Safety of Ospemifene during Real-Life Use
Nico Bruyniks1*, Fabio De Gregorio2, Trevor Gibbs2, Robert Carroll3, Kathy H Fraeman4 and Beth L Nordstrom5
1BrInPhar Ltd, Crowther Lodge, UK
2Shionogi Limited, 33 Kingsway, London WC2B 6UF, UK
3Evidera, Metro Building, UK
4Evidera, 7101 Wisconsin Avenue, US
5Evidera, 500 Totten Pond Road, US
Submission: March 21, 2018 ; Published: April 13, 2018
*Corresponding author: Nico Bruyniks, BrInPhar Ltd, Crowther Lodge, Cherrytree Lane, Iver Heath, Buckinghamshire, SL0 0EE, UK, Tel: +442030534200; Fax:+4402030534199; Email: brinphar@gmail.com
How to cite this article: Bruyniks N, DeGregorio F, Gibbs T, Carrol R, Fraeman KH, Nordstrom BL. Safety of Ospemifene during Real-Life Use. J Gynecol Women�s Health 2018; 9(3): 555762. DOI: 10.19080/JGWH.2018.09.555762
Abstract
Objective: To provide an overview of the current safety profile of Senshio® (ospemifene), a Selective Estrogen Receptor Modulator (SERMJ developed for the treatment of vulvar and vaginal atrophy (VVA), in relation to the Important Potential Risks, including Venous ThromboEmbolism (VTE), based on real world use of the product.
Methods: Review of all data from pharmacovigilance reporting for all Important Potential Risks and an analysis of the 2-year interim data of the Post Authorisation Safety Study (PASS) ospemifene. Three cohorts were compared, women newly prescribed ospemifene, a comparator SERM cohort, using other SERMs for non-oncological indications and women newly diagnosed with VVA but not treated. Only descriptive statistics were applied to this interim analysis.
Results: The incidence rates from the pharmacovigilance reporting system for the Important Potential Risks are consistently much lower than the background risks identified. In the PASS interim analysis, VTE was observed to occur at a rate of 4.02 (95% CI: 1.48-8.75) events per 1,000 person-years for ospemifene, 12.63 (95% CI: 9.49-16.48) for the comparator SERM cohort and 12.02 (95% CI: 11.50-12.57) for the untreated cohort. Of the secondary outcomes, only increased triglycerides were observed with any notable frequency..
Conclusion: The analysis of the safety of ospemifene in real-world medical practice has found a low rate of VTE and other adverse outcomes, originally identified as Important Potential Risks, with ospemifene use. The PASS findings represent an early analysis in an as-yet relatively small cohort of ospemifene initiators and their comparators.
Keywords: Vulvar and vaginal atrophy; Menopause; Ospemifene; Safety; Selective estrogen receptor modulator; Venous thrombo-embolism
Abbreviations: VVA: Vulvar and Vaginal Atrophy; SERM: Selective Estrogen Receptor Modulator; VTE: Venous Thrombo-Embolism; RMP: Risk Management Plan; EMA: European Medicines Agency; FDA: Food and Drug Administration; RMP: Risk Management Plan; RR Relative Risk; CI: Confidence Interval; WY: Women Years; NSABP: National Surgical Adjuvant Breast and Bowel Project; WHI: Women's Health Initiative Hormone Replacement Therapy randomized clinical trial; AMI: Acute Myocardial Infarction; CPN: Chronic Progressive Nephropathy; PCNA: Proliferating Cell Nuclear Antigen; PASS: Post-Authorisation Safety Study; CVE: Cerebro-Vascular Event; PBRER: Periodic Benefit and Risk Evaluation Report
Introduction
Ospemifene, a triphenylethylene Selective Estrogen Receptor Modulator (SERM) has specific agonist action on the vaginal epithelium, making it a candidate for the treatment of vulvar and vaginal atrophy [1,2]. The development programme for ospemifene was based on the FDA Guidance for Industry: Estrogen and Estrogen/Progestin Drug Products to Treat Vasomotor Symptoms and Vulvar and Vaginal Atrophy Symptoms-Recommendations for Clinical Evaluation (draft guidance, January 2003) [3] and the EMA Guideline on clinical investigation of medicinal products for hormone replacement therapy of oestrogen deficiency symptoms in postmenopausal women (EMEA/CHMP/021/97 Rev. 1, October 2005) [4]. The clinical development program for ospemifene is the largest for a product for VVA with nearly 2,500 subjects exposed to ospemifene [5] up to 800 mg daily, with 1,379 patients exposed to 60mg ospemifene during the phase 2 and 3 studies.
On 26th February 2013, ospemifene 60mg once daily oral tablet was approved by the FDA for use in the United States for the treatment of moderate to severe dyspareunia, a symptom of vulvar and vaginal atrophy, due to menopause under the trade name Osphena® [6]. On 15th January 2015, ospemifene 60 mg once daily was granted marketing authorisation by the European Commission for the use in the European Economic Area (EEA) for the treatment of moderate to severe symptomatic vulvar and vaginal atrophy (VVA) in post-menopausal women who are not candidates for local vaginal oestrogen therapy under the trade name Senshio® [7].
During the review of the ospemifene data for Marketing Authorisation, the European Medicines Agency (EMA), considered neither ospemifene's (agonist [8,9]) effect on bone nor its (antagonist [10-12]) effect on breast as important (identified or potential) risks and therefore effects on breast or bone will not be discussed here.
Based on the clinical trial program, an increase in uterine diagnostic procedures was included as an important identified risk in the Senshio® Risk Management Plan (RMP) [13]. This is likely a consequence of the clinical trial design, since the most common reason for uterine diagnostic procedures in postmenopausal women is vaginal bleeding. There was no difference in the rate of vaginal bleeding for ospemifene compared with placebo. Additionally, the bleeding incidence was very low (2.2% for ospemifene 60mg vs. 2.6% for placebo). Therefore, it is not expected that ospemifene 60mg will lead to an increase in unnecessary gynaecological procedures resulting from vaginal bleeding or spotting [5].
Important potential risks included in the Senshio® RMP are:
Venous Thromboembolic Embolism (VTE)
mg (milligram)
VTE (Venous Thrombo-embolism RR (Relative Risk)
CI (Confidence Interval)
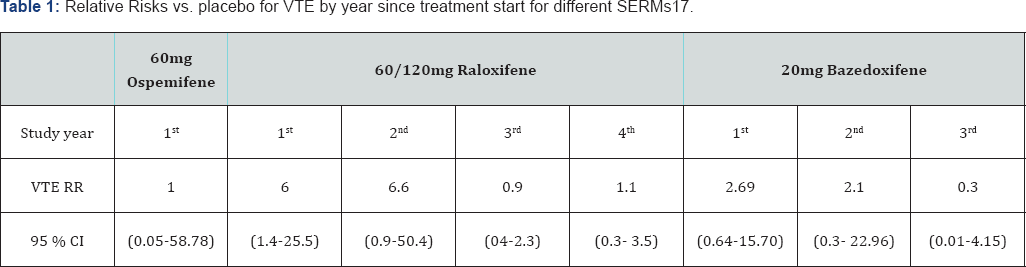
In the ospemifene clinical trial program no increased risk of VTE was observed, but the 95% confidence limits are wide (Table 1).
A 1.3 -3-fold increase in the risk of VTE, deep vein thrombosis (DVT) or pulmonary embolism (PE), has been described for oral oestrogens, particularly in the first year of use [14,15].
An increased risk of VTE has been observed for other SERMS, with a relative risk similar to HRT. Overall, the relative risk of VTE vs. placebo is reported as 2.13 for lasofoxifene (95% CI 1.343.39), 1.9 for bazedoxifene and 2.13 for raloxifene (95% CI 1.213.75) [5,16-19]. The highest rates of VTE with SERMs were also observed in the first year [20,21]. The relative risk of VTE with raloxifene is 6 (95% CI 1.4-25.5) in the first year, with the highest risk during the initial months of treatment [2 3].
A comparison of the relative risk of VTE with placebo for different SERMS by treatment duration is provided in Table 1. Long term treatment with a SERM is associated with lower risks of VTE in later years.
The development plan for Senshio® was designed to demonstrate the efficacy and safety of ospemifene for the treatment of moderate to severe symptomatic vulvar and vaginal atrophy (VVA) in post-menopausal women. No increased risk was observed for VTE in the clinical programme: 3.65 per 1000 patient years for ospemifene 60mg (95% CI: 0.44-13.19) vs. 3.66 per 1000 patient years for placebo (95% CI: 0.09-20.41). These data do not indicate a specific concern, but the confidence limits are wide. increase the” between "Because ospemifene may possibly also” and "risk of VTE, the restriction "who are not candidates for local vaginal oestrogen therapy” was included in the European indication and VTE was included as an important potential risk in the Senshio® Risk Management Plan [5].
