- Review Article
- Abstract
- Introduction
- What is Already Known?
- The Preovulatory Phase
- The Male Function
- Infertility and cfDNA
- Deoxyribonuclease: DNase
- DNase Production
- DNase Absorption and Bioavailability
- Preliminary Results of the Patented Study
- DNAse Activity Quantification
- cfDNA Isolation
- CfDNA Quantification
- Result
- Clinical Results Patented
- Discussion
- Conclusion
- References
Free Circulating Nucleic Acids and Infertility
Hazout A1, Montjean D2, Cassuto NG3, Belloc S4, Dalleac A4, Tesarik J5 and Benkhalifa M*6
1 ATL R&D Laboratory, France
2 Centre FIV Hopital Saint Joseph, France
3 Drouot Laboratory, France
4 Laboratoire lavergne/Accolab, France
5 MARGen Clinic, Spain
6 ART & Reproductive Genetics Dept and PERITOX Laboratory, France
Submission: August 17, 2018; Published: August 29, 2018
*Corresponding author: Moncef Benkhalifa, ART & Reproductive Genetics Dept and PERITOX Laboratory, University Hospital & School of Medicine. Picardie University Jules Verne, Amiens, France, Tel: 0033677867390; Email :benkhalifamoncef78@gmail.com
How to cite this article: Hazout A, Montjean D, Cassuto NG, Belloc S, Dalleac A, Tesarik J, Benkhalifa M. Free Circulating Nucleic Acids and Infertility. J Gynecol Women’s Health. 2018: 11(3): 555812. DOI: 10.19080/JGWH.2018.11.555812
- Review Article
- Abstract
- Introduction
- What is Already Known?
- The Preovulatory Phase
- The Male Function
- Infertility and cfDNA
- Deoxyribonuclease: DNase
- DNase Production
- DNase Absorption and Bioavailability
- Preliminary Results of the Patented Study
- DNAse Activity Quantification
- cfDNA Isolation
- CfDNA Quantification
- Result
- Clinical Results Patented
- Discussion
- Conclusion
- References
Abstract
Background: Human infertility is due to deficiencies in sperm quality in more than half of the cases. Several authors insist on a better exploration of the sperm but little is known about the quality of oocytes and especially about their competence at the crucial moment of transcription. Low ovarian reserve and sperm DNA damage are supposed to be the main culprits in assisted reproductive technology (ART) failures. The presence of excessive cell-free nucleic acids, mainly cell-free DNA (cfDNA), in the serum of men or women has attracted the attention of many practitioners in cases of the so-called unexplained infertility or embryo implantation failures.
Methods: Several and relevant studies are recalled as well as those of our work preceding an european patent granted in 2013 and titled: « cell freee DNA as a therapeutic target for female infertility and diagnostic marker ». The mean cfDNA value in the group of fertile women was 49,2ng/μl versus 98,5ng/μl in the infertile group, which was statistically significant. The mean cfDNA value in the group of fertile men was 60,6ng/μl and 83,34ng/μl in the infertile group which was no statistically significant difference. Comparing cfDNA in the plasma and Follicular Fluid of 42 patients we demonstrated that mean cfDNA value in the FF was less than plasma CFDNA value in the corresponding patients.
Conclusion: Although the excess of cfDNA in the etiology of infertility is not clearly established, our experience and that of other authors suggest that excess cfDNA, might be a marker of oxidative stress or apoptotic and/or necrotic processes deleterious ART outcomes. This review takes stock of the diagnostic, prognostic and therapeutic potentialities, already known and demonstrated in human reproduction
Keywords: Infertility; ART; Free circulating DNA; Oxidative stress; Apoptotic factors
Abbrevations:ART: Assisted Reproductive Technology; IVF: Invitro Fertilization; cfDNA: Cell free DNA; scfDNA: séminal cfDNA; mtDNA: mitochondrial DNA; CCNA1: cycline A1promoter methylation; DMRT1: Double and mab3 Related Transcription Factor
- Review Article
- Abstract
- Introduction
- What is Already Known?
- The Preovulatory Phase
- The Male Function
- Infertility and cfDNA
- Deoxyribonuclease: DNase
- DNase Production
- DNase Absorption and Bioavailability
- Preliminary Results of the Patented Study
- DNAse Activity Quantification
- cfDNA Isolation
- CfDNA Quantification
- Result
- Clinical Results Patented
- Discussion
- Conclusion
- References
Introduction
The cause of infertility can be determined in most cases. The known male factors include hormonal defects, genital infections, with or without genital tract obstruction, cryptorchidism, varicocele and spernm DNA fragmentation or chromatin decondensation, whereas female infertility is mainly due to a low oocyte quality. However, the occurrence of unexplained infertility varies between 0% and 6%, according to different authors [1].
Taking into consideration abnormal results of the evaluation of ovarian function, hysteroscopy, hysterosalpingography, laparoscopy and comprehensive sperm evaluation including testing of DNA and chromatin integrity, the occurrence of unexplained infertility is reduced dramatically. We also know the deleterious effects of different life-style and environmental factors (smoking, excess caffeine intake, or alcohol and drug abuse). Numerous defects which lead to problems with implantation are unknown and constitute another area of unexplained infertility. Integrins, LIF; G-CSF; GH or other growth factors may affect the percentage of patients with unexplained infertility. But this was not clearly identified.
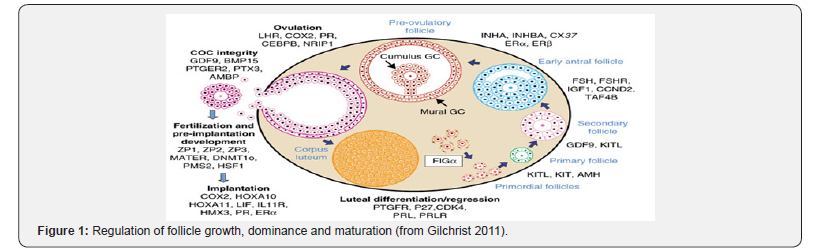
The presence of circulating cell-free DNA in human plasma was reported in 1948 by Mendel and Metais [2]. Cell-free circulating DNA (cfDNA) has been studied in a wide range of physiological and pathological conditions, including inflammatory disorders, oxidative stress and malignancy [3]. It is present in normalhealthy individuals at low concentrations (< 50ng/ml). Although the precise mechanism of DNA release into the blood remains uncertain, it probably derives from a combination of apoptosis, necrosis and active release from cells (Figure 1).
