Failure of Gnrh Agonist to Trigger Ovulation: A Clue to the Mechanism of Polycystic Ovary Syndrome?
Jan Tesarik*
MARGEN CLINIC, GRANADA, SPAIN
Submission: March 08, 2018; Published: March 29, 2018;
*Corresponding author: Jan Tesarik, MARGen Clinic, Camino de Ronda 2, 18006 Granada, Spain, Email: jtesarik@clinicamargen.com
How to cite this article: Jan Tesarik. Failure of Gnrh Agonist to Trigger Ovulation: A Clue to the Mechanism of Polycystic Ovary Syndrome?. J Gynecol Women's Health 2018; 9(2): 555759. DOI: 10.19080/JGWH.2018.09.555759.
Abstract
A series of 3 cases of unexpected failure of ovulation induction with GnRH agonist is reported. All women underwent an in vitro fertilization attempt, were young (21-25 years) and showed some features of a mild polycystic ovary syndrome (PCOS). After one cycle with a contraceptive pill, followed by ovarian stimulaton with gonadotropins using a GnRH antagonist protocol, GnRH agonist was administered to trigger ovulation.In spite of the presence of numerous large (15-18mm in diameter) follicles on the day of GnRH agonist administration, no oocyte was recovered from any of them. Luteal phase characteristics suggested the absence of LH surge (failure of endometrial transformation, the absence of luteal transformation of the punctured follicles and persisting low serum progesterone levels). Surprisingly low blood LH levels throughout the ovarian stimulation were observed in all these patients. All of them were subsequently treated with the same ovarian stimulation protocol, but using recombinant hCG to trigger ovulation, resulting in the recovery of multiple mature oocytes.
These data suggest that the GnRH agonist can fail to induce preovulatory LH surge, required for ovulation, in some women with a good ovarian reserve after ovarian stimulation with a GnRH antagonist protocol. A similar mechanism, impeding the preovulatory LH rise in response to the endogenous GnRH signal, may contribute to anovulation in some PCOS women.
Keywords: Ovulation induction; Ovulation trigger; LH surge; GnRH agonist; GnRH antagonist; Anovulation; Polycystic ovary syndrome pathogenesis
Abbreviations: Gnrh: Gonadotropin-Releasing Hormone; PCOS: Polycystic Ovary Syndrome; LH: Luteinizing Hormone; Hcg: Human Chorionic Gonadotropin; FSH: Follicle-Stimulating Hormone; Hmg: Human Menopausal Gonadotropin; AMH: Anti-Mullerian Hormone; AFC: Antral Follicle Count; IU: International Unit
Introduction
Ovarian stimulation protocols using GnRH antagonists to prevent premature ovulation, followed by a GnRH agonist bolus to induce ovulation, are commonly used for ovarian stimulation of women at risk of developing ovarian hyperstimulation syndrome [1]. Many patients currently treated with the use of these protocols suffer from polycystic ovary syndrome (PCOS). Except luteal phase insufficiency, which can be resolved by prolonged GnRH agonist treatment in the luteal phase [2,3], no other inconveniences of these protocols have been reported yet.
This study shows that ovulation induction with GnRH agonist can fail in some of these patients. A series of 3 cases falling into this category is reported. It is suggested that the failure of the female endocrine system to respond correctly to GnRH may be a contributing factor in the pathogenesis of PCOS.
Patients and Methods
This study was performed with 3 couples treated by in vitro fertilization. The female patients, aged between 21 and 25 years, had some features of PCOS, but did not show either oligomenorrhea or hirsutism. Their blood FSH and LH concentrations were normal, but all showed a reversed FSH/ LH ratio as compared with normal values (Table 1). Their blood testosterone and insulin levels were normal. All the patients had regular mestrual bleeding. A high number of small antral follicles was detected by vaginal ultrasound scan on the second day of their mestrual cycle (Table 1). These follicles were predominantly located in the ovarian subcortical area, while the ovarian stroma was hypertrophied and free of follicles.
All patients were treated with a contraceptive pill (Trigynovin, Bayer) from the 2nd day till the 22nd day of the cycle preceding the beginning of ovarian stimulation. The stimulation was started between the 2nd and the 5th day of the menstrual bleeding after the end of the contraceptive pill treatment. The starting daily gonadotropin dose was 150 U recombinant FSH (Puregon, MSD) and 75 U hMG (Menopur, Ferring). The number and size of ovarian follicles was evaluated by vaginal ultrasound on the 5th day of stimulation and then every other day until the induction of ovulation as described [4,5]. Determinations of blood estradiol and LH concentrations were performed on the same days. The daily doses of recombinant FSH and hMG were adjusted according to the results of these examinations [4,5].