Cerebrovascular events
No increased risk was observed with ospemifene for cerebrovascular events: 1.83 per 1,000 patient years for ospemifene 60mg (95% CI: 0.05-10.17) vs. 3.66 per 1,000 patient years (95% CI: 0.09-20.41) for placebo, but the confidence limits are wide. The inclusion of stroke as an important potential risk is largely based on the increase in stroke risk seen with oestrogens. The Women's Health Initiative Hormone Replacement Therapy randomized clinical trial (WHI) oestrogen-alone sub-study was stopped early because an increased risk of stroke was observed [18]. Based on centrally adjudicated data for an average follow- up of 7.1 years, the relative risk of ischemic stroke of oestrogen alone vs. placebo was 1.55 (95% CI 1.19-2.01). In the oestrogen plus progestin sub-study, after an average follow-up of 5.6 years and based on centrally adjudicated data, the relative risk of ischemic stroke was 1.44 (95% CI 1.09-1.90) [19].
Despite the potential oestrogenic effect of SERMs on clotting factors, there has been no increased risk reported for ischemic stroke with 20mg bazedoxifene [20] or 60mg raloxifene [5,21]. In the PEARL study, lasofoxifene demonstrated a decreased risk of stroke in both doses tested compared to placebo (HR 0.61 (95% CI 0.39-0.96) and 0.64 (95% CI 0.41-0.99) for 0.25 and 0.5 mg/day respectively) [22]. However, in a clinical trial of postmenopausal women with documented coronary heart disease or at increased risk for coronary events, a borderline increased risk of death due to stroke was observed after treatment with raloxifene (RR 1.49, 95% CI 1.00-2.24, p=0.05 ) [23,24]. In the Breast Cancer Prevention Trial (NSABP P-1), there was a non-statistically significant increase in stroke among patients randomized to tamoxifen (RR=1.42; 95% CI 0.822.51) [25]. But a recent publication from Taiwan demonstrated that tamoxifen use was associated with reduced risks of acute myocardial infarction (AMI), ischemic and haemorrhagic stroke and total cardiovascular events [26].
Vaginal bleeding
Vaginal bleeding, either spontaneous or upon contact, sexual intercourse, etc. in postmenopausal women requires investigation. The vast majority of postmenopausal vaginal bleeding is due to vaginal and/or endometrial atrophy, but approximately 10% (range 1-25%) of women presenting with postmenopausal bleeding will be diagnosed with endometrial carcinoma [27] and 90% of all endometrial carcinomas in postmenopausal patients present with vaginal bleeding. It is therefore an important sign, leading to gynaecological investigation. A Cochrane review in 1999 concluded that irregular bleeding was significantly more likely in patients treated with unopposed oestrogen regimens with greater effects with higher dose therapy [28].
Neither raloxifene [21], bazedoxifene [29] or ospemifene [5] are associated with an increase in vaginal bleeding, but in the NATO trial (NOLVADEX Adjuvant Trial Organization) tamoxifen users showed a 10-fold increase in vaginal bleeding (2.0%) compared to the untreated group (0.2%) [25] and another SERM, lasofoxifene, increased vaginal bleeding two-fold (2.6%) compared to the placebo arm (1.3%) [30]. As vaginal bleeding is a (partial) SERM class effect, it was included in the PASS as an adverse event of special interest.
Endometrial cancer
More than one in 20 female cancers in Europe is of the endometrium. The overall risk of endometrial cancer varies between countries, from >40 per 100,000 in Slovakia and the Czech Republic to <30 per 100,000 in France, Spain, and the United Kingdom. The risk is strongly associated with age of the woman, with the highest risk in postmenopausal women. The incidence of endometrial cancer is rising, particularly in the latter population, possibly due to a decrease in the age of menarche, shifting patterns in reproductive behaviour (reduction in the number of pregnancies), the increase in obesity and increased HRT use [31]. The 2-4 fold increased risk of endometrial cancer with oestrogens has been repeatedly demonstrated, even for low dose oestrogens [32] and combination treatment with progestagens [33].
A 1.3-7.5 fold increase in endometrial cancer has also been observed with long-term tamoxifen treatment [34], but not with raloxifene [21], bazedoxifene [20] or ospemifene [5] treatment.
Pelvic organ prolapse
The absence of a precise anatomical and symptomatic definition of pelvic organ prolapse limits research into its epidemiology among postmenopausal women. Prevalence rates from 8% to 41% have been quoted in community surveys [35] and that rate increases with parity [36]. In a cross-sectional analysis of the WHI (n = 27,342 women) a baseline pelvic examination assessed uterine prolapse, cystocele, and rectocele. In the 16,616 women with a uterus, the prevalence rate of uterine prolapse was 14.2%; the rate of cystocele was 34.3%; and the rate of rectocele was 18.6%. For the 10,727 women who had undergone hysterectomy, the prevalence of cystocele was 32.9% and of rectocele was 18.3%. The authors admit that this may be an underestimation due to various reasons [37], and an analysis of the prevalence of pelvic organ prolapse at one site in the WHI where all women were examined with the Pelvic Organ Prolapse Quantification examination during a maximal Valsalva maneuver and in addition completed a questionnaire, reported a 97.7% prevalence of pelvic organ prolapse [38].
Although the atrophy due to the drop in circulating oestrogen levels following the loss of ovarian function in postmenopausal women has often been associated with pelvic organ prolapse, there is remarkably little evidence that oestrogens, either local or systemic, are effective in alleviating pelvic organ prolapse [39,40].
Raloxifene, bazedoxifene, tamoxifen and ospemifene are generally not associated with an increase in prolapse. However, a small study comparing tamoxifen 20mg, raloxifene 60mg and conjugated equine oestrogen 0.625mg with placebo showed that tamoxifen and raloxifene worsened prolapse compared with conjugated equine oestrogen and placebo [41]. On the other hand, in a meta-analysis of three trials with raloxifene, it was found that raloxifene reduces the need for pelvic surgery in women >60 years of age [42]. However, two SERMs in development, idoxifene and levormeloxifene, were found to be associated with pelvic organ prolapse which has contributed to the discontinuation of their development [43-45]. Another SERM, lasofoxifene, also demonstrated a statistically non-significant increase in prolapse during clinical development. Overall, the results concerning the effect of SERMs on pelvic organ prolapse are contradictory [46]. However, as pelvic organ prolapse has been noted as a concern with some early SERMs during their development, it was included in the PASS as an adverse event of special interest [47,48].
Urinary incontinence
Despite the presence of oestrogen receptors in the urogenital tissues, the association of urinary incontinence and the menopause is anything but clear. Although it is difficult to draw the distinction between the effects of menopause and those of ageing, most studies have concluded that the effect of menopause, if any, was modest compared to e.g. modifiable factors such as weight gain and changes in weight distribution. Diabetes was also associated with developing more frequent incontinence [49,50]. Pregnancy, childbirth, past history of pelvic and perineal surgery, obesity and age was more important than menopausal status [51,52]. Urinary incontinence however is highly prevalent in postmenopausal women. Of the 27,347 women randomised in the WHI, 16,417 (60%) complained of incontinence at baseline [53].
The results of studies investigating the impact of oestrogens on incontinence vary. Results from a number of large studies unequivocally demonstrated that systemic oestrogens increase the risk of urinary incontinence, but it may be improved by local oestrogens [52-54].
Although lasofoxifene, idoxifene and levormeloxifene use was associated with an increase in urinary incontinence [47,55,56], the effects of tamoxifen [57] are controversial and raloxifene had no effect on urinary incontinence [44,58]. A recent study by Schiavi et al. [59] suggests that ospemifene could be an effective therapy for postmenopausal women with VVA, improving overactive bladder symptoms and quality of life.
Cholecystitis and gallbladder events
Epidemiological investigations have found, and clinical studies have confirmed, that at all ages, women are twice as likely as men to form cholesterol gallstones, suggesting that oestrogen may be an important risk factor for the formation of cholesterol gallstones in humans [60,61]. It has been known for more than 40 years that there is a significant association between oestrogen containing drugs and gall bladder disease [62]. This has been confirmed in a number of large clinical studies, retrospective [63] and prospective [64] cohort studies as well in the prospective randomised WHI study [65]. The proposed mechanism is that oestrogens increase the risk of developing cholesterol gallstones by increasing the saturation of cholesterol in bile by increasing hepatic secretion.
Some SERMs have been associated with an increased risk of cholelithiasis. Raloxifene is known to increase the risk of cholelithiasis by about 26% [23], although this side-effect in itself is rare. The reports on tamoxifen are conflicting. In a retrospective cohort study in Turkey it was demonstrated that adjuvant tamoxifen therapy leads to gallstone formation in postmenopausal breast cancer patients and is most apparent after 3 years of treatment [66] whilst a prospective cohort study in India showed no significant association between tamoxifen and gallstones [67].
Atrial fibrillation
The prevalence and incidence of atrial fibrillation increases with age and is higher in men than in women [68]. The WHI study demonstrated that the risk of atrial fibrillation is modestly elevated in hysterectomized women randomized to postmenopausal oestrogen-only treatment, and in the pooled group randomized to oestrogen-only treatment or oestrogen plus progestin. The trend in women with intact uterus receiving oestrogen plus progestin, considered separately, was not statistically significant [69].
Although one study in Italy reported an increased risk of atrial fibrillation in women receiving tamoxifen for the prevention of breast cancer [70], the much larger NSABP Breast Cancer Prevention Trial (BCPT) demonstrated that tamoxifen, when used for breast cancer prevention in women with or without heart disease, is not associated with beneficial or adverse cardiovascular effects [71]. SERM use is not generally associated with an increased risk of atrial fibrillation. No increased risk of atrial fibrillation has been seen in the Senshio clinical trials [5].