The clearance of cfDNA from the bloodstream occurs rapidly: fetal DNA disappeared from the blood of mothers after delivery with a half life time of 16.3 minutes [4]. It is known that cfDNA is sensitive to plasma nucleases (for example DNase 1), but renal and hepatic clearance are also involved in the elimination of cfDNA. Cultured cells have been shown to release double stranded DNA into the media, and cfDNA might be incorporated into cells [3]. However, this hypothesis remains to be proven.
Circulating DNA can be isolated from both plasma and serum [5]. Recently, It was shown that less than 10% of the 6-fold higher serum DNA levels were due to contamination by other sources (i.e. release from leucocytes during the separation of serum) [6,7]. Cell-free circulating DNA harbors the potential of a useful biomarker. DNA levels and fragmentation patterns offer interesting possibilities for diagnostic and prognostic purposes. Here, we present literature data as well as our recent findings on cfDNA in infertile female and male patients with regard to the choice of diagnostic methods, prognostic information and treatment.
- Review Article
- Abstract
- Introduction
- What is Already Known?
- The Preovulatory Phase
- The Male Function
- Infertility and cfDNA
- Deoxyribonuclease: DNase
- DNase Production
- DNase Absorption and Bioavailability
- Preliminary Results of the Patented Study
- DNAse Activity Quantification
- cfDNA Isolation
- CfDNA Quantification
- Result
- Clinical Results Patented
- Discussion
- Conclusion
- References
What is Already Known?
The ovarian function
From the primordial follicles until the ovulation, the follicles are submitted to a lot of growth factors participating in the regulation of follicle growth, dominance and oocyte maturation, and the list of currently known factors is not exhaustive (Table 1).
As in the male, female gametes production comprise mitosis, meiosis and oocyte maturation. They are named oogonia during this process, and they stop mitosis once they have reached their first meiotic division. At that time-point they become primary oocytes. As they enter meiosis, the primary oocytes become surrounded by ovarian mesenchymal cells to form primordial follicles.
From puberty, a few primordial follicles begin to grow every day. The follicle first grows quickly. Most of this growth occurs in the primary oocyte. This is the moment of a major protein synthesis. Zona pellucida is constituted with gap junctions between adjacent granulosa cells and between the granulosa cells and the oocyte.
Cells of the ovarian stroma condense on the membrana propria to form the theca, which is vascularized. FSH and LH receptors develop; if not, follicules undergo atresia. FSH and LH convert the preantral follicles to antral follicles (Graafian follicles). The theca divides into two layers: the theca interna, glandular and highly vascular, surrounded by the theca externa, a fibrous capsule. Fluid starts to appear between the granulosa cells, creating follicular fluid within the follicular antrum.
The follicle makes androgens and oestrogens. The principal androgens are androstenedione and testosterone; the principal oestrogen is Estradiol 17ß. Only the theca interna has LH receptors. The granulosa cells receive these androgens and aromatize them to oestrogens. The combination of oestrogen and FSH causes LH receptors to develop on the granulosa cells.
- Review Article
- Abstract
- Introduction
- What is Already Known?
- The Preovulatory Phase
- The Male Function
- Infertility and cfDNA
- Deoxyribonuclease: DNase
- DNase Production
- DNase Absorption and Bioavailability
- Preliminary Results of the Patented Study
- DNAse Activity Quantification
- cfDNA Isolation
- CfDNA Quantification
- Result
- Clinical Results Patented
- Discussion
- Conclusion
- References
The Preovulatory Phase
The LH surge has two effects
a) The oocyte, whose first meiotic division has been interrupted in prophase, resumes meiosis and undergoes metaphase and anaphase. After the separation of the first polar body, the oocyte enters rapidly the second meiotic division which becomes arrested again at the metaphase stage until fertilization. On the same time, cytoplasmic maturation occurs.
b) The follicle itself matures. The granulosa cells no longer converts androgen to estrogen but instead synthetizes progesterone. LH also stimulates this progesterone synthesis. To be fertilizable the oocyte has to acquire a real competence during follicular development; Oocyte competence is defined as the intrinsic ability to complete meiotic maturation, undergo fertilization, start embryonic development, and establish a successful pregnancy.
Moreover it is well known that pregnancy rates (PR) in women over 35 years of age are significantly lower, both naturally and with assisted reproduction. With the exception of growth hormone treatment during ovarian stimulation there is currently no known intervention to improve pregnancy outcome of elderly women [8].
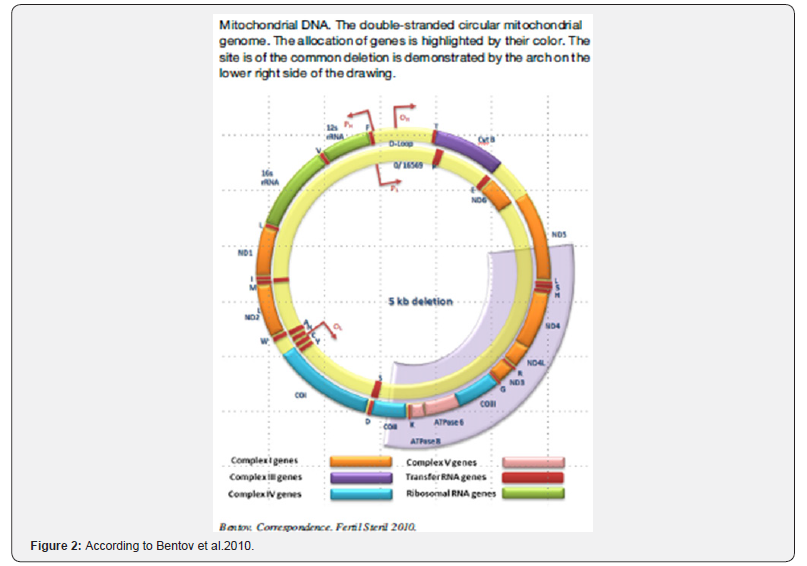
Aging and age-related pathologies are frequently associated with loss of mitochondrial function mainly due to the accumulation of mtDNA mutations and deletions. In oocytes, low levels of mitochondrial oxidative phosphorylation may occur for up to 40 years before follicle maturation and ovulation, further increasing the risk for mtDNA mutations.
One of the more frequent mtDNA deletions is the ‘‘common deletion’’of 4977 base pairs, almost a third of the whole mtDNA genome. This deletion was shown to have a high prevalence in unfertilized oocytes and oocytes from elderly women [9] (Figure 2).
- Review Article
- Abstract
- Introduction
- What is Already Known?
