Beta Fibrinogen (Rs1800790) and Angiotensin I Converting Enzyme (Rs4646994) Gene Polymorphisms in Recurrent Pregnancy Loss: A Case Control Study
Noha M Issa1, Lama M El-Attar1*, Dalia A Moaty El-Neily2 and Sally S El Tawab3
1Department of Human Genetics, Alexandria University, Egypt.
2Department of Clinical Pathology, Alexandria University, Egypt
3Department of Obstetrics and Gynecology, Alexandria University, Egypt<sup>/i>
Submission: September 1, 2019;Published: September 17, 2019
*Corresponding author: Lama M El-Attar, Department of Human Genetics, Alexandria University, Egypt
Noha M Issa1, Lama M El-Attar, Dalia A Moaty El-Neily, Sally S El Tawab. Beta Fibrinogen (Rs1800790) and Angiotensin I Converting Enzyme (Rs4646994) Gene Polymorphisms in Recurrent Pregnancy Loss: A Case Control Study. J Gynecol Women’s Health. 2019: 16(4):555942. DOI: 10.19080/JGWH.2019.16.555942
Abstract
Background: Recurrent pregnancy loss (RPL) is a devastating and stressful problem for conceived women all over the world. Considering the tendency to thrombosis during the course of pregnancy, genetic analysis for inherited thrombophilia can be helpful. The aim of the study is to investigate the correlation between RPL and common polymorphisms of Beta fibrinogen (rs1800790) and Angiotensin I converting enzyme (ACE) (rs4646994) genes in women who had RPL of unknown cause.
Methods: Eighty women with RPLs and eighty women without a previous history of abortion were included in the current case control study. Genotyping of Beta fibrinogen (rs1800790) and ACE (rs4646994) gene polymorphisms was done by PCR-RFLP.
Result: The frequency of DD, ID, II genotypes were 47.5%,38.7%,13.7% in women with RPL respectively, versus 31.25%, 46.25%, 22.5% in control group. (P=0.086). D allele frequency was 0.67in RPL group and 0.54 in control (P 0.022, OR 1.694; 95% CI: 1.08-2.67). There was lack of association between B fibrinogen gene polymorphism and RPL in the studied groups.
Conclusion: Genotyping for ACE I/D gene polymorphism is valuable during screening for inherited thrombophila in women with RPLas D allele carriers are more prone to RPL than I allele ones.
Keywords: Recurrent pregnancy loss; ACE gene; B-fibrinogen gene; Polymorphism; Thrombophila
Abbreviations: ASRM: American Society for Reproductive Medicine; RPL: Recurrent pregnancy loss; ESHRE: European Society of Human Reproduction and Embryology; APS; Anti-Phospholipid Syndrome; ACE: Angiotensin I Converting Enzyme.
Introduction
has been defined over decades as three or more miscarriages before 24 weeks of gestation [1]. The definition was revised by the American Society for Reproductive Medicine (ASRM) in 2013, and again by European Society of Human Reproduction and Embryology (ESHRE) in 2018; as having two or more failed pregnancies. It was believed that this new definition will facilitate research, shared decision-making and psychological support to couples. In addition, testing for anti-phospholipid syndrome (APS); a treatable cause of RPL, can be performed after two losses [2,3].
The worldwide prevalence of RPL is about 1-2% in married couples. Several causes are involved in the etiology, including endocrinal, infectious factors, uterine anatomical abnormalities, parental chromosomal aberrations and immunological factors. However, in many cases, routine gynecological, endocrinal and cytogenetic diagnostics could not clarify the reason [1-3]. Among these, the heritable factors of thrombophilia that may predispose to obstetric complications attract a great attention.
Angiotensin I converting enzyme (ACE) gene was first discovered by Rigat et al in 1990. It is mapped to the long arm of chromosome 17 (17q23) and encodes ACE enzyme which plays a major role in the control of renin angiotensin system (RAS) and fibrinolysis regulation in the body through its conversion of angiotensin I to angiotensin II. The well known ACE I/D gene polymorphism (SNP rs4646994) results frominsertion-I or deletion-D of a 287 bpAlu repeat element at intron 16 in ACE gene resulting in three genotypes DD, ID and II [4]. The importance of RAS and blood homeostasis during pregnancy made this polymorphism and its effect on the pregnancy outcome a target to scientists all over the world [5].