Increased triglycerides
Oestrogens increase HDL cholesterol and lower LDL and total cholesterol, all of which are considered positive for cardiovascular risk. However, oral oestrogen also increases triglycerides [72,73] which is not considered beneficial. In rare cases, the hypertriglyceridemia, caused by oestrogens, can lead to acute pancreatitis [74].
Triglyceride levels are increased during tamoxifen use, but rarely to clinically significant levels [75,76]. Both clomiphene [77] and tamoxifen [78] use have been associated with incidental severe cases of hypertriglyceridemia. Raloxifene on the other hand did not increase serum triglycerides in postmenopausal women, except in those whose body mass was in the upper tertile [79]. In a two year osteoporosis prevention trial [80] and a three year osteoporosis treatment trial [81], significant increases from baseline in median concentrations of triglycerides were observed among women receiving bazedoxifene 20mg, bazedoxifene 40 mg, and placebo, with no significant difference found among the groups. In a study with 4 different doses of levormeloxifene no significant change in triglyceride levels was observed [82]. A study comparing the effect of toremifene and tamoxifen on triglycerides found that triglycerides significantly increased in the tamoxifen group but significantly decreased in the toremifene group in the 12th month of administration and that overall the effect on lipid metabolism showed different profiles between the two SERMs and that toremifene gave better results than tamoxifen [83]. A recent analysis by Archer et al. [84] demonstrated that the effect of ospemifene on triglyceride levels were similar to placebo after 3, 6 and 12 months of treatment.
Liver tumours
Liver cancer is the fifth most common cancer worldwide [85] and the third-leading cause of cancer-related deaths [86]. In the 2-year rat study with ospemifene, an increase in hepatocellular tumours was recorded at all ospemifene dose levels. The type of tumours and their incidences were comparable to those seen in the carcinogenicity studies with other SERMs [5]. Especially for tamoxifen, a high incidence of rat liver adenomas and carcinomas was reported which is likely to be due both to DNA adduct formation and an oestrogen agonistic activity [87]. Ospemifene did not induce DNA adduct formation in the rat liver [5].
A male to female ratio between 3:1 and 5:1 is a constant finding in hepato cellular carcinoma (HCC) [85]. The liver is sensitive to the action of oestrogens, but the role of oestrogens in liver cancer is unclear, as oestrogens have been implied as promotors as well as inhibitors of liver cancer [85,86,88].
There is as yet no indication that tamoxifen is associated with an increase in the risk of liver cancer in humans.
Thymic epithelial tumours
Tumors ofthymus gland are rare and account for 0.2% to 1.5% of all the neoplasms. They constitute a heterogeneous group that has an unknown etiology and a complex as well as varied biology [89]. In the 2-year rat carcinogenicity study with doses from 10300mg/kg/day, a clear increase in mostly benign thymic tumours was recorded at all ospemifene dose levels. Thymomas have not been seen with other SERMs and there was no increase in the incidence of thymoma in the 2-year mouse study [5]. Thymomas are common in the rat used in the carcinogenicity studies [90] (Wistar rats) and it is likely that ospemifene has an antagonistic effect on the thymus. Oestrogen induces thymus involution [91] and opposing the oestrogen action leads to an attenuation of the physiological thymic involution (atrophy) process induced by oestrogens starting during puberty. In postmenopausal women, the thymus is predominantly atrophic[91]. Tumour development in these studies is believed to be the result of rodent specific hormonal mechanisms when treated during their reproductive lives; these findings are unlikely to have any clinical relevance in postmenopausal women [92].
Renal carcinoma and adenoma
Although oestrogen receptors have been identified more than 40 years ago in human renal cell carcinoma [93], the impact of oestrogen on renal cancer is less clear. Oestrogens have been implicated in the induction of renal cell carcinoma [94], but more recently have also been found to be protective [95]. Kidney cancer is more common in males than in females [95].
Tamoxifen has been used in the palliative setting of renal cell carcinoma with variable success [96-99]. Recently, kidney cancer was found in preclinical studies with bazedoxifene in both rats and monkeys. These findings are unlikely to be of relevance for humans because the incidence of renal carcinoma in these studies is roughly in line with few data found in the literature. Due to the slow growth characteristics of renal carcinoma, the carcinomas observed at study end in the monkeys have very likely been present prior to the start of the study and the age of the monkeys (13.5 to 14.2 years out of a lifespan of 20 to 25 years) is comparable to the higher age in humans, the age at which renal cell carcinoma occurs most commonly in humans. In addition, histochemical staining did not show evidence of possible bazedoxifene-related renal injury such as basement membrane thickening or fibrosis, bazedoxifene-induced DNA- adduct formation or a relevant increase in renal cell proliferation as measured by Ki-67 and PCNA [100].
Renal failure
Bazedoxifene caused corticomedullar nephrocalcinosis and enhanced spontaneous chronic progressive nephropathy (CPN) in male rats and, although not seen in the clinical studies, renal damage in humans cannot be completely ruled out to date. Similar observations were made with raloxifene albeit to a quantitatively lesser extent at comparable exposures [100]. No such changes have been seen with ospemifene in the clinical or preclinical studies.
Off label use
As the indications in the USA and Europe for ospemifene are slightly different, relevant off-label use will be looked at when more European data for the PASS become available.
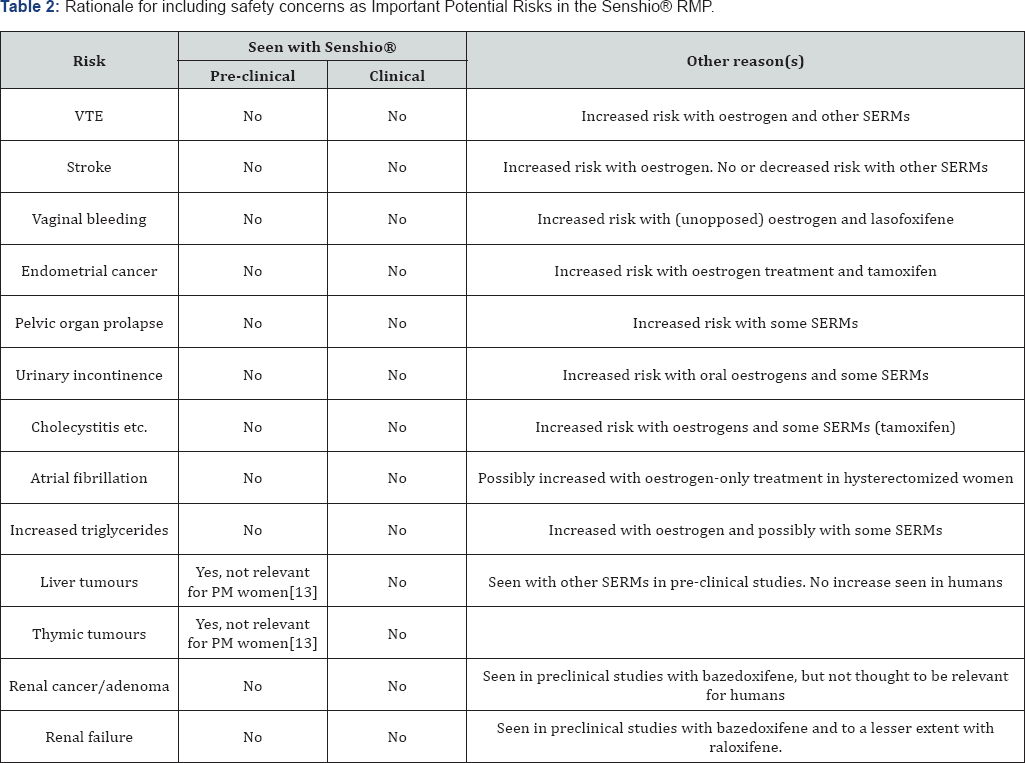
A summary of the rationale for including certain risks as potential risks in the Senshio® RMP can be found in Table 2.
SERM (Selective Estrogen Receptor Modulator)
The safety data from the clinical development program for ospemifene have been thoroughly reviewed by the FDA [101], the EMA [5] and have also recently been published [102]. Following Marketing Authorisation in the EU, the Marketing Authorisation Holder (Shionogi Ltd) proposed and agreed to perform a PostAuthorisation Safety Study (PASS) to evaluate the incidence of venous thromboembolism and other adverse events, as agreed in the risk management plan, in VVA patients treated with ospemifene as compared to 1) patients newly prescribed SERMs for oestrogen-deficiency conditions or breast cancer prevention and 2) patients with untreated VVA. Here we review the safety data from real life use for the specific conditions identified as important potential risks with ospemifene, collected via pharmacovigilance reporting and the PASS.
Methods
Post-marketing Adverse Event (AE) reports for ospemifene received from all sources, including spontaneous notification, regulatory authorities, medical/scientific literature, and solicited sources are recorded in the Shionogi Global Safety Database. The data are used for signal detection and evaluation. Shionogi Ltd submits to the European Medicines Agency (EMA) a Periodic Benefit and Risk Evaluation Report (PBRER) at least every six months during the first two years following the initial placing on the market and once a year for the following two years. Thereafter, the reports shall be submitted at three-yearly intervals, or immediately upon request [103]. The last PBRER, covering the period of 27 February 2017 to 26 August 2017 was submitted 20th October 2017. The cumulative data in this report cover the period from 26 February 2013 (International Birth Date, first approval of ospemifene for marketing worldwide in the United States of America) to 26 August 2017. Adverse event reporting on the basis of voluntary reports cannot be used to accurately establish an incidence proportion or rate; nevertheless, rates for the Important Potential Risks are calculated, based on the number of events reported and the years of exposure based on the number of tablets sold or delivered as samples.
