serum levels of pro-apoptotic microRNAs [13,14].
Aim of the Work
To evaluate and assess embryonic development and quality levels, besides the genetic expression of apoptosis-related genes and microRNAs in embryo in accordance to sperm DNA fragmentation categorical level.
Methodology
A prospective clinical research trail held in Tanta University teaching hospital in the ART unit of gynecology department through a year of time from January to December 2018, on which semen and germinal Vesicle (GV) oocytes were obtained after written consent (60 ICSI management cycles) from whom at least two and maximum of 15% of punctured oocytes were in the GV stage after ovarian stimulation. Inclusive research criteria were male factor and unexplained infertility issues; and exclusive research criteria was female factor infertility. Long protocol was implemented for ovarian stimulation in all study subjects. cases age range was 25 till 45 years in men and women recruited. duration of abstinence was from 3 to 5 days research groups were categorized according to sperm DNA fragmentation SDF <30% and SDF> 30% research groups. Semen sample collection and preparation Semen samples have been obtained by masturbation, liquefied at 37degree Celsius and 5% CO2 for around 30min, and prepared by density gradient centrifugation protocol.
Sperm DNA Fragmentation Evaluation
Processed semen sample have been mixed with 1% low gelling agarose, layered on glass slide pre-coated with standard agarose and placed in 4 degree Celsius for 5min. HCl solution have been added and then the slide incubated within lysis buffer solution for 15min). The buffer was added (2min) and next, the slide dehydrated in 70, 90 and 100% of alcohol, consecutively, 2min for each concentration. After staining with Diff-quick, halo size of 300 spermatozoa analyzed under light microscope. Four dispersion pattern considered; large, medium, small sizes and without halo or degenerated nuclei. Sperm cells with small or without halo considered as sperm containing fragmented DNA.
Ovarian stimulation followed by ICSI With the Reactive oxygen species assessment
In culture medium The ROS production was evaluated by the chemiluminescence assay using luminol the media (400mL) was mixed with luminol (10mL) and located into a Luminometer in the integrated mode for 15min. The results were expressed in relative light units (RLU) per sec.
Evaluation of embryo quality and developmental rate
The embryos with symmetrical blastomeres and less than 10% of cytoplasmic fragmentation categorized as grade A embryos and score 3 was given to them. Score 2 was used to the grade B embryos with equal size blastomeres and 10-50% of cytoplasmic fragmentation. Score 1 was given to grade C embryos with unsymmetrical blastomeres and above 50% of fragmentation. The total embryo quality score was assessed as a sum of the embryo quality scores in each ICSI cycle to the total number of developed embryos. Cleavage rate was assessed as the number of cleaved embryos in day 3 to total number of fertilized oocytes.
Total RNA extraction and cDNA synthesis of human embryo
Four embryos in morula stage were transferred to microtube containing lysis buffer (1.5mL) for total RNA extraction. Next, complementary DNA (cDNA) synthesis was performed by adding Poly N (2mL), nuclease free water (5mL), primers of miR 15a and miR 16-1 and snord 47 (2mL of each) to 2mL of each embryo sample. The reaction accomplished in a thermocycler (5 min at 75 _C). Reverse transcription (RT) carried out by addition of 5mL of RT buffer, 3mL of 10mM dNTP, 1mL of 200u RT enzyme and 0.25mL of 10u RNase inhibitor to reaction tube and set out as follow: 25 degree Celsius for 10min, 37 degree Celsius for 15min, 42 degree Celsius for 45min, and 72 degree Celsius for 10min. The synthesized cDNA was kept in 4 degree Celsius overnight.
Quantitative real time PCR (q-RT PCR)
The expression level of apoptotic related genes (BCL-2 and BAX) and miroRNAs (miR 15a and miR 16-1) was assessed by q-RT PCR using Rotor Gene Q instrument. Briefly, cDNA (1mL), DEPS water (4.5mL) and specific primer for each genes and miRNAs (1 mL of each them) were subjected to q-RT PCR via DNA Master SYBR Green I mix for a total volume of 13mL according to instructions in manual. Reactions took place at 95 _C for 30 sec in order to initiate denaturation, and then be amplified in 40 cycles using a denaturing temperature of 95 degree Celsius for 3min, and annealing/ extension temperature of 60 _C for 30sec. Melting curve analysis was performed to confirm a single genespecific peak of all individual amplification reactions and lack of primer dimers. For normalization, the b-actin gene for apoptotic related genes and also, snord 47 for pro-apoptotic miRNAs were used as reference genes. Primers sequence, product size and annealing temperature were listed in our previous study.