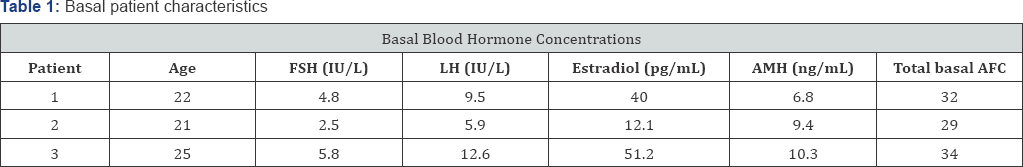
Abbreviations: AMH: Anti-Mullerian Hormone; AFC: Antral Follicle Count
Explanations: Blood FSH; LH; Estradiol And AMH And AFC Were Determined On The 2nd Day Of The Cycle.
Daily administration of GnRH antagonist ganirelix (Orgalutran, MSD) was started when at least one follicle reached 12 mm in diameter. The last injection of GnRH antagonist was administered on the day preceding the ovulation trigger. When at least 3 follicles had reached 18 mm in diameter, ovulation was triggered either with a single dose of 0.2 mg triptorelin (Decapeptyl, Ipsen) or of 250 mg recombinant hCG (Ovitrelle, Merck Serono). Ultrasound-guided follicular punture and aspiration was performed 36 h later. Previously described techniques were used for the recovery of oocytes from the follicular aspirates and their evaluation [4,5]. On the 7th day after follicular punture, blood concentrations of estradiol and progesterone were determined, and the endometrial and ovarian morphology was assessed by vaginal ultrasound.
Result
Ovarian puncture, performed 36 h after the injection of GnRH agonist, resulted in the total absence of oocytes in the folicular aspirates in all of the 3 women included in this report, in spite of the presence of multiple large follicles in their ovaries and the high blood estradiol levels achieved in the final phase of ovarian stimulation (Table 2). The blood levels of LH remained unusually low (<0.5 IU/L) from the beginning to the end of ovarian stimulation. The punctured follicles refilled with fluid after the puncture, as evidenced by ultrasound scan performed 7 days later. However, no sonographic signs of luteal transformation were observed at that time. In agreement with these observations, blood concentrations of progesterone remained low on the 7th day after ovarian puncture in all these women (Table 2).
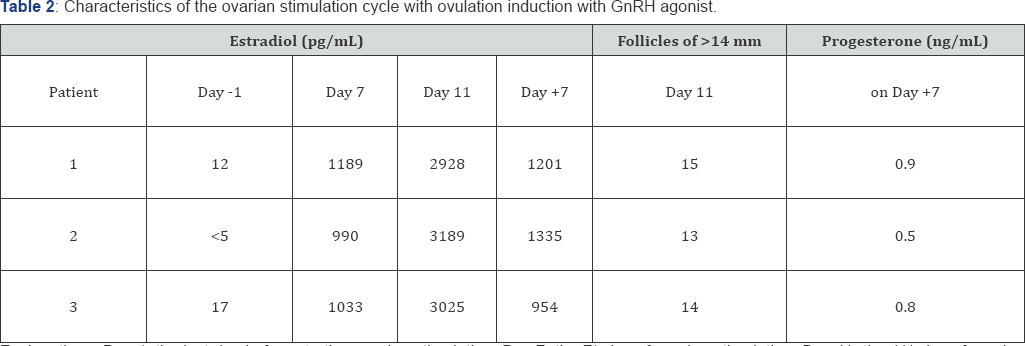
Explanations: Day -1, the last day before starting ovarian stimulation; Day 7: the 7th day of ovarian stimulation; Day 11: the 11th day of ovarian stimulation; Day +7: the 7th day after ovarian puncture
In all of the 3 women included in this study, the in vitro fertilization attempt was repeated 2-3 months later. The protocol of the ovarian stimulation was the same as in the previous attempt, but for the use of recombinant hCG, instead of GnRH agonist, as ovulation triggering agent . The growth of the ovarian follicles and the evolution of the blood estradiol levels were similar as in the previous attempt (Table 3), and the same unusually low levels of blood LH concentration were detected throughout the ovarian stimulation. However, oocytes were recovered in all of the 3 patients in this second attempt. Mature oocytes were fertilized by intracytoplasmic sperm injection, and an ongoing clinical pregnancy was achieved in 2 of the 3 patients.