The PASS is a retrospective cohort study using data captured in existing electronic medical record (EMR) and claims databases. The duration of the study will be up to 5 years, conducted on a "rolling” basis, that is, incorporating annual data updates to include data on new patients eligible for the cohorts and additional follow-up of the existing patients in the cohorts. The PASS is being undertaken to assess the safety of ospemifene in real life over a period of five years. The population for this study includes:
a. postmenopausal women who are being treated with ospemifene
b. postmenopausal women diagnosed with VVA but not treated for it and
c. postmenopausal women being treated with SERMs (for non-cancer, non-infertility indications or breast cancer prevention). The SERM comparison cohort will therefore consist of patients treated with raloxifene, bazedoxifene or tamoxifen (in the context of reducing breast cancer risk).
The inclusion criteria were: postmenopausal women (defined as females, ≥54 years of age) with at least one dispensing of ospemifene, raloxifene, bazedoxifene, or tamoxifen, or with a new diagnosis of VVA within the cohort definition period (1 May 2013 through 2 October 2015) and at least 12 months of medical history prior to the index date and one day after. The index date (date of cohort entry) was defined for each patient as the first dispensing of a qualified drug for the two treatment-related cohorts, and first diagnosis of VVA for the untreated for VVA cohort. Exclusion criteria were: any of the following diagnoses in the 12month baseline period: VTE, CVE, myocardial infarction (MI), endometrial hyperplasia, untreated uterine polyps, uterine prolapse, or cancers; or antineoplastic treatment during the baseline period and dispensing of ospemifene, any other SERM, or oestrogen therapy in the baseline period, other than the dispensing of the index therapy on the index date.
The primary analysis examined outcomes during the first continuous course of treatment (or continuous untreated time for the untreated for VVA cohort), defined as sequential dispensings of the study medication with no more than a 30-day gap. Person- days for an individual were defined as the total number of days in the treatment episode, ending at the earliest of the date of the initial event, end of the treatment episode, death, end of enrolment in the database, end of the study period, or initiation of oestrogen therapy or other non-comparator SERM. The primary outcome of the study was the first occurrence during the follow-up period of VTE, including deep vein thrombosis (DVT), pulmonary embolism (PE), and retinal vein thrombosis (RVT). Secondary outcomes include cerebro-vascular events (CVE), endometrial hyperplasia, endometrial cancer, pelvic organ prolapse, urinary incontinence, gall bladder events, atrial fibrillation, renal failure, renal carcinoma, renal adenoma, liver tumours, thymic epithelial tumours, increased triglycerides, vaginal bleeding and uterine diagnostic tests and procedures. Outcomes were identified through ICD-9 diagnosis codes and ICD or CPT procedure codes.
For the first continuous treatment episode, incidence proportion (%) of VTE and each of the secondary outcomes is calculated by dividing the number of patients with the outcome with the number of patients eligible for the outcome (excluding those with the outcome event at baseline). The incidence rate (per 1,000 person-years) for this period is calculated by dividing the number of first occurrences of the event in the cohort by the total aggregate person-time accrued in that cohort at the time of analysis. Poisson 95% confidence intervals were computed around each incidence rate.
Additional sensitivity analyses examined the first occurrence of each event during all follow-up time, with incidence of each event linked to the exposure categories (current, recent, or past ospemifene or comparator SERM) across the cohorts into which patients fell at the time of the event. Because separation into current, recent, and past exposure periods does not allow a view of any lingering effects of a drug after current exposure ends, the exposure periods were also combined.
The results presented here are limited to the US data from 1 May 2013 to 31 December 2015 from the planned five-year PASS and examines data from the US - MarketScan Databases (Commercial and Medicare Supplemental), as scant data are available to date on ospemifene users from the EU.
Results
Based on a cumulative total of 35,315,939 tablets distributed and 11,767,105 tablets delivered as samples between 26 February 2013 and 26 August 2017, the cumulative postmarketing exposure in the USA and EU is approximately 128,995 women-years [104-107].
Incidence rates for Important Potential Risks, based on pharmacovigilance post-marketing adverse event reporting, can be found inTable 3. The incidence rates are based on the number of cases reported during marketed use between 26th February 2013 and the 27th August 2017, divided by the number of women years represented by the total number of tablets distributed and delivered as samples between those dates. This does not provide an accurate assessment of the true incidence of these adverse events because the latter may not accurately reflect ospemifene usage and the spontaneous reporting, upon which these data are based, is subject to reporting bias, commonly leading to underreporting [108]. An assessment of the background incidence in the population of women using ospemifene is also provided but should not be used for direct comparison. For example, no degree of spontaneous reporting, even zero, is likely to remove an Important Potential Risk from the RMP .
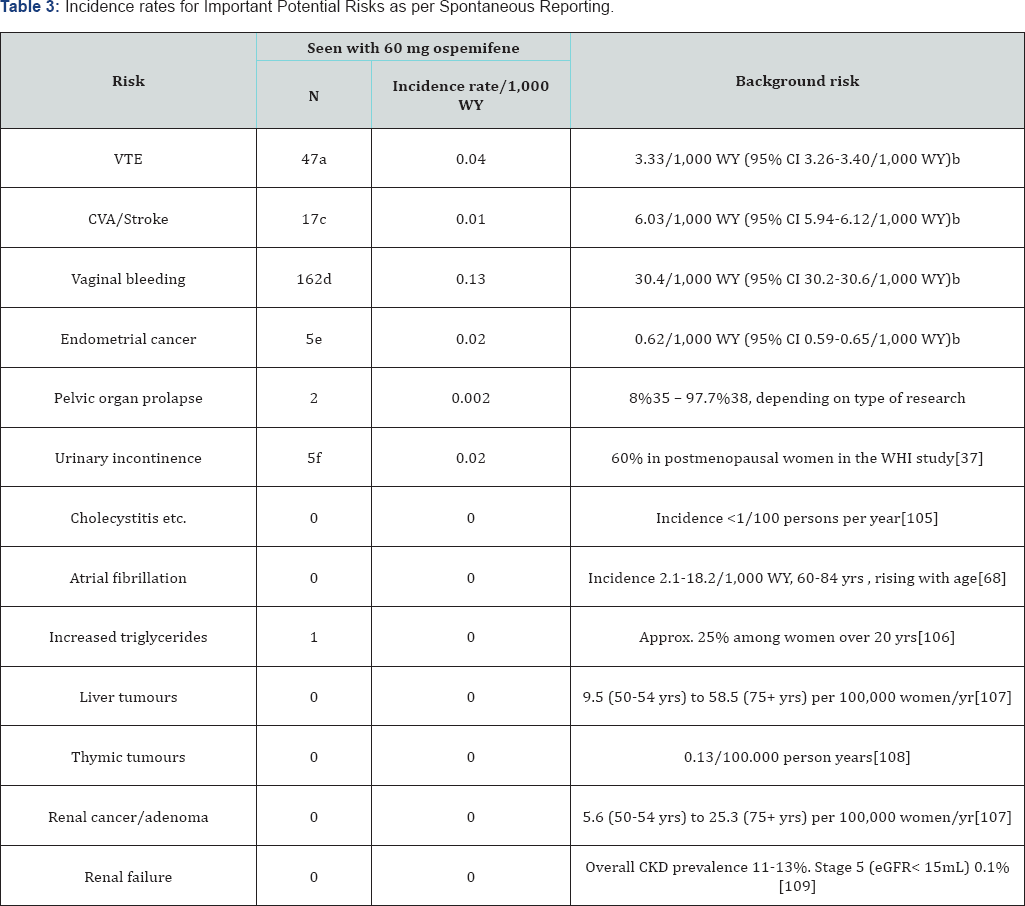
a. Includes deep vein thrombosis, thrombophlebitis, thrombosis, pulmonary embolism, retinal and portal vein thrombosis.
b. Based on a cohort of women who turned 50 years old during 2010-2015 from USA MarketScan data.
c. Includes cerebral haemorrhage, Cerebral venous thrombosis, Cerebrovascular accident, Ischaemic stroke, haemorrhagic stroke and Transient Ischaemic Attack.
d. Includes uterine haemorrhage, vaginal haemorrhage, genital haemorrhage, metrorrhagia, or postmenopausal haemorrhage.
e. Includes endometrial adenocarcinoma, endometrial cancer and uterine cancer
f. Includes incontinence, stress urinary incontinence, urge and urinary incontinence.