- The Preovulatory Phase
- The Male Function
- Infertility and cfDNA
- Deoxyribonuclease: DNase
- DNase Production
- DNase Absorption and Bioavailability
- Preliminary Results of the Patented Study
- DNAse Activity Quantification
- cfDNA Isolation
- CfDNA Quantification
- Result
- Clinical Results Patented
- Discussion
- Conclusion
- References
The Male Function
To produce, maintain and transport spermatozoa and seminal plasma to discharge sperm within the female reproductive tract during sexual intercourse. To produce male sexual hormones responsible for maintening the male reproductive function. The testes secrete large amounts of androgens principally testosterone, but they also secrete small amounts of estrogens.
In the seminiferous tubules, spermatozoa are formed from the primitive germ cells. Then, they acquire maturation in the epididymus and, crossing the vas deferens, there are ready to be ejaculate. Each spermatozoon has its proper morphology suggesting structure variations and DNA decays according to the particularity of the head shape, basis and nucleus abnormalities [10].
Epigenetic alterations should be considered among the idiopathic male infertility etiologies because hyper- and hypo-methylation of imprinted and non-imprinted genes in spermatozoa have been associated with oligo-, astheno-, and/or teratozoospermia [11].
cf DNA has been detected in human semen [12]. Interestingly, its concentration in semen is much higher than in other body fluids. As reported by Chou et al. [13], cf-DNA concentration in semen is related with sperm parameters linked to normal sperm function such as velocity or morphology. cf-DNA level has been shown to be higher in seminal plasma of azoospermic than in that of normozoospermic patients [14].
Wu et al. [14] showed the existence of a correlation between methylation of specific promoters in cell-free seminal DNA and sperm physiopathology. In their study, methylation of seminal plasma cf-DNA was shown to be higher in the hypo spermatogenesis group than in other groups with spermatogenic defects and normozoospermic patients
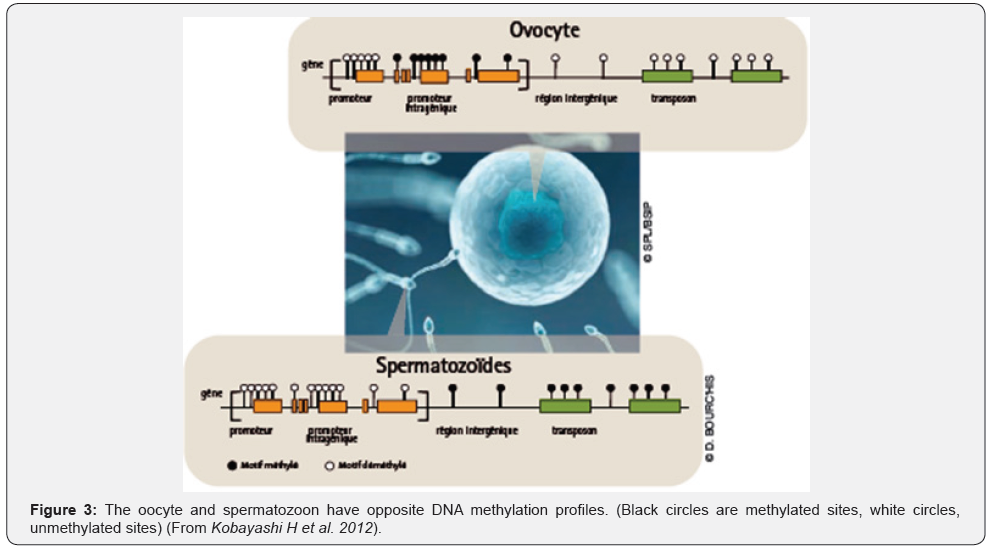
As expected, methylation profiles of female and male gametes are quite different. This parental inequality is known as genomic imprinting. At the moment of gonads colonization, germ cells are subject to a massive wave of demethylation, allowing a discount to zero methylation profiles, in a way similar to the reboot function of a computer. This phase of reprogramming is a prerequisite to the acquisition of sex-specific methylation profiles.
So, at birth, DNA of male germ cells is already methylated. Then, remethylation of oocyte DNA occurs in post-pubertal females. During each ovulatory cycle, the oocyte cohort engaged towards ovulation acquires female-type methylation patterns.
Consequently, men appear to be more likely to develop methylation disorders in their sperm when exposed to toxic products, (neuroendocrine disruption during fetal life [15]. In contrast, similar effects in women could only occur during adult life (Figure 3).
- Review Article
- Abstract
- Introduction
- What is Already Known?
- The Preovulatory Phase
- The Male Function
- Infertility and cfDNA
- Deoxyribonuclease: DNase
- DNase Production
- DNase Absorption and Bioavailability
- Preliminary Results of the Patented Study
- DNAse Activity Quantification
- cfDNA Isolation
- CfDNA Quantification
- Result
- Clinical Results Patented
- Discussion
- Conclusion
- References
Infertility and cfDNA
The presence of cell-free nucleic acids in human plasma and their importance as candidate biomarkers were recognized in the early 1990s after the study published by Sorenson et al. [16]. They include cell-free DNAs (cf-DNAs) and cell-free RNAs (cf-RNAs), which comprises messenger RNAs (mRNAs) and three major small non coding RNAs: microRNAs (miRNAs), piwiinteracting RNAs (piRNAs) and small interfering RNAs (siRNAs).
Cf-DNAs circulate in the bloodstream following their release from apoptotic and/or necrotic cells [3]. Circulating cf-DNA is a non-invasive source of material that can be used to collect genetic and epigenetic information on these cells [17]. Cf-RNAs have been detected in many biological fluids. Like cf-DNA, they can be released by dying cells or actively secreted by living cells.
They also may represent promising sources of material for assessing the gene expression profile of cells and tissues. Moreover, it has been demonstrated that extracellular small non coding RNAs may function as signaling molecules in cell-cell communication [18].
Infertility is defined as the inability to achieve a clinical pregnancy after one year of regular and unprotected sexual intercourse. Worldwide, it affects nearly 15% of couples in age to procreate and trying to conceive [19]. Male-related causes are involved in 59% of cases. One third is due to known female and male causes, whereas the cause cannot be idenfied clearly in the rest. It can be hypothesized that exposure of infertile women to risks factors, such as intrafollicular cells apoptosis, oxidative stress with the generation of immuno-inflammatory factors, cytoplasmic and mitochondrial DNA damages may provide increased cfDNA in blood of exposed subjects. The fact that cfDNA can be obtained without invasive or painful procedures makes it particularly suitable for studies on infertile women and men.
cfDNA is present in healthy subjects at concentrations between 0 and 100ng/ml of blood with an average of 30ng/ml [20]. Assuming that the DNA content of a normal cell amounts to 6.6pg, these values represent an average of 0-15,000 genome equivalents per ml of blood with an average of 5000 genomes per ml. Most of this DNA is double-stranded and is apparently in the form of nucleoprotein complex.