Beta fibrinogen gene (FGB)is located at 4q31.3 and encodes Beta chain of fibrinogen. Enzymatic cleavage of fibrinogen (factor 1) by thrombin will form fibrin that is the corner stone for blood clot formation after tissue injury. In addition, fibrinogen has its role in cell adhesion regulation, vasoconstriction and angiogenesis. A common variant of Beta fibrinogen genecaused by G/A substitution in the promoter region is found to be associated with 7-10% higher plasma fibrinogen levels [6]. In pregnant women this increase may enhance the physiologically elevated fibrinogen levels tendency to thrombosis [7].
For normal implantation and intrauterine fetal development, adequate feto-placental circulation is a must depending on the balance between coagulation and fibrinolysis cascades. Conflicting results were seen in finding the link between genetic risk factors and tendency to thrombosis in women with unexplained RPL. The aim of this study was to investigate whether the common variants of β-fibrinogen (rs1800790)and Angiotensin I converting enzyme (ACE) gene(rs4646994) are associated with unexplained RPL.
Materials and Methods
Study design
The current case control study included 160 women, divided into two groups: group one included 80 females who had at least three RPLs ≤24 weeks of gestation referred from El-Shatby Teaching Hospital , Faculty of medicine to the Genetic Clinic, Medical Research institute; University of Alexandria, Egypt; for genetic evaluation after exclusion of other causes of recurrent abortion or obstetrical complications during the period between May 2016 to May 2017. Group 2 included age and ethnicity matched 80 healthy women with at least two live births and without a previous history of abortion as a control group.
A written informed consent was obtained from all participants, and approval for conducting the study was obtained by the local ethical committees of the Faculty of Medicine and the Medical research institute. The study protocol was in agreement with Declaration of Helsinki guidelines. All women with RPL accepted to participate in the study were subjected to a complete history and pedigree analysis with full clinical assessment and investigations to exclude other causes of recurrent pregnancy loss as structural uterine abnormalities, infectious diseases, immunological causes and hormonal disturbances at least 6 months after last pregnancy loss. Women with hypertension or Diabetes Mellitus were also excluded from the study.
Sample Collection
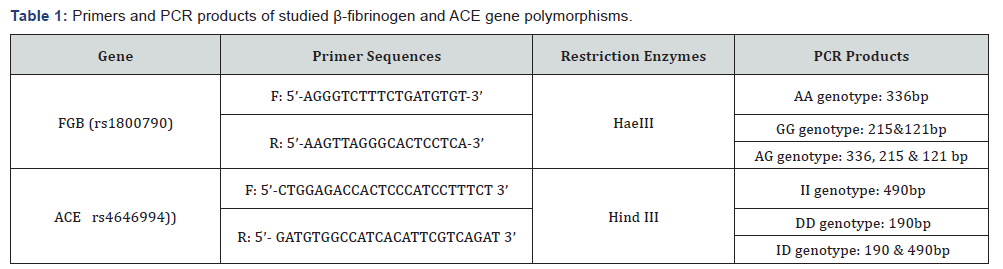
2ml blood were collected from recruited women and their husbands on sodium heparin vacutained tubes for chromosome analysis by trypsin G-banding technique with some modifications to exclude chromosomal aberrations [8]. And 2ml blood samples were collected on EDTA tubes for genomic DNA extraction using QIA Amp ® DNA Blood Mini kits (QIAGEN Hilden, Germany) following the manufacturer’s instructions and stored at -20 0C for use. DNA concentration and purity were measured by Nano Drop TM 1000 spectrophotometer. PCR reaction was done in a total 25μl volume for each SNP, 30ng of genomic DNA were mixed with specific primers according to previous reports [9,10] as illustrated in Table 1.