WY (Women Years)
CI (Confidence Interval)
eGFR (estimated Glomerular Filtration Rate)
WHI (Women's Health Initiative) mL (millilitre)
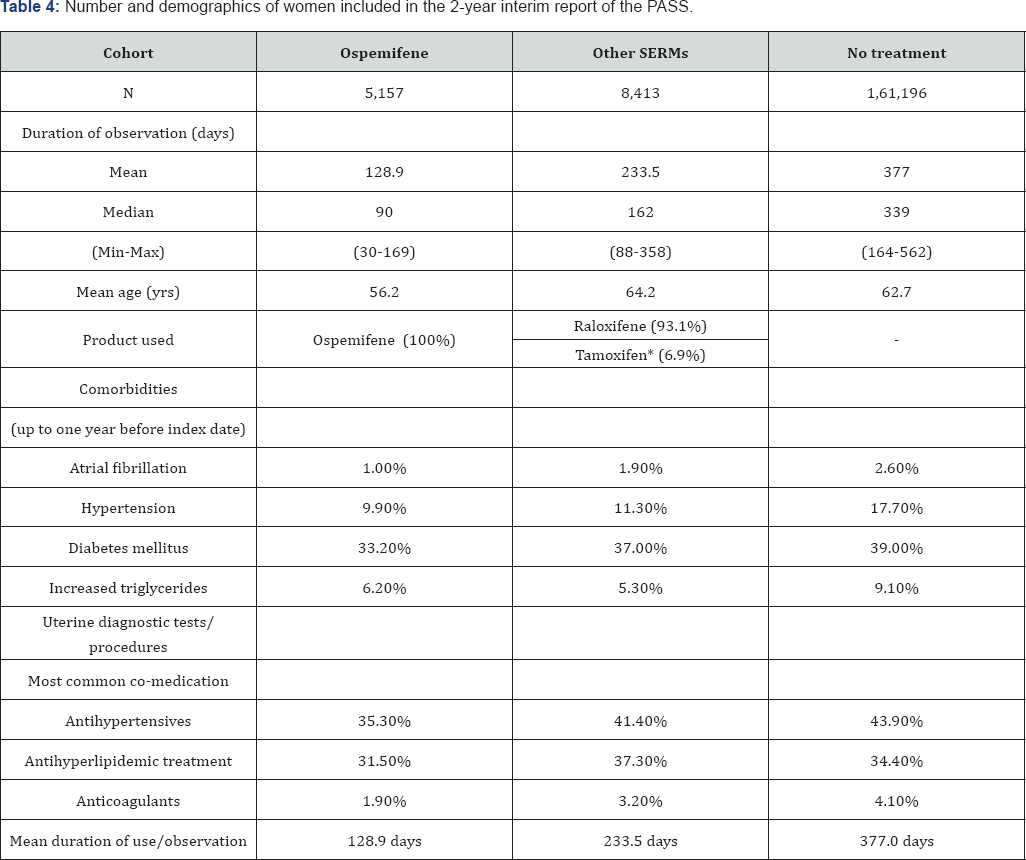
None of the Important Potential Risks incidence rates exceeds the incidence in the population; although, for reasons already outlined, the PASS study provides a more robust comparison. The PASS offers a direct comparison between women using ospemifene 60mg, women using other SERMs, not for cancer indications, and women with VVA without treatment. The numbers and demographic data from the women included Table 4: Number and demographics of women included in the 2-year i in the 2-year interim report of the PASS can be found in Table 4. Women in the ospemifene cohort are generally younger than the other two cohorts. As expected, most co-morbidities were also somewhat higher in the latter populations. The mean duration of use was shortest in the ospemifene cohort and the period of observation (untreated) was longest in the untreated cohort.
*No breast cancer patients were enrolled in this study. Both tamoxifen and raloxifene are licensed for the prevention of breast cancer in the US.
SERM (Selective Estrogen Receptor Modulator)
Ideally, all cohorts should have a similar number of patients included and similar follow-up time on drug/observation time. However, since ospemifene has only been introduced in the US 4.5 years ago, it is inevitable that the ospemifene cohort is much smaller than the other cohorts. The difference with the number of patients in the ospemifene cohort vs. the comparator SERM cohort is a factor 1.6, but vs. the untreated VVA cohort a factor 31.3. The difference in total exposure is even greater, 3.0 and 91.4 respectively. The duration of use of ospemifene is also shorter than that of other SERMs or the observation period for the notreatment population, which seems inherent to the indication [109,110].
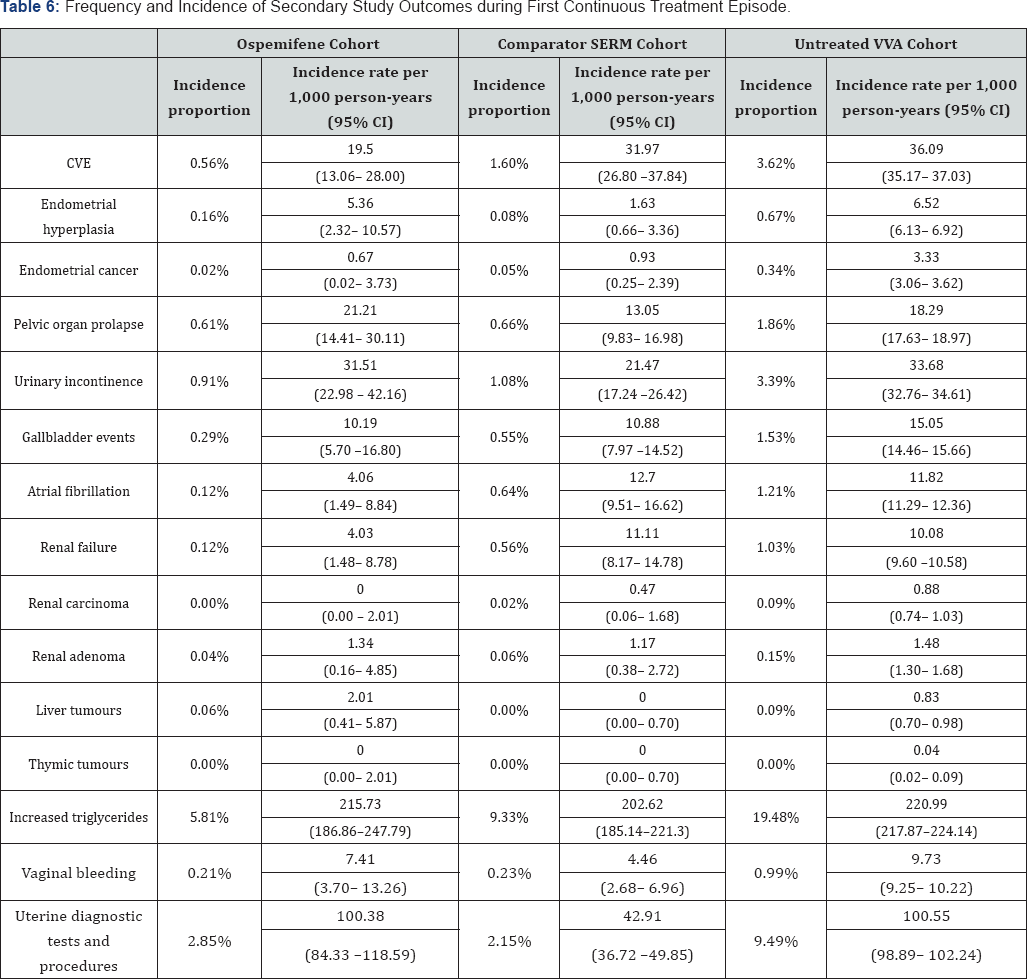
The 2-year interim results of the primary endpoint of the PASS are summarized in Table 5 and the secondary endpoints in Table 6. The incidence rate, rather than the incidence proportion, corrects for difference in exposure/observation time. Statistical comparisons have not been made in this descriptive analysis.