Clearance of cfDNA from the bloodstream seems to be rapid. cfDNA is sensitive to plasma endonucleases.
The biological mechanisms of release of free DNA in blood are not fully understood. Two main mechanisms have been proposed: apoptosis/necrosis, or release of intact cells into the bloodstream and their subsequent lysis. It is common to detect large, quasi-genome size DNA fragments. Mutant DNA fragments may originate from necrotic cells that have been engulfed by macrophages which then release partially digested DNA.
- Review Article
- Abstract
- Introduction
- What is Already Known?
- The Preovulatory Phase
- The Male Function
- Infertility and cfDNA
- Deoxyribonuclease: DNase
- DNase Production
- DNase Absorption and Bioavailability
- Preliminary Results of the Patented Study
- DNAse Activity Quantification
- cfDNA Isolation
- CfDNA Quantification
- Result
- Clinical Results Patented
- Discussion
- Conclusion
- References
Deoxyribonuclease: DNase
Deoxyribonuclease (DNase) is an enzyme that catalyzes hydrolytic cleavage of phosphodiester linkages in the DNA. Some DNases cleave only double-stranded DNA, others are specific for single-stranded molecules, and sothers are in business to both. The DNase I preferentially cleaves single-stranded DNA, doublestranded DNA, and chromatin. DNases II, alpha and beta has the same function. Recombinant human DNase I is a more frequently used.
- Review Article
- Abstract
- Introduction
- What is Already Known?
- The Preovulatory Phase
- The Male Function
- Infertility and cfDNA
- Deoxyribonuclease: DNase
- DNase Production
- DNase Absorption and Bioavailability
- Preliminary Results of the Patented Study
- DNAse Activity Quantification
- cfDNA Isolation
- CfDNA Quantification
- Result
- Clinical Results Patented
- Discussion
- Conclusion
- References
DNase Production
DNase I is produced by recombinant means in an organism with less endogenous RNase greatly facilitating purification of an enzyme with no RNase. Dornase alfa: Inhalation solution (Pulmozyme®: Roche Laboratory) is a sterile, highly purified solution of recombinant human DNaseI.
The protein is produced by genetically engineered Chinese Hamster Ovary (CHO) cells containing DNA encoding for the native human protein, deoxyribonuclease I (DNase). The purified glycoprotein contains 260 amino acids with a molecular weight of 37,000 daltons. This DNase is easily obtained with Escherichia Coli but since the recent introduction of glycosylation system into Yeast, this last microorganism is more attractive.
- Review Article
- Abstract
- Introduction
- What is Already Known?
- The Preovulatory Phase
- The Male Function
- Infertility and cfDNA
- Deoxyribonuclease: DNase
- DNase Production
- DNase Absorption and Bioavailability
- Preliminary Results of the Patented Study
- DNAse Activity Quantification
- cfDNA Isolation
- CfDNA Quantification
- Result
- Clinical Results Patented
- Discussion
- Conclusion
- References
DNase Absorption and Bioavailability
Animal studies (rats and non human primates) show a low percentage of dornase systemic absorption (< 15%) after inhalation. In human DNase administred to patients as an inhaled aerosol also shows low systemic exposure. These latter studies have shown that, following intravenous administration, DNase was rapidly cleared from the serum. Human intravenous studies suggest an elimination half life from serum of 3-4 hours. There was not systemic toxicicity.
The patented invention of Bartoov et al. [21] was based on the unexpected finding that high levels of cf DNA present in men’s blood circulation are associated with subfertility and that administration of exogenous cfDNA reduces sperm quality and causes sub-fertility. Moreover they have have found that providing sub-fertile males with a DNase may improve semen quality and fertility potential. Czamansky-Cohen et al. [22], in a prospective study, examined the cfDNA concentrations during ovarian stimulation and the relationship between cfDNA concentration and pregnancy rates in women undergoing IVFembryo transfer. Thirty seven women underwent IVF treatment. cfDNA concentrations were measured by a direct fluorescence assay, pregnancy rates were identified by plasma β human chorionic gonadotrophin (HCG) concentrations and verified by vaginal ultrasound to determine gestational sac and fetal heart beats.
On the day of βHCG test in patients undergoing IVF-ET, plasma cfDNA concentrations were statistically significantly higher among women who did not conceive in comparison to those who conceived. The authors concluded than plasma cfDNA may reflect the presence of factors which interfere with embryo implantation.
The patented invention of Hazout et al. [20] after several preliminary studies in serum of healthy and infertile women and males, found a statistically significant difference in cfDNA concentrations between fertile and infertile women. The authors demonstrated a beneficial effect, in terms of ongoing pregnancies and live births, of the treatment with exogenous DNase, in women with more than two (2 to 8) implantation failures after previous transfers of good embryos (more than 50%).
In contrast, Hazout and al. did not find, in a preliminary study, a significant difference in cfDNA concentration between two groups of fertile and infertile males. The aim of this latter study was triple. First, to identify, in unexplained infertile women and men (unexplained infertility since at least two years with a history of more than 4 IVF/ICSI with embryos available for transfer and no pregnancy, the level of cfDNA compared to fertile women (AMH > 2ng/ml and/or a history of pregnancy).
Secondly, to verify the efficacy of the injection of one vial of alpha Dornase on the blood level of cfDNA in infertile women. Third, to treat at least 10 infertile women with a high level of cfDNA (more than 50ng/ml) twice a day, for the last seven days of luteal phase in a preceding cycle before ovarian stimulation for ART and to follow up these women in terms of IVF/ICSI results and outcomes.
- Review Article
- Abstract
- Introduction
- What is Already Known?
- The Preovulatory Phase
- The Male Function
- Infertility and cfDNA
- Deoxyribonuclease: DNase
- DNase Production
- DNase Absorption and Bioavailability
- Preliminary Results of the Patented Study
- DNAse Activity Quantification
- cfDNA Isolation
- CfDNA Quantification
- Result
- Clinical Results Patented
- Discussion
- Conclusion
- References
Preliminary Results of the Patented Study
A total of 161 men < 50 were included: 73 fertile ones and 88 infertile ones. The authors first tried to prove the concept of an association of male infertility with high levels of cfDNA, assuming the fact that DNA fragmentation might contribute to cfDNA increase.
In a second phase cfDNA quantity in plasma was measured in 94 fertile women (AMH>2ng/ml) and in 96 infertile women of less than 37 years of age. A genomic study was made to verify the origin of the cfDNA particularly in infertile women (Figure 1) All the genome was concerned.