Genotyping
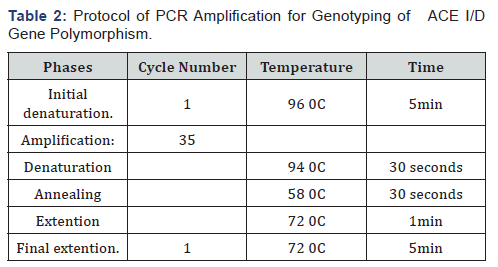
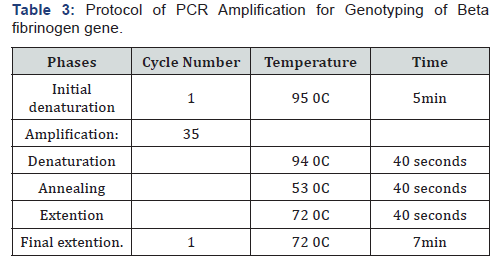
Genotyping of Beta fibrinogen - 455G>A (rs1800790) and ACE I/D (rs4646994) gene polymorphisms was performed using PCR-RFLP method (polymerase chain reaction (PCR)/ restriction fragment length polymorphism (RFLP) according to the protocol mentioned in Tables 2 & 3. At 37 0C for 16 hours, the PCR products were digested with HaeIII restriction enzyme (Thermo Scientific Inc, USA®) for β-fibrinogen gene and by Hind III restriction enzyme (Thermo Scientific Inc, USA®) for ACE gene, then the cleaved bands were visualized on 2% agarose gel electrophoresis after staining with ethidium bromide under UV illumination. The results were confirmed after the repeat of some samples which were randomly selected.
Statistical Analysis
Statistical analyses were performed using IBM SPSS software package version 20.0. (Armonk, NY: IBM Corp).The Hardy- Weinberg equilibrium (HWE) equation was used to calculate the expected genotypes frequencies. Difference between expected and observed genotypes was assessed by Chi square (X2) test. The frequency of the alleles and genotypes was compared between the studied groups by Chi square (X2) test or Fisher exact test depending on the obtained data. The odds ratio (OR) and 95% confidence interval (CI) were estimated in order to assess the risk of RPL in association with the studied gene variants. P-values less than 0.05 were considered statistically significant.
Result
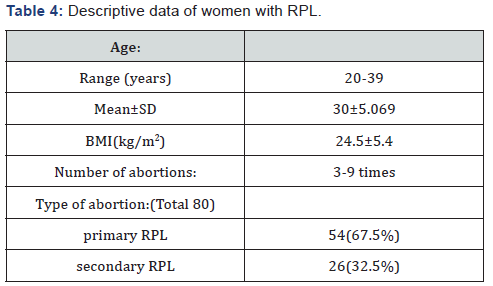
The mean age of women with RPL was 30±5.069 years and in control women it was31±4.076 years. The descriptive data of women with RPL enrolled in the current study were summarized in Table 4.
Genotype and allele frequency of ACEI/D (rs4646994) gene polymorphisms gene polymorphism
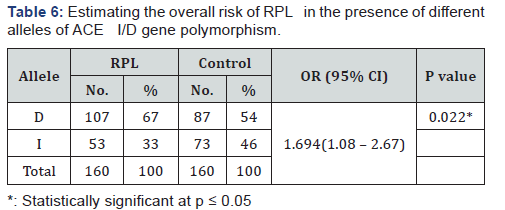
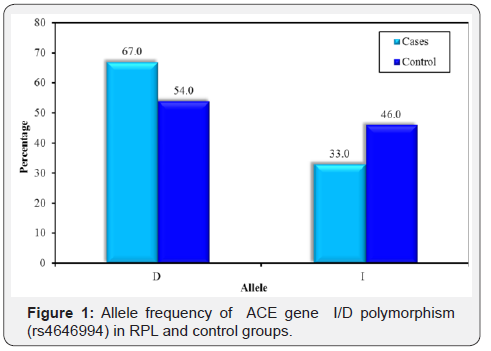
The obtained data regarding ACE I/D gene polymorphism were illustrated in Table 5 in which RPL group as well as control group agreed with Hardy-Weinberg equilibrium (P=0.262 and P=0.543 respectively). The frequency of homozygous DD genotype was found to be higher in RPL group (47.5%) than control group(31.25%) and II genotype was more frequent in control than in RPL (22.5% versus 13.7%) group. The frequency of the heterozygous ID genotype was higher in the control group (46.25%) than RPL group than (38.7%). Although these observations were statistically on significant. (P=0.086). Comparing the allele frequency in RPL and control groups revealed statistically significant increase in the frequency of the D allele in RPL group than control group (0.67 vs. 0.54) (P=0.022). By risk assessment, there was a significant association between D allele and RPL in which carrier women of D allele were at 1.7fold risk to have RPL. (OR 1.694; 95% CI: 1.08 - 2.67) (Table 6) (Figure 1).