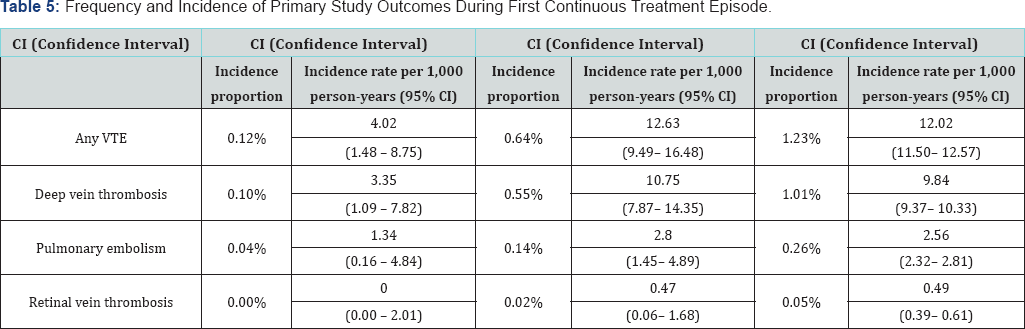
VTE (Venous Thrombo-embolism)
CI (Confidence Interval)
CI (Confidence Interval)
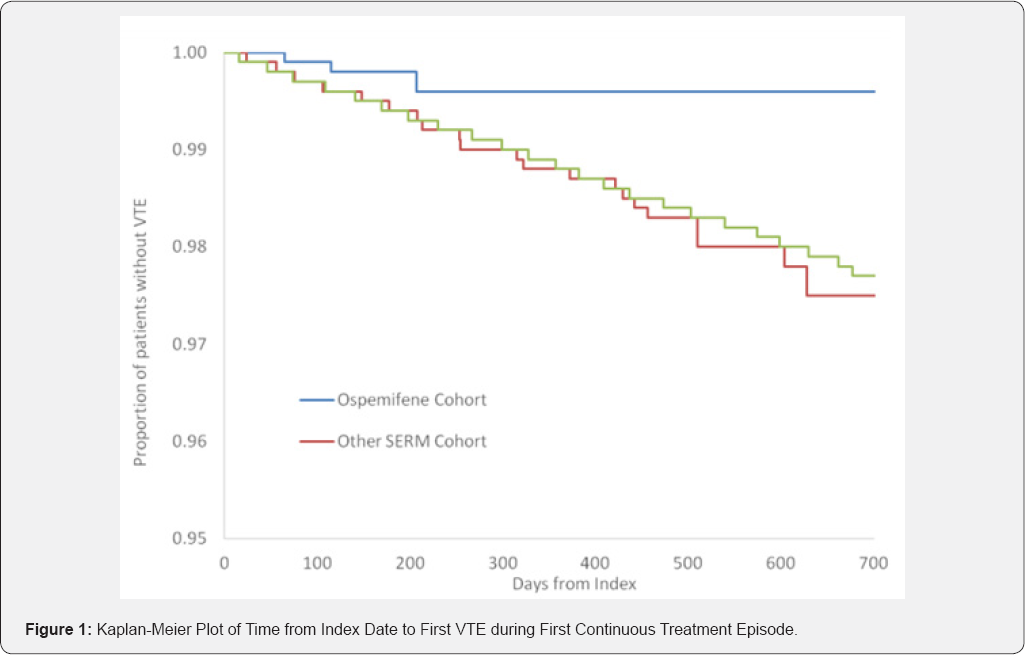
The incidence of DVT is lower in the ospemifene cohort than any of the other cohorts with no overlap in the 95% confidence intervals. The incidence of pulmonary embolism is also lower in the ospemifene cohort, but the 95% confidence limits overlap with the other two cohorts. There were no cases of RVT in the ospemifene cohort. For the primary outcome of any VTE, a Kaplan-Meier plot was constructed to illustrate the time to first event (in days) in each cohort during the first treatment episode (Figure 1). This suggests the limited impact of the shorter duration of exposure/observation in the ospemifene cohort compared to the other two cohorts as the Kaplan-Meier plot of the ospemifene cohort separates from the other two cohorts very early after the start of the first continuous treatment episode.
The incidence rates of the Important Potential Risks included as secondary endpoints can be found in Table 6 and are also lower in the ospemifene cohort than the other two cohorts, expect for pelvic organ prolapse and liver tumours, but the 95% confidence intervals overlapped substantially for both conditions with those of the other cohorts.
The incidence rates for endometrial hyperplasia, pelvic organ prolapse, urinary incontinence, liver tumours, increased triglycerides, vaginal bleeding and uterine diagnostic tests and procedures were the lowest in the comparator SERM cohort, but only for the latter did the 95% confidence interval not overlap with those of the ospemifene cohort.
Discussion
Post marketing spontaneous report data are largely used for case review and signal detection; due to the nature of their voluntary reporting, they cannot be used to exclude certain increases in risk in commonly observed events in patients at risk of those events [111]. Whilst we note that the calculated incidences of spontaneous cases for the Potential Important Risks, identified in Senshio®'s RMP, are well below the background risks in the population, a more robust comparison is expected from the formal PASS study for events with a high background rate. Conversely, because of the limited number of women included in the PASS, rare events risk being either neglected or over weighted, increasing the chances of type 2 errors. Data from spontaneous reporting systems (postmarketing surveillance) may be better for detecting signals of rare, unusual adverse events [112,113].
Thus the combination of the spontaneous reporting system, the PASS, in addition to the clinical trial data [102], provides a more balanced overview of the current knowledge of the safety of ospemifene than can be obtained through a single source of data.
Thus far, none of the Important Potential Risks identified in the Senshio® RMP has either been flagged in the post marketing spontaneous reporting system, nor has shown to be increased over and above the incidence of these risks in a population using comparator SERMs or in patients with a diagnosis of VVA without any treatment. The incidence of vaginal bleeding and uterine diagnostic tests and procedures, albeit somewhat higher than the rate in the comparator SERM population, is lower than in the untreated population. Double blind, placebo controlled, randomised studies have shown the bleeding incidence with ospemifene to be very low and comparable to placebo (2.2% for ospemifene 60mg vs. 2.6% for placebo) [5].
In the 2 year annual report of the PASS data, descriptive statistics only were applied; a full analysis is planned at the end of the 5 year study. It is important to note that the ospemifene users appear to be younger on average and with lower prevalence of some co-morbidities than the members of the other two cohorts. The lower incidence rates for VTE in the ospemifene cohort may be (partly) related to these baseline differences. The final comparative analyses will adjust for age as well as other potential confounders, allowing a more appropriate examination of the differences in rate of VTE that may be associated with ospemifene use. It is also possible that the differences in mean period of use/observation between the three cohorts have contributed to some of the differences observed, although the Kaplan-Meier plot would suggest that this influence is small.
Nevertheless, a simple comparison of the numeric results reveals no indication of an increased rate of VTE among the ospemifene users relative to the comparator SERM cohort or patients who were untreated for VVA; if anything, the rate appears lowest in the ospemifene cohort. This pattern holds true in both the primary analysis examining the first continuous course of treatment and the sensitivity analysis of time spent in categories of current, recent, and past exposure to ospemifene and comparator SERM (data not shown). Rates of all of the secondary outcomes were relatively low, with only one outcome (increased triglycerides) occurring in more than 5% of the ospemifene users; this outcome was the most frequent in both of the comparator cohorts as well.
Limitations of the present PASS analysis include the relatively small amount of person-time of exposure to ospemifene accrued to date. These analyses examine claims data only Claims diagnoses are entered for billing purposes and may be erroneous; in this database the diagnoses cannot be confirmed through chart review or other validation approaches. Conditions such as hypertension may be under-reported in claims data, and over the counter medications are not recorded. Follow-up duration for cancers, which can have a long latency period, may be insufficient.
The low number of cases for some of the endpoints also contributes to wide confidence intervals and following the continuation of the PASS, when more cases are likely to be collected, may bring some more certainty about their true incidence.
Conclusion
Despite the limitations of the methods employed to assess the real-life use safety of ospemifene, there is no reason to suggest that the risk of VTE is increased with the use of ospemifene. Also the incidence of other important potential risks does not seem to be increased above the risks seen in an untreated VVA population.
Conflicts of Interest
The study is supported by Shionogi Ltd, UK. FDG is an employee of Shionogi Ltd, NNB and TG are consultants to Shionogi Ltd, RC, KHF and BLN are employees of Evidera which conducts the study on behalf of Shionogi Ltd.
References
- Wurz GT, Kao CJ, DeGregorio MW (2014) Safety and efficacy of ospemifene for the treatment of dyspareunia associated with vulvar and vaginal atrophy due to menopause. Clin Interv Aging 9: 19391950.
- Shulman LP (2010) Selective estrogen receptor modulators and vulvovaginal atrophy: can we improve the lives of our patients with new therapeutic options? Menopause 17(3): 452.
- Guidance for Industry: Estrogen and Estrogen/Progestin Drug Products to Treat Vasomotor Symptoms and Vulvar and Vaginal Atrophy Symptoms - Recommendations for Clinical Evaluation, Accessed 14th August 2017.
- EMA Guideline on clinical investigation of medicinal products for hormone replacement therapy of oestrogen deficiency symptoms in postmenopausal women.
- http://www.ema.europa.eu/docs/en_GB/document_library/EPAR__ Public_assessment_report/human/002780/WC500182777.pdf
- https:/ /www.accessdata.fda.gov/drugsatfda_docs/ appletter/2013/203505Orig1s000ltr.pdf
- https://ec.europa.eu/health/documents/community- register/2015/20150115130408/dec_130408_en.pdf
- Komi J, Lankinen KS, DeGregorio M, Heikkinen J, Saarikoski S, et al. (2016) Effects of ospemifene and raloxifene on biochemical markers of bone turnover in postmenopausal women. J Bone Miner Metab 24(4): 314-318.
- Constantine GD, Kagan R and Miller PD (2016) Effects of ospemifene on bone parameters including clinical biomarkers in postmenopausal women. Menopause 23(6): 638-644.
- Berga SL (2013) Profile of Ospemifene in the Breast. Reprod Sci 20(10): 1130-1136.
- Soe LH, Wurz GT, Kao CJ, Degregorio MW (2013) Ospemifene for the treatment of dyspareunia associated with vulvar and vaginal atrophy: potential benefits in bone and breast. Int J Womens Health 5: 605-611.
- Eigeliene N, Kangas L, Hellmer C, Kauko T, Erkkola R, et al. (2016) Effects of ospemifene, a novel selective estrogen-receptor modulator, on human breast tissue ex vivo. Menopause 23(7): 719-730.
- Summary of the risk management plan (RMP) for Senshio (ospemifene).
- Canonico M, Plu-Bureau G, Lowe GD, Scarabin PY (2008) Hormone replacement therapy and risk of venous thromboembolism in postmenopausal women: systematic review and meta-analysis. BMJ 336(7655): 1227-1231.
- SmPC Premarin® tablets.