When samples were available, cfDNA quantification was also performed in follicular fluid from both spontaneous and stimulated cycles in corresponding infertile women. A total of 37 follicular fluid samples were included in this study: most of the patients were stimulated for ART using a long agonist protocol or antagonist protocols with recombinant FSH or HMG. The ovulation was triggered with recombinant or urinary HCG, 36 hours before oocyte retrieval. Each follicular fluid aspirated frome one, two or three dominant follicles was isolated and centrifuged before storage. Then cfDNA content was evaluated using the same method that used for plasma/serum (Figure 2).
- Review Article
- Abstract
- Introduction
- What is Already Known?
- The Preovulatory Phase
- The Male Function
- Infertility and cfDNA
- Deoxyribonuclease: DNase
- DNase Production
- DNase Absorption and Bioavailability
- Preliminary Results of the Patented Study
- DNAse Activity Quantification
- cfDNA Isolation
- CfDNA Quantification
- Result
- Clinical Results Patented
- Discussion
- Conclusion
- References
DNAse Activity Quantification
DNase activity was first determined using Immunometric Enzyme Immunoassay kit (Orgentec) 100μl of calibrators (using separate pipette tips), controls and prediluted patient samples were loaded into the wells of a microplate. The microplate was closed (parafilm, sealing foil or lid) and incubated for 60 minutes at 37 °C. The contents of the microwells were discarded and washed 3 times with 300μl of wash solution. 100μl of enzyme conjugate solution was added into each well and incubated for 15 minutes at room temperature. The contents of the microwells were discarded and washed 3 times with 300μl of wash solution. 100μl of TMB substrate solution were dispensed into each well and incubated for 15 minutes at room temperature. 100μl of stop solution was added to each well of the modules and left untouched for 5 minutes. The optical density was read, no longer than after 30 minutes after of incubation, at 450nm and the results were calculated with the use of standard absorption samples (provided with the kit).
- Review Article
- Abstract
- Introduction
- What is Already Known?
- The Preovulatory Phase
- The Male Function
- Infertility and cfDNA
- Deoxyribonuclease: DNase
- DNase Production
- DNase Absorption and Bioavailability
- Preliminary Results of the Patented Study
- DNAse Activity Quantification
- cfDNA Isolation
- CfDNA Quantification
- Result
- Clinical Results Patented
- Discussion
- Conclusion
- References
cfDNA Isolation
Plasma cfDNA was isolated using high pure PCR template preparation kit (Roche) following manufacturer’s recommendation: Elution buffer was diluted to a 20% solution by using ddH20 and prewarmed at 70 °C. Samples were centrifuged at 16,000g for 5min, 400μl of plasma were transfered to a 2ml Eppendorf tube avoiding cellular debris. 400μl of binding buffer and 40μl of reconstituted proteinase K were mixed to the samples. After a brief vortex, the tubes were incubated for 10min at 70 °C. After incubation 200μl of 100% isopropanol was mixed with the samples that were subsequenly transferred to the upper reservoir of a high pure filter collection tube provided in the kit. The column was centrifuged at 8,000g for 1min at room temperature. The flow-through and collection tubes were discarded and the filter was combined to a new collection tube. This loading step was repeated until the entire sample had been loaded to the filter. 500μl of inhibitor removal buffer were added to the upper reservoir and centrifuged for 1min at 8,000g at room temperature. The flow-through and collection tubes were discarded and the filter as combined to a new collection tube. The tubes were washed twice by adding 500μl of wash buffer to the upper reservoir and centrifuged for 1min at room temperature. Columns were dried by centrifuging at maximum speed (approximately 13,000g) for 10s, transferred to a new 1.5ml Effendorf tube and warmed for 5min at 70 °C in an incubator. 100μl of pre-warmed 20% elution buffer was carefully added to the filter. The tube and filter were placed in the incubator at 70 °C and shook at low speed (approximately 400rpm) for 5min. DNA samples were eluted from the columns by centrifuging at 8,000g for 5min and subsequently stored at 4 °C before being used or frozen at -70 °C for long term storage.
PCR amplification: The master mix used to amplify JmJC2 and DXS1285 loci contained 200mM of each dNTP, 1X Taq polymerase buffer, 2μM of each primer sets, 1.5mM MgCl2, and 0,5U of Biotaq™ DNA polymerase (Bioline) in a 15μl reaction volume. The sequences of primers used to amplify JmJC2 (marker of Y chromosome) and DXS1285 (marker of X chromosome) are
5’-GAGTATGCGACCAGT-3’,
5’-TGGCACACCATGGGA-3’
5’-CGTGCTTAGGCTTAATCCC-3’
5’-GAACTGACTGTAGAGAAGG-3’, respectively, with a 60 °C annealing.
- Review Article
- Abstract
- Introduction
- What is Already Known?
- The Preovulatory Phase
- The Male Function
- Infertility and cfDNA
- Deoxyribonuclease: DNase
- DNase Production
- DNase Absorption and Bioavailability
- Preliminary Results of the Patented Study
- DNAse Activity Quantification
- cfDNA Isolation
- CfDNA Quantification
- Result
- Clinical Results Patented
- Discussion
- Conclusion
- References
CfDNA Quantification
Blood samples were collected in EDTA-containing vacutainer tubes. They were centrifuged (3,400rpm for 15 minutes) for plasma isolation. Before cfDNA quantification, plasma and follicular fluid sample were centrifuged at 3,400g for 20min. Samples have to be transparent with no red blood cells. Indeed cfDNA quantification can be altered in couloured coloured samples. Standard DNA solution was diluted to 20, 50, 100 and 500ng/ml in 166μl to draw the standard curve. 166μl of 1N HCLO4 (Perchloric acid) and 664μl of diphenylamine were added to each 166μl of plasma or follicular fluid supernatant samples. Samples were incubated at 37 °C for 20h, subsequently centrifuged at 15,000g for 10 minutes. 300μl of the supernatant was transferred to a 96-well plate and measures in spectrophotometer (Tecan, Genios) at 600nm.
- Review Article
- Abstract
- Introduction
- What is Already Known?
- The Preovulatory Phase
- The Male Function
- Infertility and cfDNA
- Deoxyribonuclease: DNase
- DNase Production
- DNase Absorption and Bioavailability
- Preliminary Results of the Patented Study
- DNAse Activity Quantification
- cfDNA Isolation
- CfDNA Quantification
- Result
- Clinical Results Patented
- Discussion
- Conclusion
- References
Result
The mean cfDNA value in the group of fertile men was 60,6ng/ μl and 83,34ng/μl in the infertile group, a difference which was not statistically significant. The mean cfDNA value in the group of fertile women was 49,2ng/μl versus 98,5ng/μl in the infertile group, which was statistically significant (Figure 4).