455G>A (rs1800790) gene polymorphism
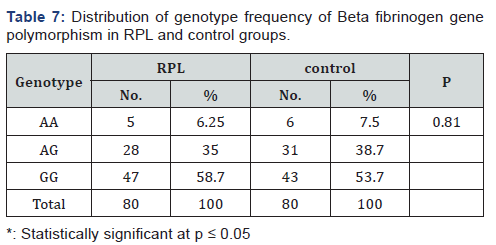
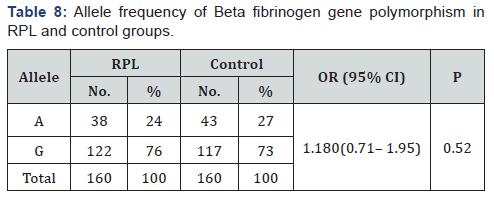
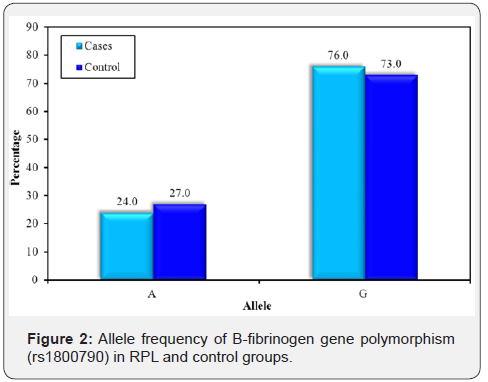
The genotype distribution of Beta fibrinogen gene was in agreement with Hardy-Weinberg equilibrium in RPL affected women and controls (P=0.887 and P=0.899 respectively). There was no statistically significant difference in genotype frequency of Beta fibrinogen gene polymorphism between RPL and control groups (P = 0.810)as illustrated in Table 7, again no significant difference in allele frequency of BFG in RPL group compared to control one(P = 0.520) (Table 8) & (Figure 2).
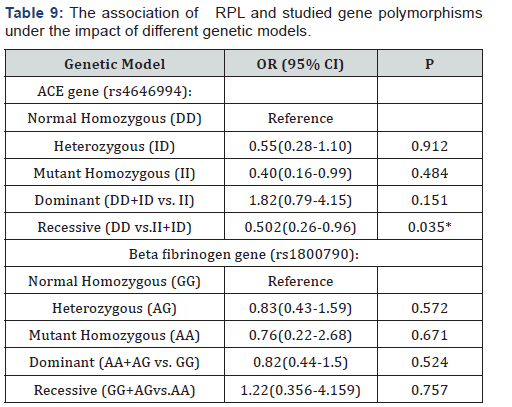
3.3 The impact of different genetic models of Beta fibrinogen– 455G>A (rs1800790) and ACE I/D (rs4646994) gene variants on RPL was assessed by Odds and 95% CI. We observed an increased risk of RPL in patients with the dominant (DD+ID)model (OR:1.82(0.79-4.15)) againit was statistically non significant (P=0.151). A low risk of RPL was observed with recessive (II+ID) model (OR: 0.502(0.26-0.96), P =0.035).Regarding Bfibrinogen gene, there was lack of association of RPL with any genetic model (Table 9).
Discussion
There are several physiological changes that occur during normal pregnancy, hypercoaggulabilty state is one of them in which some coagulation factors increased in blood in harmony with decreased blood level of others. These changes are needed for normal fetal growth and development with complete return to the normal physiological state after labor and purperium without leaving any harm to the mother. However, hypercoaggulabilty state of pregnancy may be exacerbated by alterations in the coagulation/fibrinolysis cascades together with associated venous stasis and hormonal induced endothelial injury throughout the course of pregnancy
The balance between coagulation and fibrinolysis is maintained by the influence of several genetic loci. Gene mutations (whether inherited or acquired) may exacerbate hypercoagulability and predispose to placental vascular thrombosis and subsequent adverse pregnancy outcomes [11] An emerging interest in the recent years was growing to identify the possible role of thrombophilic as well as hypofibrinolytic genes on the susceptibility to RPL in different populations [12,13]. This may guide against the widespread use of therapeutic anticoagulants as a prophylactic regimen in cases of RPL of unidentified cause.