Statistical Analysis
Data were collected, revised, coded and entered to the Statistical Package for Social Science (IBM SPSS) version 23. The quantitative data were presented as mean, standard deviations and ranges when their distribution found parametric. Also qualitative data were presented as number and percentages. The comparison between two independent groups with qualitative data was done by using Chi-square test while comparison between two independent groups with quantitative data and parametric distribution was done by using Independent t-test. Pearson correlation coefficients were used to assess the correlation between two quantitative parameters in the same group. The confidence interval was set to 95% and the margin of error accepted was set to 5%. So, the p-value was considered significant at the level of < 0.05.
Result
Table 1 and Figures 1-5 reveal and display the comparative statistical analysis between SDF ≤ 30% group and SDF > 30% research groups as regards the investigated parameters in which there was no statistical significant difference as regards Female age (years), Male age (years), BMI (Kg/m2), Causes of infertility, Average oocytes retrieved, Cleavage rate (%), Morula formulation rate (%), Number of total cells, Apoptotic cell rate ( p values=0.230, 0.114, 0.648, 0.516, 0.870, 0.061, 0.253, 0.375, 0.155 consecutively) however there was statistically significantly higher levels of Fertilization rate (%), ROS level (x106cpm), Embryo quality score (p values <0.001).
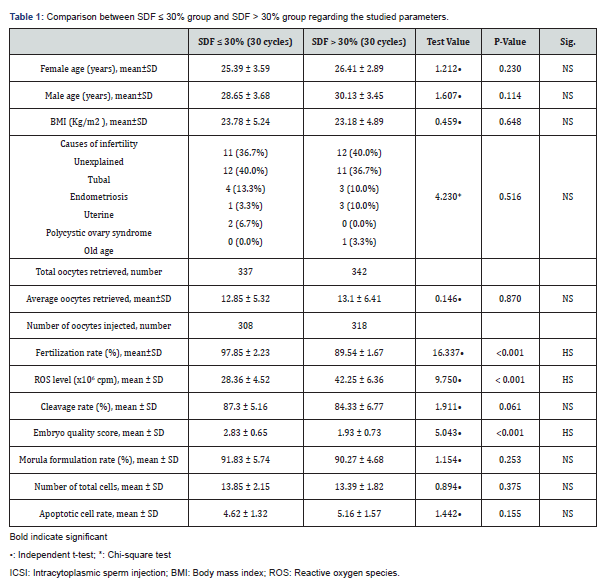





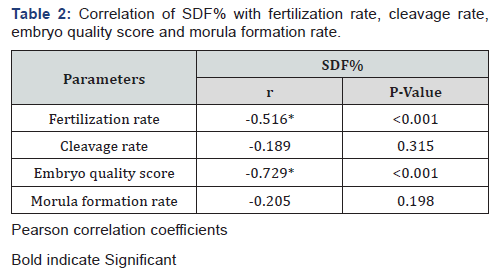
Table 2 reveals and displays that the statistical correlation SDF% with fertilization rate, cleavage rate, embryo quality score and morula formation rate have shown statistical significance concerning Fertilization rate, embryo quality score (p values <0.001) and no statistical significance as regards Cleavage and Morula formation rates (p values =0.315, 0.198 consecutively).
Discussion
Male factor infertility represents half of all etiological of infertility. A growing research interest have been conducted in investigating sperm DNA fragmentation as an underlying etiology for of unexplained infertility issues. The raised DNA damage levels in sperms derived from males having oligozoospermia appears to be correlated and linked to reduced probability of natural conception and a raised risk for early pregnancy loss. Sperm DNA quality is an integral factor that affects the embryonic DNA quality and following development of the embryo at molecular and cellular levels [1,3,7,9].
A prior research study investigating the frequency of apoptosis among embryos derived from oocytes according to sperm DNA integrity levels, showing among their research study result that there is lower rate of embryonic quality scoring besides they have revealed s higher apoptosis rate with cases having sperm DNA fragmentation above 30% in comparison to research subjects having to sperm DNA fragmentation below 30% [2,5,8].
Another prior research effort has displayed that SDF had a negative correlation with embryonic quality and blastocyst formation. Interestingly in harmony with the current research study results it was observed that the fertilization rate is reduced in IVM-MII oocytes in presence of high levels of sperm DNA fragmentation besides it was revealed that as sperm DNA fragmentation is increased there is, defective rate of decompensation [4,10,11].