Comparing cfDNA in the plasma and Follicular Fluid (FF) of 42 patients we demonstrated that mean cfDNA value in the FF was lower than plasma cfDNA value in the corresponding patients. As to the cfDNA, in plasma and FF, levels with regard to the outcome of 32 FIV/ICSI patients either after ovarian stimulation or spontaneous cycles (Figure 5&6) we found that:
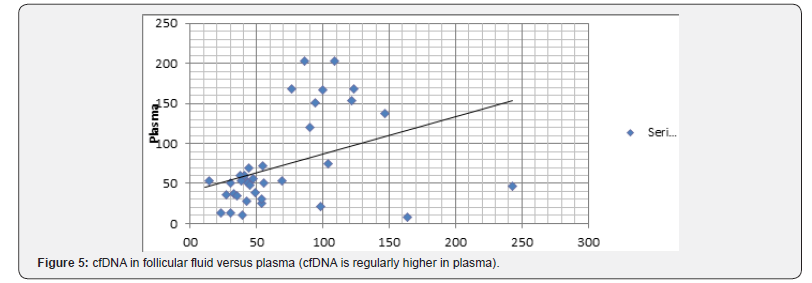
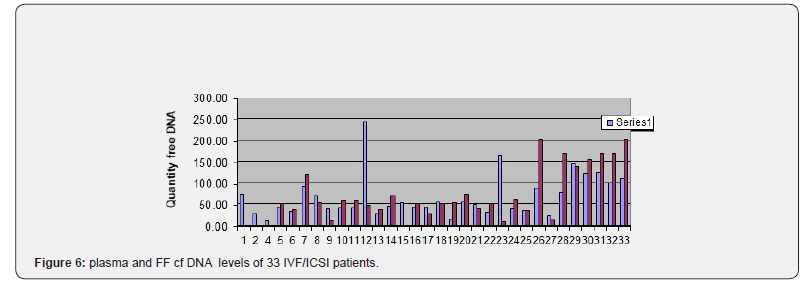
a) the patients number 5, 9, 15, 17, 19, 24, were pregnant and delivered.
b) The majority (6/6) had low levels of CFDNA in the plasma and FF (less than 60ng/microliter)
c) the patients 11, 16, 21 deplored miscarriages (for probably male reasons?) with normal plasma and FF CFDNA levels (< 50)
d) the patient 12 had a normal plasma CFDNA level (46,) but a very high FF CFDNA level (242,7ng/μl) with only one oocyte from three punctured follicles and no fertilization.
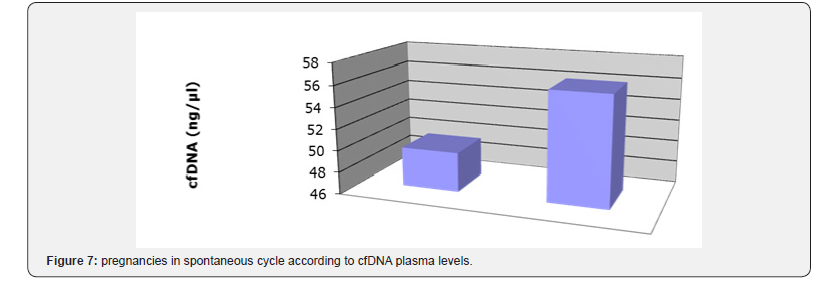
e) six patients were monitored and punctured on spontaneous cycles after several standard FIV/ICSI failures: patients number: 1,2,4,15 ,22,23; with respectively 72,5; 27,4; 11,3; 43,5; 30,3;8,5ng/μl in the plasma and 72; 27;11; 53,6; 30,3; 163,1ng/l in the FF.
Patients 1 and 4 had no oocyte (1 with high levels in the plasma and FF, the other with normal level). Patient 15 was pregnant and delivered (with normal levels in the plasma and FF) (Figure 7).
- Review Article
- Abstract
- Introduction
- What is Already Known?
- The Preovulatory Phase
- The Male Function
- Infertility and cfDNA
- Deoxyribonuclease: DNase
- DNase Production
- DNase Absorption and Bioavailability
- Preliminary Results of the Patented Study
- DNAse Activity Quantification
- cfDNA Isolation
- CfDNA Quantification
- Result
- Clinical Results Patented
- Discussion
- Conclusion
- References
Clinical Results Patented
We selected 30 patients with several FIV/ICSI failures but 27 with a high levels of cfDNA in their plasma and 3 with normal levels (less than 50ng/μl) and then we decided to treat 10 patients among the two groups with the highest cfDNA plasma levels (Table 1).

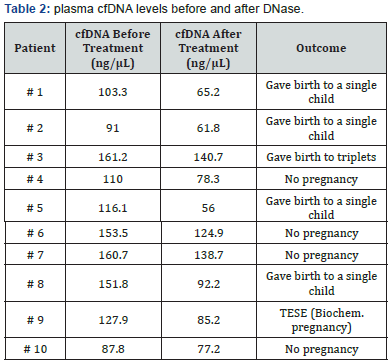
All these patients were young women (less than 37) infertile since 4 years and/or had an history of several ART failures without obvious explanation. All of them were treated with one intramuscular injection, twice a day, of DNAse I (5000IU/day) for seven days in the late luteal phase of the cycle preceding ovarian stimulation and IVF/ICSI (Table 2). From four patients treated in Algeria 3 were pregnant and delivered. From 6 patients treated in Tunisia 3 were pregnant (2 live births and one abortion).
- Review Article
- Abstract
- Introduction
- What is Already Known?
- The Preovulatory Phase
- The Male Function
- Infertility and cfDNA
- Deoxyribonuclease: DNase
- DNase Production
- DNase Absorption and Bioavailability
- Preliminary Results of the Patented Study
- DNAse Activity Quantification
- cfDNA Isolation
- CfDNA Quantification
- Result
- Clinical Results Patented
- Discussion
- Conclusion
- References
Discussion
Based on a few studies exploring the relationship of cfDNA and outcome of ART in infertile couples, and in the light of our experience, we suggest that there are more failures in assisted reproduction when the level of blood cfDNA is in excess in women and / or men.
Boissière A. et al. [23] showed that little has been clarified about circulating nucleic acids in spermatogenesis, and in male infertility and provided a complete summary of the consistent data on circulating nucleic acids and intracellular miRNAs in male infertilty.