Based on these findings, we encouraged to investigate ACE I/D and Beta fibrinogen–455G>A gene polymorphisms to assess their association with RPL. In the current study we recruited 80 females with RPL and 80 healthy females as control. Regarding ACE I/D gene polymorphism, D allele frequency was significantly higher in RPL group than control with allele frequency (0.67 and 0.54 respectively) (P= 0.022) and 1.8 fold increased risk to RPL in D allele carrier women than I allele carrier women. We found D allele frequency in our cohort was similar to that reported in Arab populations (0.60–0.76) [14] and slightly higher than that in Caucasians (0.46–0.51) [15]. In Egypt, a genetic study from Cairo found D allele frequency was 0.71 [16]. While in other study it was 0.68 in Ismailia and 0.66 in Sinai [14].
The frequency DD genotype in the present study was increased in women with RPL than control (47.5% vs.31.25%), although there was no statistically significant difference between both groups which can be related to relatively small sample size (P=0.086). These findings were further supported by other studies. [17-19] On the other hand, other studies did not find any association between D allele of ACE I/D gene polymorphism and RPL [20-22].
Despite that ACE I/D gene polymorphism (rs4646994) is well studied in different populations, its exact role in the predisposition to RPL is still unclear due to contradictory results which may be attributed to ethnic differences and prevalence of this polymorphism in different populations. Other factors related to study design, definition of repeated abortion whether 2 times or three times of pregnancy loss before 20th week of gestation, inclusion and exclusion criteria of studied cases to be considered. Genetic factors (gene-gene interaction, geneenvironment interaction and epigenetic regulations) and their effects cannot be ignored.
It is well known that ACE gene encodes ACE enzyme which plays an important role in the renin angiotensin system. During normal pregnancy ACE has its impact on maternal and fetal health. As regards maternal side: ACE is an essential regulator of blood pressure and water-electrolyte hemostasis while on the fetal side it has its own fingerprint on implantation, fetoplacental microvasculature and fetal development [23]. Carrier individuals with D allele have higher plasma level of ACE enzyme than carriers of I allele. High ACE level enhances the conversion of angiotensin I to angiotensin II, increases endothelial plasminogen activator inhibitor-1 (PAI-1), placental plasminogen activator inhibitor-2 (PAI-2) and bradykinines production lead to reduced nitric oxide levels. These alterations result in hypofibrinolysis and increase tendency to intravascular thrombosis [24]. In order to study the relative risk to RPL under different genetic models of Beta fibrinogen – 455G>A (rs1800790) and ACE (rs4646994) gene variants, ORs and 95% CIs were used in the current study. We foundthe risk was significantly low with II+IDrecessive model (OR: 0.502(0.26-0.96), P=0.035). The risk was 1.82highinDD+ID dominantmodel (OR: 1.82(0.79-4.15),but it was statistically non significant (P=0.151). Overall, we cannot assume the recessive model of ACE I/D polymorphism exerts a protective role against RP Lunless these observations are confirmed by a large comprehensive study.
The most common variant of Beta-fibrinogen gene results from-455G/A substitution at the promoter region is found to be associated with high levels of fibrinogen in plasma and subsequent increased risk of thrombosis and fibrin clot formation [25]. In the present study, there was lack of association between RPL and Beta fibrinogen – 455G>A gene polymorphisms; neither genotypes nor alleles in our population. The same was reported by Alastal et al. [26] A meta-analysis done by Li et al. [27] concluded no significant association between Beta fibrinogen– 455G>A polymorphism and recurrent pregnancy loss under different genetic models, in the Asian and Caucasian subgroups. Hardly we found one Egyptian study reported a significant association between RPL and Beta fibrinogen–455G>A genotypes and G allele [28] which matched with Ticconi et al. [6] and Torabi et al. [29].
There was paucity of studies concerning Beta fibrinogen– 455G>A gene polymorphism and risk of RPL especially in Arab countries. Overall, most studies failed to find meaningful association of Beta fibrinogen– 455G>A polymorphism with RPL. But when screening for this polymorphism was done in combination with other thrombophilic genes, significant results were obtained in some studies [30].
Finally, the discrepancy between studies regarding the genetic predisposition of thrombophilia made many clinicians more satisfied to routine genetic screening for thrombophila and prophylactic anticoagulant use in cases of RPL. However, the new ESHRE guidelines published in 2018 [3] recommended that genetic screening should not be done routinely in any woman with RPL unless there is evidence of other risk factors for thromboembolism in association with inherited thrombophila; in such cases antithrombotic prophylaxis (heparin and low-dose aspirin) may improve the live birth rate.