An interesting fact that justifies the current study findings is that reduced embryonic quality could be due to failure to repair the sperm DNA damage after fertilization making SDF a detectability tool for embryonic quality molecular and cellular highly complex embryonic development process that is highly sensitive to reactive oxygen species. a prior research group of investigators have revealed that the apoptosis pathway is triggered within embryos derived from IVM oocytes and high levels of sperm DNA fragmentation molecular level of DNA repair is a crucial part of all embryonic genome formation processes [7,12]. Another prior research effort mentioned among their high quality oocytes -matured in in vivo condition could avoid the negative impact of sperm DNA damage on gestation probably due to high level of required sperm DNA repair enzymatic system in these oocytes [5,9,10].
Conclusions and Recommendations
High sperm DNA fragmentation is directly correlated to reduced fertilization rates and embryo quality scores. However, the current research study results require verification by multicentric fashion research efforts in a larger sample sizes with consideration of racial and ethnic differences that could affect the results.
References
- Gonzalez-Marin C, Gosalvez J, Roy R (2012) Types, causes, detection and repair of DNA fragmentation in animal and human sperm cells, Int J Mol Sci 13(11) 14026e-14052.
- Zhao J, Zhang Q, Wang Y, Li Y (2014) Whether sperm deoxyribonucleic acid fragmentation has an effect on pregnancy and miscarriage after in vitro fertilization/ intracytoplasmic sperm injection: a systematic review and metaanalysis. Fertil Steril 102(4): 998-1005.
- Haghpanah T, Salehi M, Ghaffari NM, Farahani RM, Fadaei-Fathabadi F, et al. (2015) Does sperm DNA fragmentation affect the developmental potential and the incidence of apoptosis following blastomere biopsy? Syst Biol Reprod Med 62(1): 1-10.
- Farsi MM, Kamali N, Pourghasem M (2013) Embryological aspects of oocyte invitro Int J Mol Cell Med 2(3): 99-109.
- Haghpanah T, Eslami-Arshaghi T, Afarinesh M, Salehi M (2017) Decreased fertilization: human sperm DNA fragmentation and invitro maturation of oocyte in stimulated ICSI cycles. Acta Endocrinol (Buchar) 13(1): 23-31.
- Saeedabadi S, Abazari-Kia AH, Rajabi H, Parivar K, Salehi M, et al. (2018) Melatonin improves the developmental competence of goat oocytes, Int J Fertil Steril 12(2): 157-163.
- Zheng W, Song G, Wang Q, Liu S, Zhu X, et al. (2018) Sperm DNA damage has a negative effect on early embryonic development following invitro fertilization, Asian J Androl 20(1): 75-79.
- Meseguer M, Santiso R, Garrido N, Garcia-Herrero S, Remohi J, et al. (2011) Effect of sperm DNA fragmentation on pregnancy outcome depends on oocyte quality. Fertil Steril 95(1): 124-128.
- Virant-Klun I, Knez K, Tomazevic T, Skutella T (2013) Gene expression profiling of human oocytes developed and matured invivo or invitro. BioMed Res Int.
- Jungwirth A, Giwercman A, Tournaye H, Diemer T, Kopa Z, et al. (2012) European Association of Urology guidelines on Male Infertility: the 2012 up-date. Eur Urol 62(2): 324-332.
- Agarwal A, Majzoub A, Esteves SC, Ko E, Ramasamy R, et al. (2016) Clinical utility of sperm DNA fragmentation testing: practice recommendations based on clinical scenarios. Transl Androl Urol 5(6): 935-950.
- Cissen M, Wely MV, Scholten I, Mansell S, Bruin JP, et al. (2016) Measuring sperm DNA fragmentation and clinical outcomes of medically assisted reproduction: a systematic review and meta-analysis. PLoS One 11(11): e0165125.
- Borges EJr, Zanetti BF, Setti AS, Paesde Almeida FBD, Provenza RR, et al. (2019) Sperm DNA fragmentation is correlated with poor embryo devel-opment, lower implantation rate and higher miscarriage rate in reproductive cycles of non-male factor infertility. Fertil Steril 112(3): 483-490.
- Practice Committee of the American Society for Reproductive (2013) The clinical utility of sperm DNA integrity testing: a guideline. Fertil Steril 99(3): 673-677.






