- Grady D, Ettinger B, Moscarelli E, Plouffe L Jr, Sarkar S, et al. (2004) Multiple Outcomes of Raloxifene Evaluation Investigators. Safety and adverse effects associated with raloxifene: multiple outcomes of raloxifene evaluation. Obstet Gynecol 104(4): 837-844.
- Rabe T, Bruyniks N, Merkle E, Damann-Hanser B, Hadji P, et al. (2015) Selective Estrogen Receptor Modulators: an Update. Joint Statement by the German Society for Gynecological Endocrinology and Reproductive Medicine (DGGEF) and the German Professional Association of Gynecologists (BVF). J Reproduktionsmed Endokrinol 12(4): 287-317.
- Anderson GL, Limacher M, Assaf AR (2004) Women's Health Initiative Steering Committee. Effects of conjugated equine estrogen in postmenopausal women with hysterectomy: the Women's Health Initiative randomized controlled trial. JAMA 291(14): 1701-1712.
- Premarin® conjugated estrogen tablets USP.
- SmPC Conbriza®.
- EPAR Evista®.
- Ensrud K, LoCroix A, Thompson JR, Thompson DD, Eastell R, et al. (2010) Lasofoxifene and Cardiovascular Events in Postmenopausal Women With Osteoporosis. Circulation 122: 1716-1724.
- Evista USPI.
- Barrett-Connor E, Mosca L, Collins P (2006) Raloxifene Use for The Heart (RUTH) Trial Investigators. Effects of raloxifene on cardiovascular events and breast cancer in postmenopausal women. N Engl J Med 355(2): 125-137.
- Tamoxifen citrate tablets USP.
- Yang TL, Wu TC, Huang CC, Huang PH, Chung CM, et al. (2014) Association of tamoxifen use and reduced cardiovascular events among asian females with breast cancer. Circ J 78(1): 135-140.
- Burbos N, Musonda P, Giarenis I, Shiner AM, Giamougiannis P, et al. (2010) Predicting the risk of endometrial cancer in postmenopausal women presenting with vaginal bleeding: the Norwich DEFAB risk assessment tool. BJ Cancer 102(8): 1201-1206.
- Lethaby A, Suckling J, Barlow D, Farquhar CM, Jepson RG, et al.(2004) Hormone replacement therapy in postmenopausal women: endometrial hyperplasia and irregular bleeding. Cochrane Database Syst Rev (3): CD000402.
- Archer DF, Pinkerton JV, Utian WH, Menegoci JC, de Villiers TJ, et al. (2009) Bazedoxifene, a selective estrogen receptor modulator: effects on the endometrium, ovaries, and breast from a randomized controlled trial in osteoporotic postmenopausal women. Menopause 16(6): 1109-1115.
- SmPC Fablyn.
- Bray F, Dos Santos Silva I, Moller H, Weiderpass E (2005) Endometrial cancer incidence trends in Europe: underlying determinants and prospects for prevention. Cancer Epidemiol Biomarkers Prev 14(5): 1132-1142.
- Weiderpass E, Baron JA, Adami HO, Magnusson C, Lindgren A, et al. (1999) Low-potency oestrogen and risk of endometrial cancer: a case-control study. Lancet 353(9167): 1824-1828.
- Pike MC, Peters RK, Cozen W, Probst-Hensch NM, Felix JC, et al. (1997) Estrogen-Progestin Replacement Therapy and Endometrial Cancer. J Natl Cancer Inst 89(15): 1110-1116.
- Polin SA, Ascher SM (2008) The effect of tamoxifen on the genital tract. Cancer Imaging 8: 135-145.
- Kenton K, Mueller ER (2006) The global burden of female pelvic floor disorders. BJU Int 98(Suppl 1): 1-5.
- Nygaard I, Barber MD, Burgio KL, Kenton K, Meikle S, et al. (2008) Prevalence of symptomatic pelvic floor disorders in US women. JAMA 300(11): 1311-1316.
- Hendrix SL, Clark A, Nygaard I, Aragaki A, Barnabei V, et al. (2002) Pelvic organ prolapse in the Women's Health Initiative: gravity and gravidity. Am J Obstet Gynecol 186(6): 1160-1162.
- Nygaard I, Bradley C, Brandt D (2004) Pelvic organ prolapse in older women: prevalence and risk Factors. Obstet Gynecol 104(3): 489-497.
- Weber MA, Kleijn MH, Langendam M, Limpens J, Heineman MJ, et al. (2015) Local Oestrogen for Pelvic Floor Disorders: A Systematic Review. PLoS One 10(9): e0136265.
- Ismail SI, Bain C, Hagen S (2010) Oestrogens for treatment or prevention of pelvic organ prolapse in postmenopausal women. Cochrane Database Syst Rev (9): CD007063.
- Vardy MD, Lindsay R, Scotti RJ, Mikhail M, Richart RM, et al. (2003) Short-term urogenital effects of raloxifene, tamoxifen, and estrogen. Am J Obstet Gynecol 189(1): 81-88.
- Goldstein SR, Neven P, Zhou L, Taylor YL, Ciaccia AV, et al. (2001) Raloxifene effect on frequency of surgery for pelvic floor relaxation. Obstet Gynecol 98(1): 91-96.
- Ravn P, Nielsen TF, Christiansen C (2006) What can be learned from the levormeloxifene experience? Acta Obstet Gynecol Scand 85(2): 135-142.
- Hendrix SL, McNeeley G (2001) Effects of selective receptor modulators on reproductive tissues other than endometrium. Ann NY Acad Sci 949: 243-250.
- Goldstein SR, Nanavati N (2002) Adverse events that are associated with the selective estrogen receptor modulator levormeloxifene in an aborted phase III osteoporosis treatment study. Am J Obstet Gynecol 187(3): 521-527.
- Zbucka-Kretowska M, Marcus-Braun N, Eboue C, Abeguile G, Wolczynski S, et al. (2011) Expression of estrogen receptors in the pelvic floor of pre- and postmenopausal women presenting pelvic organ prolapse. Folia Histochem Cytobiol 49(3): 521-527.
- Lasofoxifene Background Document for Meeting of Advisory Committee for Reproductive Health Drugs.
- Waetjen LE, Ye J, Feng WY, Johnson WO, Greendale GA, et al. (2009) Association between menopausal transition stages and developing urinary incontinence. Obstetrics and Gynecology 114(5): 989-998.
- Waetjen LE, Feng WY, Ye J, Johnson WO, Greendale GA, et al. (2008) Factors associated with worsening and improving urinary incontinence across the menopausal transition. Obstet Gynecol 111(3): 667-677.
- Legendre G, Ringa V, Fauconnier A, Fritel X (2013) Menopause, hormone treatment and urinary incontinence at midlife. Maturitas 74(1): 26-30.
- Mishra GD, Cardozo L, Kuh D (2010) Menopausal transition and the risk of urinary incontinence: results from a British prospective cohort. BJU International 106(8): 1170-1175.
- Hendrix SL, Cochrane BB, Nygaard IE, Handa VL, Barnabei VM, et al. (2005) Effects of estrogen with and without progestin on urinary incontinence. JAMA 293(8): 935-948.
- Steinauer JE, Waetjen LE, Vittinghoff E, Subak LL, Hulley SB, et al.(2005) Postenopausal hormone therapy: does it cause incontinence? Obstet Gynecol 106(5 Pt 1): 940-945.
- Cody JD, Jacobs ML, Richardson K, Moehrer B, Hextall A (2012) Oestrogen therapy for urinary incontinence in post-menopausal women. Cochrane Database Syst Rev 10: CD001405.
- Warming L, Christoffersen C, Riis BJ, Stakkestad JA, Delmas PD, et al. (2003) Adverse effects of a SERM (Levormeloxifene) Safety parameters and bone mineral density 12 months after treatment withdrawal. Maturitas 44(3): 189-199.
- Silfen SL, Ciaccia AV, Bryant HU (1999) Selective estrogen receptor modulators: tissue specificity and differential uterine effects. Climacteric 2(4): 268-283.
- Yildirim Y, Toz E (2007) The effect of long-term tamoxifen usage on the lower part of female genital tract in breast cancer survivors: a review. Marmara Medical Journal 20(3): 196-201.
- Waetjen LE, Brown JS, Modelska K, Blackwell T, Vittinghoff E, et al. (2004) Effect of raloxifene on urinary incontinence: a randomized controlled trial. Obstet Gynecol 103(2): 261-266.
- Schiavi MC, Zullo MA, Faiano P, D'Oria O, Prata G, et al. (2017) Retrospective analysis in 46 women with vulvovaginal atrophy treated with ospemifene for 12 weeks: improvement in overactive bladder symptoms. Gynecol Endocrinol 33(12): 942-945.
- Wang HH, Afdhal NH, Wang DQ (2006) Overexpression of estrogen receptor alpha increases hepatic cholesterogenesis, leading to biliary hypersecretion in mice. J Lipid Res 47(4): 778-786.
- Radha K Dhiman, Yogesh K Chawla (2006) Is there a link between oestrogen therapy and gallbladder disease? Expert Opinion on Drug Safety 5(1): 117-129.
- Boston Collaborative Drug Surveillance Program (Bcdsp) (1974) Surgically confirmed gallbladder disease, venous thromboembolism, and breast tumors in relation to postmenopausal oestrogen therapy. N Engl J Med 290(1): 15-19.