Traver S et al. [24] demonstrated that follicular fluid cfDNA level was an independent and significant predictive factor for pregnancy outcome (adjusted odds ratio=0.69 [0.5;0.96], p=0.03). In multivariate analysis, the Receiving Operator Curve (ROC) analysis showed that the performance of FF cfDNA in predicting clinical pregnancy reached 0.73 (0.66-0.87) with 88% specificity and 60% sensitivity. The authors concluded that CfDNA might constitute a promising biomarker of follicular micro-environment quality which could be used to predict IVF prognosis and to enhance female infertility management. However, the main limitation of this study was that all FF samples from the same patient were pooled.
A previous study did not find a statistically significant difference in cfDNA concentrations comparing women who conceived in IVF and those who did not. However, in this latter study the determination of cfDNA was performed one week after embryo transfer. Czamanski-Cohen et al. [22] demonstrated an increase of plasma cfDNA associated with low pregnancy rates among women undergoing IVF-Embryo transfer.
In our study all the pregnant women had a decrease of cfDNA after DNase therapy. The only abortion is probably due to the bad quality of one testicular sperm from an obstructive azoospermic man. In the non-pregnant group the fall of cfDNA was moderate suggesting that the dose of DNase I must to be increased in certain cases with very high levels of cfDNA.
Our clinical results are encouraging and raise a question of the real DNase I mode of action. The decrease in apoptotic factors in the plasma, may, promote a better oocyte competence, but also a better endometrial receptivity. All women treated deplored more than 4 years of unexplained infertility or many embryo transfers, at early stage of development, without implantation. Liu and Li (2010) looked at the relationship between apoptosis in granulosa cells and IVF–embryo transfer success and speculated that oxidative stress in granulosa cells had an effect on IVFembryo transfer failure, and also connected the higher apoptotic rate to lower oocyte quality.
Díaz-Fontdevila et al. [25] examined the apoptotic rate of cumulus cells in relation to infertility diagnosis and spermatozoa exposure and found that cumulus cells of women with a diagnosis of endometriosis and those exposed to spermatozoa had higher rates of apoptosis. Protein p53 was involved in initiation of apoptosis Choisi-Rossi et al. [26] and implantation failure [27].
Given the large number of failures in ART, our new and effective approach seems to be highly promising.
Cell-free DNA has also been detected in human semen. Interestingly, its concentration in semen is much higher than in other body fluids. As reported by Chou et al. seminal cfDNA (scf-DNA) is associated to sperm parameters linked to normal sperm function such as velocity or morphology. Sperm cf-DNA (scfDNA) level has been shown to be higher in seminal plasma of azoospermic than normozoospermic patients.
These observations suggest that scf-DNA could be used in a search for biomarkers of sperm quality. Otherwise, it has been hypothesized that epigenetic alterations could cause male infertility. The presence of epigenetic information has been shown on seminal cfDNA [14] This epigenetic information should reflect testicular epigenetic aberrations as semen is a mixture of secretions from the two testes, epididymes, seminal vesicles, bulbo-urethral glands and prostate. Indeed, Wu et al. [14] showed also the existence of a correlation between methylation of specific promoters (such as CCNA1 and DMRT1) in cell-free DNA seminal and sperm physiopathology. In that study, the cf- DNA methylation of these promoters was shown to be higher in the hypospermatogenesis group than in other groups with spermatogenic defects and normozoospermic patients.
These findings indicate that seminal cfDNA contains the epigenetic information of the male genital tract and could be a novel, non-invasive biomarker to detect spermatogenesis abnormalities. The mice mutants for the process of germinal methylation are sterile in both sexes; this demonstrates the essential character of methylation of gametes for the reproduction. The absence of methylation induces complete arrest of spermatogenesis well upstream [28]. The females suffer from systematic spontaneous abortions of embryos from fertilization of their oocytes devoid of methylation. So while the methylation of DNA has an immediate function for the integrity of male gametes, it does not really matter for the oocyte, but conditions the viability of the future embryos.
Embryos lacking maternal methylation suffer from expression disorders of genes essential for development in utero; hence the systematic miscarriage occurs at the moment when the embryo becomes dependent of maternal supply via the placenta.
So, oocytes and spermatozoa ensure the continuity of the species by delivering to the next generation a genetic capital and a reversible epigenetic inheritance. DNA methylation, in particular, confers intrinsic properties of stability and heritability allowing its transmission from parental gametes to the embryo, « Methylation helps to give life and can take it away. In reality, without methylation, there would be no life at all [29-36].
- Review Article
- Abstract
- Introduction
- What is Already Known?
- The Preovulatory Phase
- The Male Function
- Infertility and cfDNA
- Deoxyribonuclease: DNase
- DNase Production
- DNase Absorption and Bioavailability
- Preliminary Results of the Patented Study
- DNAse Activity Quantification
- cfDNA Isolation
- CfDNA Quantification
- Result
- Clinical Results Patented
- Discussion
- Conclusion
- References
Conclusion
Excess free nucleic acids in the serum of infertile couples seems to be a marker of their decreased ability to conceive even if mechanical causes are excluded. The methylation status of these free DNA fragments adds an additional, non-invasive factor of predictability. This need to be confirmed in larger studies as much as therapeutic solutions are available to change the fate of male and female gametes.
- Review Article
- Abstract
- Introduction
- What is Already Known?
- The Preovulatory Phase
- The Male Function
- Infertility and cfDNA
- Deoxyribonuclease: DNase
- DNase Production
- DNase Absorption and Bioavailability
- Preliminary Results of the Patented Study
- DNAse Activity Quantification
- cfDNA Isolation
- CfDNA Quantification
- Result
- Clinical Results Patented
- Discussion
- Conclusion
- References
References
- Gelbaya TA, Potdar N, Jeve YB, Nardo LG (2014) Definition and epidemiology of unexplained infertility. Obstet Gynecol Surv 69(2):109- 115.
- Mandel P, Metais P (1948) les acides nucléiques du plasma sanguin chez l’homme. CR Séances Soc Biol Ses Fil 142: 241-243.
- Stroun M, Lyautey J, Lederrey C, Olson-Sand A, Anker P, et al. (2001) About the possible origin and mechanism of circulating DNA. Clin Chim Acta 313(1-2): 139-142.
- Wong BC, Lo YM (2003) Cell-free DNA and RNA in plasma as new tools for molecular diagnostics. Expert Rev Mol Diagn 3(6): 785-797
- Wong FC, Sun K, Jiang P, Cheng YK, Chan KC, et al. (2016) cell free DNA in maternal plasma and serum: a comparison of quantity, quality and tissu origin using genomic and epigenomic approaches. Clin Biochem 49(18): 1379-1386.
- Umetani N, Hiramatsu S, Hoon DS (2006) higher amount of free circulating DNA in serum than in plasma is not mainly caused by contaminated extraneous DNA during separation. Ann NY Acad Sci 1075: 299-307.