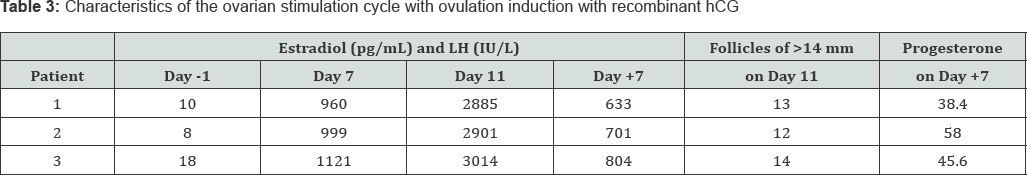
Explanations: Day -1, the last day before starting ovarian stimulation; Day 7: the 7th day of ovarian stimulation; Day 11: the 11th day of ovarian stimulation; Day +7: the 7th day after ovarian puncture
Discussion
The present observations show that ovarian puncture can fail to recover oocytes after GnRH-antagonist controlled ovarian stimulation followed by ovulation induction with GnRH agonist in some women with good ovarian reserve. The same ovarian stimulation protocol, but using recombinant human hCG instead of GnRH agonist to trigger ovulation, allowed the recovery of the expected number of fertilizable oocytes in the same patients. These data strongly suggest that GnRH agonist failed to produce the preovulatory LH surge in these women. This explanation is further corroborated by the inability to detect the luteal transformation of the punctured follicles with the use of ultrasound scan and by low blood progesterone concentrations one week after ovarian puncture.
The reason why the women included in this study did not respond to the bolus of GnRH agonist by producing an LH surge is not clear. Interestingly, all the three women had blood LH concentrations deeply and persistently suppressed after one month of contraceptive pill treatment, a rather unusual condition in this category of patients. All the three women had some features of PCOS, but did not show the fully developed syndrome. It can be hypothesized that occasional repeated failures of the endogenous GnRH signals to produce an ovulatory LH surge may contribute to the pathogenesis of PCOS.
Genetic variations of the GnRH receptor may be involved in the pathogenesis of PCOS [6-8]. Further research is needed to determine whether the failure of LH surge in response to GnRH or GnRH agonist is associated with a mutation or genetic modification of the GnRH receptor or whether other, non-genetic factors are responsible for this condition.
References
- Itskovitz-Eldor J, Kol S, Mannaerts B (2000) Use of a single bolus of GnRH agonist triptorelin to trigger ovulation after GnRH antagonist ganirelix treatment in women undergoing ovarian stimulation for assisted reproduction, with special reference to the prevention of ovarian hypersimulation syndrome: preliminary report. Hum Reprod 15(9): 1965-1968.
- Bar-Hava I, Mizrachi Y, Karfunkel-Doron D, Omer Y (2016) The GnRH- analog combination, a novel approach to avoid OHSS and enable fresh embryo transfer in high responders. Fertil Steril 106: 330-333.
- Tesarik J, Mendoza-Tesarik R, Mendoza N (2016) Gonadotropin- releasing hormone agonist for luteal phase support: the origin of the concept, current experience, mechanism of action and future perspectives. Fertil Steril 106(2): 268-269.
- Tesarik J, Mendoza C (2002) Effects of exogenous LH administration during ovarian stimulation of pituitary down-regulated young oocyte donors on oocyte yield and developmental competence. Hum Reprod 17(12): 3129-3137.
- Tesarik J (2017) Customized Assisted Reproduction Enhancement (CARE) for Women with Extremely Poor Ovarian Reserve (EPOR). J Gynecol Women's Health 3(4): 555625.
- Li Q, Yang G, Wang Y, Zhang X, Sang Q, et al. (2011) Common genetic variations in the 3'-untranslated region of gonadotropin-releasing hormone receptor regulates gene expression in cella and is associated with thyroid function, insulin secretion as well as insulin sensitivity in polycystic ovary syndrome patients. Hum Genet 129(5): 553-561.
- Chen WY, Du YQ, Guan X, Zhang HY, Liu T (2017) Efect of GnRHR polymorphisms on in vitro fertilization and embryo transfer in patients with polycystic ovary syndrome. J Hum Genet 62(12): 1065-1071.
- Caburet S, Fruchter RB, Legois B, Fellous M, Shalev S, et al. (2017) A homozygous mutation of GNRHR in a familial case diagnosed with polycystic ovary syndrome. Eur J Endocrinol 176(5): K9-K14.






