- Mamdani MM, Tu K, van Walraven C, Austin PC, Naylor DC (2000) Postmenopausal estrogen replacement therapy and increased rates of cholecystectomy and appendectomy. CMAJ 162(10): 1421-1424.
- Racine A, Bijon A, Fournier A, Mesrine S, Clavel-Chapelon F, et al. (2103) Menopausal hormone therapy and risk of cholecystectomy: a prospective study based on the French E3N cohort. CMAJ 185(7): 555-561.
- Manson JE, Chlebowski RT, Stefanick ML, Aragaki AK, Rossouw JE, et al. (2013) Menopausal Hormone Therapy and Health Outcomes During the Intervention and Extended Poststopping Phases of the Women's Health Initiative Randomized Trials. JAMA 310(13): 1353-1368.
- Akin ML, Uluutku H, Erenoglu C, Karadag A, Gulluoglu BM, et al. (2003) Tamoxifen and gallstone formation in postmenopausal breast cancer patients: retrospective cohort study. World J Surg 27(4): 395-399.
- Mohamed A, Kadambari D, Bhuvaneswari V (2009) Tamoxifen use and gallstone formation in postmenopausal breast cancer patients in south Indian population. Indian J Cancer 46(2): 151-154.
- Heeringa J, van der Kuip DA, Hofman A, Kors JA, van Herpen G, et al.(2006) Prevlence, incidence and lifetime risk of atrial fibrillation: the Rotterdam study. Eur Heart J 27(8): 949-953.
- Perez MV, Wang PJ, Larson JC, Virnig BA, Cochrane B, et al. (2012) Effects of postmenopausal hormone therapy on incident atrial fibrillation: the Women's Health Initiative randomized controlled trials. Circ Arrhythm Electrophysiol 5(6): 1108-1116.
- Veronesi U, Maisonneuve P, Rotmensz N, Bonanni B, Boyle P, et al.(2007) Tamoxifen for the prevention of breast cancer: late results of the Italian Randomized Tamoxifen Prevention Trial among women with hysterectomy. J Natl Cancer Inst 99(9): 727-737.
- Reis SE, Costantino JP, Wickerham DL, Tan-Chiu E, Wang J, et al. (2001) Cardiovascular Effects of Tamoxifen in Women With and Without Heart Disease: Breast Cancer Prevention Trial. J Natl Cancer Inst 93(1): 16-21.
- Gupta S, Rymer J (1996) Hormone replacement therapy and cardiovascular disease (review article). Int J Gynecol Obstet 52: 119125.
- Nanda S, Gupta N, Mehta HC, Sangwan K (2003) Effect of oestrogen replacement therapy on serum lipid profile. Aust N Z J Obstet Gynaecol 43(3): 213-216.
- Lee J, Goldberg IJ (2008) Hypertriglyceridemia-induced pancreatitis created by oral estrogen and in vitro fertilization ovulation induction. J Clin Lipidol 2(1): 63-66.
- Liu CL, Yang TL (2003) Sequential changes in serum triglyceride levels during adjuvant tamoxifen therapy in breast cancer patients and the effect of dose reduction. Breast Cancer Res Treat 79(1): 11-16.
- Gupta S, Tandon VR, Kapoor B, Gupta A, Gupta GD, et al. (2006) Effects of Tamoxifen Therapy on Plasma Lipid Profile in Patients of Breast Cancer. JAPI 54: 183-186.
- Castro MR, Nguyen TT, O'Brien T (1999) Clomiphene-induced severe hypertriglyceridemia and pancreatitis. Mayo Clin Proc 74(11): 11251128.
- Hozumi Y, Kawano M, Miyata M (1997) Severe hypertriglyceridemia caused by tamoxifen-treatment after breast cancer surgery. Endocr J 44(5): 745-749.
- Mosca L, Harper K, Sarkar S, O'Gorman J, Anderson PW, et al. (2001). Effect of raloxifene on serum triglycerides in postmenopausal women: influence of predisposing factors for hypertriglyceridemia. Clin Ther 23(9): 1552-1565.
- Miller PD, Chines AA, Christiansen C, Hoeck HC, Kendler DL, et al. (2008) Effects of bazedoxifene on BMD and bone turnover in postmenopausal women: 2-yr results of a randomized, double-blind, placebo-, and active-controlled study. J Bone Miner Res 23(4): 525535.
- Christiansen C1, Chesnut CH, Adachi JD, Brown JP, Fernandes CE, et al. (2010) Safety of bazedoxifene in a randomized, double-blind, placebo- and active-controlled Phase 3 study of postmenopausal women with osteoporosis. BMC Musculoskelet Disord 11: 130.
- Alexandersen P, Riis BJ, Stakkestad JA, Delmas PD, Christiansen C (2001) Efficacy of Levormeloxifene in the Prevention of Postmenopausal Bone Loss and on the Lipid Profile Compared to Low Dose Hormone Replacement Therapy. J Clin Endocrinol Metab 86(2): 755-760.
- Kusama M, Miyauchi K, Aoyama H (2004) Effects of toremifene (TOR) and tamoxifen (TAM) on serum lipids in postmenopausal patients with breast cancer. Breast Cancer Res Treat 88(1): 1-8.
- Archer DF, Altomare C, Jiang W, Cort S (2017) Ospemifene's effects on lipids and coagulation factors: a post hoc analysis of phase 2 and 3 clinical trial data. Menopause 24(10): 1167-1174.
- Villa E (2008) Role of estrogen in liver cancer. Womens Health (Lond) 4: 41-50.
- Shi L, Feng Y, Lin H, Ma R, Cai X (2014) Role of estrogen in hepatocellular carcinoma: is inflammation the key? Journal of Translational Medicine 12: 93.
- IARC Monograph 100A.
- Giannitrapani L, Soresi M, La Spada E, Cervello M, D'Alessandro N, et al. (2006) Sex hormones and risk of liver tumor. Ann N Y Acad Sci 1089: 228-236.
- Sharma S, Dawson LA (2017) Rare Tumor with a Very Rare Initial Presentation: Thymic Carcinoma as Bone Marrow Metastasis. Case Rep Pathol 2017: 6497376.
- Carlus M, Elies L, Fouque MC, Maliver P, Schorsch F (2013) Historical control data of neoplastic lesions in the Wistar Hannover Rat among eight 2-year carcinogenicity studies. Exp Toxicol Pathol 65(3): 243253.
- Chinn IK, Blackburn CC, Manley NR, Sempowski GD (2012) Changes in primary lymphoid organs with aging. Semin Immunol 24(5): 309320.
- SmPC Senshio®.
- Bojar H, Dreyfurst R, Balzer K, Staib W, Wittliff JL (1976) Oestrogen- binding components in human renal cell carcinoma. J Clin Chem Clin Biochem 14(11): 521-526.
- Nissenkorn I, Servadio C, Avidor I (1979) Oestrogen-induced renal carcinoma. Br J Urol 51(1): 6-9.
- Yu CP, Ho JY, Huang YT, Cha TL, Sun GH, et al. (2013) Estrogen inhibits renal cell carcinoma cell progression through estrogen receptor-p activation. PLoS One 8(2): e56667.
- Al-Sarraf M, Eyre H, Bonnet J, Saiki J, Gagliano R, et al. (1981) Study of tamoxifen in metastatic renal cell carcinoma and the influence of certain prognostic factors: a Southwest Oncology Group Study. Cancer Treat Rep 65(5-6): 447-451.
- Henriksson R, Nilsson S, Colleen S, Wersall P, Helsing M, et al. (1998) Survival in renal cell carcinoma-a randomized evaluation of tamoxifen vs interleukin 2, alpha-interferon (leucocyte) and tamoxifen. Br J Cancer 77(8): 1311-1317.
- Papac RJ, Keohane MF (1993) Hormonal therapy for metastatic renal cell carcinoma combined androgen and provera followed by high dose tamoxifen. Eur J Cancer 29A(7): 997-999.
- Ferrazzi E, Salvagno L, Fornasiero A, Cartei G, Fiorentino M (1980) Tamoxifen treatment for advanced renal cell cancer. Tumori 66(5): 601-605.
- EPAR Conbriza®.
- Medical Review Osphena®.
- Simon JA, Altomare C, Cort S, Jiang W, Pinkerton JV (2107) Overall Safety of Ospemifene in Postmenopausal Women from Placebo- Controlled Phase 2 and 3 Trials. J Womens Health (Larchmt) 27(1): 14-23.
- Article 104(6) of Directive 2004/27/EC of the European Parliament and the Council.
- Hazel L and Shakir SAW (2006) Under-Reporting of Adverse Drug Reactions: A Systematic Review. Drug Safety 29(5): 385-396.
- Aerts R, Penninckx F (2003) The burden of gallstone disease in Europe. Aliment Pharmacol Ther 18 (Suppl 3): 49-53.
- Ford ES, Giles WH, Dietz WH (2002) Prevalence of the metabolic syndrome among US adults: findings from the third National Health and Nutrition Examination Survey. JAMA 287(3): 356-359.
- http://globocan.iarc.fr/old/agespecific_table_r.






