Conclusion
There was a clear evidence that D allele of ACE I/D gene polymorphism is a potential risk factor for RPL in our study in absence of evident role of Beta fibrinogen – 455G>A gene polymorphism. In view of mentioned previous data, we believe that ACE I/D gene need to be added to the screening gene panel of thrombophilia for women with RPL. Indeed, more studies are required to clarify the hidden role of inherited thrombophilia and its relation to antithrombotic drug response in women with RPL under genetic background.
Declaration
a) Ethics approval and consent to participate:
b) The study was approved by the ethics committee of the Medical Research Institute, Alexandria University, Egypt.
c) A written informed consent was obtained from all women before participation in the study according to the Declaration of Helsinki.
Consent for Publication
a) A written consent for publication was obtained from all participants.
b) Availability of data and material:
c) All data generated or analyzed during this study are included in this published article.
Competing Interests
The authors declare that they have no competing interests.
Funding
This research did not receive any specific grant from funding agencies in the public, commercial, or not-for-profit sectors.
Acknowledgment
We are thankful to all females who participated in this study.
Author Contributions
a) Noha M Issa: Collected primary data, providing the study patients, shared in lab work, final review and approval of the manuscript to be published
b) Lama M El-Attar: Conceptualized and designed the study, shared in lab work, interpretation of results, drafted the initial manuscript
c) Dalia AM El-Neely: Shared in the lab work, supervised the statistical analysis, and revised the initial manuscript.
d) Sally S El-Tawab: Shared in the selection of participants, clinical examination of the studied cases, revised the clinical points in the initial manuscript.
All authors reviewed and approved the final manuscript as submitted and agreed to be accountable for all aspects of the work.
References
- RCOG (2011) Recurrent Miscarriage, Investigation and Treatment of Couples.
- Pfeifer S, Goldberg J, Lobo R, Thomas M, Widra E, et al. (2013) Definition of infertility and recurrent pregnancy loss: A Committee Opinion. Fertil Steril 99(1): 63.
- Atik RB, Christiansen OB, Elson J, Kolte AM, Lewis S, et al. (2018) ESHRE guideline: recurrent pregnancy loss. Human Reprod Open 2: 4.
- Rigat B, Hubert C, Alhenc-Gelas F, Cambien F, Corvol P, et al. (1990) An insertion/deletion polymorphism in the angiotensin I-converting enzyme gene accounting for half the variance of serum enzyme levels. J Clin Invest 86(4): 1343-1346.
- Kusmierska-Urban K, Rytlewski K, Huras H (2015) Associations of ACE I/D and AGT M235T Gene Polymorphisms with the Gestational Hypertension and the Fetal Growth. Obstet Gynecol Int J 2(1): 26-36.
- Tybjaerg-Hansen A, Agerholm-Larsen B, Humphries SE, Abildgaard S, Schnohr P, et al. (1997) A common mutation (G-455→A) in the β-fibrinogen promoter is an independent predictor of plasma fibrinogen, but not of ischemic heart disease. A study of 9,127 individuals based on the Copenhagen City Heart Study. J Clin Invest 99(12): 3034-3039.
- Ticconi C, Mancinelli F, Gravina P, Federici G, Piccione E, et al. (2011) Beta-fibrinogen G-455A polymorphisms and recurrent miscarriage. Gyecol Obstet Invest 71(3): 198-201.
- SeaBright M (1971) A rapid banding technique for human chromosomes. Lancet 2(7731): 971-972.
- Yaren A, Turgut S, Kursunluoglu R, Oztop I, Turgut G, et al. (2006) Association between the polymorphism of the angiotensin-converting enzyme gene and tumor size of breast cancer in premenopausal patients. Tohoku J Exp Med 210(2): 109-116.
- Xiang-feng L, Hong-jiang Y, Xiao-yang Z, Lai-yuan W, Jian-feng H, et al. (2008) Influence of fibrinogen β-chain gene variations on risk of myocardial infarction in a Chinese Han population. Chin Med J 121(16): 1549-1553.
- Bremme KA (2003) Haemostatic changes in pregnancy. Best Pract Res ClinHaematol 16(2):153-168.
- Kelly MN, Feroza D, Roy F (2012) Recurrent miscarriage and thrombophilia. Current Opinion in Obstetrics and Gynecology 24: 229-234.
- Farahmand K, Totonchi M, Hashemi M, Reyhani SF, Kalantari H, et al. (2016) Thrombophilic genes alterations as risk factor for recurrent pregnancy loss. J Matern Fetal Neonatal Med 29(8): 1269-1273.