- Breitbach S, Tug S, Helmig S, Zahn D, Kubiak T, et al. (2014) Direct quantification of cell-free, circulating DNA from unpurified plasma. PLoS One 9(4): e87838.
- Tesarik J, Hazout A, Mendoza C (2005) improvement of delivery and live birth rates ater ICSI in women aged >40 years by ovarian costimulation with growth hormone. Hum Reprod 20(9): 2536-2541.
- Bentov Y, Esfandiari N, Burstein E, Casper RF (2010) the use of mitochondrial nutrients to improve the outcome of infertility treatment in older patients. Fertil Steril 93(1): 272-275.
- Cassuto NG, Hazout A, Hammoud I, Balet R, Bouret D, et al. (2012) Correlation between DNA defect and sperm-head morphology. Reprod Biomed Online 24(2): 211-218.
- Portela A, Esteller M (2010) Epigenetic modifications and human disease. Nat Biotechnol 28(10): 1057-1068.
- Li HG, Huang SY, Zhou H, Liao AH, Xiong CL, et al. (2009) Quick recovery and characterization of cell-free DNA in seminal plasma of normozoospermia and azoospermia: implications for non-invasive genetic utilities. Asian J Androl 11(6): 703‑709.
- Chou JS, Jacobson JD, Patton WC, King A, Chan PJ, et al. (2004) modified isocratic capillary electrophoresis detection of cell-free DNA in semen. J Assist Reprod Genet 21(11): 397-400.
- Wu C, Ding X, Tan H, Li H, Xiong C, et al. (2016) alterations of testisspecific promoter methylation in cell-free seminal deoxyribonucleic acid of idiopathic nonobstructive azoospermic men with different testicular phenotypes. Fertil Steril 106(6): 1331-1337.
- Walker DM, Gore AC (2011) Transgenerational neuroendocrine disruption of reproduction. Nat Rev Endocrinol 7(4): 197-207.
- Sorenson GD, Pribish DM, Valone FH, Memoli VA, Bzik DJ, et al. (1994) Soluble normal and mutated DNA sequences from single-copy genes in human blood. Cancer Epidemiol Biomarkers Prev 3(1): 67-71.
- Warton K, Lin V, Navin T, Armstrong NJ, Kaplan W, et al. (2014) methylation capture and next-generation sequencing of free circulating DNA from human plasma. BMC Genomics 15: 476.
- Belleannée C (2015) Extracellular microRNAs from the epididymis as potential mediators of cell-to-cell communication. Asian J Androl 17(5): 730-736.
- Thoma ME, McLain AC, Louis JF, King RB, Trumble AC, et al. (2013) prevalence of infertility in the United States as estimated by the current duration approach and a traditional constructed approach. Fertil Steril 99(5): 1324-1331.
- Hazout A, Benkhalifa M (2013) Cell-Free DNA as a therapeutic target for female infertility and diagnostic marker. European Patent n°13156626.
- Bartoov B, Yehuda R, Dobroslav (2008) method and Pharmacological composition for the diagnosis and treatment of male sub-fertility. International Patent n° WO 2008/047364 A2.
- Czamanski-Cohen J, Sarid O, Cwikel J, Lunenfeld E, Douvdevani A, et al. (2013) Increased plasma cell-free DNA is associated with low pregnancy rates among women undergoing IVF-embryo transfer. RBM online 26(1): 36-41.
- Boissière A, Gala A, Ferrières H, Mullet T, Baillet S, et al. (2017) Cellfree and intracellular nucleic acids: new non-invasive biomarkers to explore male infertility. Basic Clin Androl 27: 7.
- Traver S, Scalici E, Mullet T, Molinari N, Vincens C, et al. (2015) Cellfree DNA in Human Follicular Microenvironment: New Prognostic Biomarker to Predict in vitro Fertilization Outcomes. PLoS One 10(8): e0136172.
- Díaz-Fontdevila M, Pommer R, Smith R (2009) Cumulus cell apoptosis changes with exposure to spermatozoa and pathologies involved in infertility. Fertil Steril 91(5 Suppl): 2061-2068.
- Choisy-Rossi C, Reisdorf P, Yonish-Rouach E (1999) The p53 tumor suppressor gene: structure, function and mechanism of action. Results Probl Cell Differ 23: 145-172.
- Kang HJ, Feng Z, Sun Y, Atwal G, Murphy ME, et al. (2009) Singlenucleotide polymorphisms in the p53 pathway regulate fertility in humans. Proc Natl Acad Sci USA 106(24): 9761-9766.
- Bourc’his D, Bestor TH (2004) meiotic catastrophe and retrotransposon reactivation in male germ cells lacking Dnmt3l. Nature 2: 41.
- Cooney CA (2006) Germ cells carry the epigenetic benefits of grandmother’s diet. Proc Natl Acad Sci USA 103(46): 17071-17072.
- Gilchrist RB (2011) Recent insights into oocyte-follicle cell interactions provide opportunities for the development of new approaches to in vitro maturation. Reprod Fertil Dev 23(1): 23-31.
- Hong Y, Wang C, Fu Z, Liang H, Zhang S, et al. (2016) Systematic characterization of seminal plasma piRNAs as molecular biomarkers for male infertility. Sci Rep 6: 24229.
- Kobayashi H, Kono T (2012) DNA methylation analysis of germ cells by using bisulfite-based sequencing methods. Methods Mol Biol 825: 223-235.
- McDonald JS, Milosevic D, Reddi HV, Grebe SK, Algeciras-Schimnich A, et al. (2011) Analysis of circulating microRNA: preanalytical and analytical challenges. Clin Chem 57(6): 833-840.
- Vickers KC, Palmisano BT, Shoucri BM, Shamburek RD, Remaley AT, et al. (2011) microRNAs are transported in plasma and delivered to recipient cells by high-density lipoproteins. Nat Cell Biol 13(4): 423- 433.
- Wu W, Qin Y, Li Z, Dong J, Dai J, et al. (2013) Genome-wide microRNA expression profiling in idiopathic non-obstructive azoospermia: significant up-regulation of miR-141, miR-429 and miR-7-1-3p. Hum Reprod 28(7): 1827-1836.
- Wu C, Ding X, Tan H, Li H, Xiong C, et al. (2016) Alterations of testisspecific promoter methylation in cell-free seminal deoxyribonucleic acid of idiopathic nonobstructive azoospermic men with different testicular phenotypes. Fertil Steril 106(6): 1331-1337.






