- Salem AH, Batzer MA (1999) High frequency of the D allele of the angiotensin-converting enzyme gene in Arabic populations. BMC Res Notes 2: 99.
- Ulu A, Elsobky E, Elsayed M, Yıldız Z, Tekin M, et al. (2006) Frequency of five thrombophilic polymorphisms in the Egyptian population. Turk J Haematol 23(2):100-103.
- Yang C, Fangfang W, Jie L, Yanlong Y, Jie W, et al. (2012) Angiotensin-converting enzyme insertion/deletion (I/D) polymorphisms and recurrent pregnancy loss: a meta-analysis. J Assist Reprod Genet 29(11): 1167-1173.
- Corbo RM, Ulizzi L, Piombo L, Scacchi R (2011) Association of ACE I/D polymorphism and recurrent miscarriages in an Italian population with a pre-modern reproductive pattern. Ann Hum Biol 38(1): 102-105.
- Gumus E (2018) The powerful association of angiotensin-converting enzyme insertion/deletion polymorphism and idiopathic recurrent pregnancy loss. Gine kologia Polska 89(10): 573-576.
- Fazelnia S, Farazmandfar T, Hashemi-Soteh SMB (2016) Significant correlation of angiotensin converting enzyme and glycoprotein IIIa genes polymorphisms with unexplained recurrent pregnancy loss in north of Iran. Int J Reprod Biomed 14(5): 323-328.
- Chatzidimitriou M, Chatzidimitriou D, Mavridou M, Anetakis C, Chatzopoulou F, et al. (2017) Thrombophilic gene polymorphisms and recurrent pregnancy loss in Greek women. Int J Lab Hematol 39(6): 590-595.
- Kurzawińska G, Barlik M, Drews K, Różycka A, Seremak-Mrozikiewicz A, et al. (2016) Coexistence of ACE (I/D) and PAI-1 (4G/5G) gene variants in recurrent miscarriage in Polish population. Ginekol Pol 87(4): 271-276.
- Pereza N, Ostojić S, Zdravčević M, Volk M, Kapović M, et al. (2016) Insertion/deletion polymorphism in intron 16 of ACE gene in idiopathic recurrent spontaneous abortion: case-control study, systematic review and meta-analysis. Reprod Biomed Online 32(2): 237-246.
- Lumbers ER, Pringle KG (2014) Roles of the circulating rennin angiotensin-aldosterone system in human pregnancy. Am J Physiol Regul Integr Comp Physiol 306(2): R91-R101.
- Goodman C, Hur J, Goodman CS, Jeyendran RS, Coulam C, et al. (2009) Are polymorphisms in the ACE and PAI-1 genes associated with recurrent spontaneous miscarriages? Am J ReprodImmunol 62(6): 365-370.
- Kerlin B, Cooley BC, Isermann BH, Hernandez I, Sood R, et al. (2004) Cause-effect relation between hyperfibrinogenemia and vascular disease. Blood 103(5): 1728-1734.
- Al-Astal MG, Sharif FA (2014) Beta-fibrinogen (-455 G/A) and Integrin beta-3 (PLA1/A2) polymorphisms and recurrent pregnancy loss in Gaza strip-Palestine. Int J Reprod Contracept Obstet Gynecol 3: 134-138.
- Li J, Wu H, Chen Y, Wu H, Xu H, et al. (2015) Genetic association between FXIII and β fibrinogen genes and women with recurrent spontaneous abortion: a meta- analysis. J Assist Reprod Genet 32(5): 817-825.
- Wafa YA, Oof TF, Hassanien RA, Farahat MI (2016) Relationship between Plasminogen Activator Inhibitor-1 (PAI-1) and Beta Fibrinogen Polymorphisms with Unexplained Recurrent Pregnancy Loss. Med j Cairo Univ 84: 89-95.
- Torabi R, Zarei S, Zeraati H, Zarnani AH, Akhondi MM, et al. (2012) Combination of thrombophilic gene polymorphisms as a cause of increased the risk of recurrent pregnancy loss. J ReprodInfertil 13(2): 89-94.
- Karami F, Askari M, Modarressi MH (2018) Investigating Association of rs5918 Human Platelets Antigen 1 and rs1800790 Fibrinogen β Chain as Critical Players with Recurrent Pregnancy Loss. Med Sci (Basel) 6(4): 98.






























