Should Gynecologists Test for Pain Sensitization?
John Jarrell1* and Lars Arendt-Nielsen2
1Department of Obstetrics and Gynaecology, University of Calgary, Canada
2Institute for Sensory-Motor Interaction, Aalborg University, Europe
Submission: April 06, 2017; Published: August 30, 2017
*Corresponding author: John Jarrell, Department of Obstetrics and Gynaecology, University of Calgary, Canada, Email: john.jarrell@ahs.ca
How to cite this article: John J, Lars A. Should Gynecologists Test for Pain Sensitization?. J Gynecol Women's Health 2017; 6(5): 555698. DOI: 10.19080/JGWH.2017.6.555698
Abstract
Although operative laparoscopy is a successful treatment in many cases involving pelvic pain, there is increasing awareness the procedure is often unsatisfactory as no lesions may be identified and in some instances there is continued chronic pelvic pain after removal of identified lesions. One of the emerging fields that may help to understand and address this problem is pain sensitization. This condition results in persistent pain and develops from prior pain experience such as severe dysmenorrhea. It can be associated with both visible and non-visible conditions at gynecological laparoscopy. Sensitization can only be inferred from quantitative clinical testing that the clinician can undertake at the bedside with minimal equipment requirements. The basis of the test is the identification of allodynia which is pain from a non-painful stimulus. The stimulus is the cotton-tipped applicator. The objective of this paper is to introduce pain sensitization to the field of gynecological laparoscopic surgery for non-acute pain and briefly describe the tests that are to identify allodynia in an effort to improve the outcomes of women having single or repeated surgical procedures in the pelvis for visible and non-visible lesions. There are practical benefits for the woman to the identification of pain sensitization that explain and validate their pain experience, and may improve prediction of postoperative pain experience.
Keywords: Laparoscopy; Pain testing; Pelvic pain; Pain sensitization; Allodynia; Hyperalgesia
Introduction
One of the most common procedures undertaken in the field of gynecology is operative laparoscopy in the diagnosis and management of acute and chronic pelvic pain [1]. In the former situation, the diagnosis is usually apparent: hemorrhage emanating from an ectopic pregnancy or ruptured ovarian cyst, inflammation from pelvic infection or mechanical torsion of a visceral structure. In chronic states however, there is less specificity. Reports consistently demonstrate there is a "negative” rate of laparoscopy of 25-40% in cases of chronic pelvic pain [2,3]. This can be particularly upsetting if the woman is simply told that "nothing was found”. Even when there is evidence of a lesion in the pelvis, the effect of surgery may not have any bearing on the change in pain postoperatively as no clear associations are found between size of pathology and intensity of pain [4]. It is possible the cause of pain may be attributed to a visible lesion while in fact the cause of pain is due to a non-visible lesion. This is a perspective commonly seen in many other conditions e.g. revision surgery after otherwise technically successful total knee replacement, revision of prior back surgery, nerve damage, or gastric ulcers [5,6]. The specific objective of this presentation is to explore the possible reasons for these outcomes and propose options that may act to mitigate them.
Pain Sensitization- Detectable Pre-Operatively but Invisible at Laparoscopy
Increasingly pain sensitization is being recognized as a condition that can contribute to an erroneous attribution to and misinterpretation of the cause of pain at the time of surgery. Sensitization is deemed peripheral when there is increased responsiveness and reduced threshold of stimulated nociceptive neurons in the periphery [7]. Central sensitization is increased responsiveness of nociceptive neurons in the central nervous system to their normal or sub-threshold afferent input. Sensitization may be due to visible conditions in the pelvis such as endometriosis but can also be caused by invisible conditions such as severe dysmenorrhea. It this instance the surgical assessment may indicate no obvious and known pathology and the procedure is termed a "negative laparoscpy”. This process of senitization has also been associated with a large number of medical and surgical conditions such as osteoarthritis, fibromyalgia, rheumatoid arthritis, headache, neuropathic pain, temporomandibular pain, complex regional pain, musculoskeletal pain, post-surgical pain and a variety of visceral pain syndromes [8]. It has been shown that repeated surgeries further enhance the sensitization. The relationship of endometriosis to pain sensitization has been reported [9,10].
Visceral pain syndromes resulting in pain sensitization also include dysmenorrhea. In a review of 185 women with chronic pelvic pain for more than 6 months, the overall prevalence of severe dysmenorrhea was 74%. Among women with sensitization as defined by the presence of allodynia, severe dysmenorrhea was significantly more frequent compared to women without sensitization (89% vs. 68%). Notably sensitization appears to develop over the duration of severe dysmenorrhea. The evaluation of pain sensitization using pressure pain thresholds on the lower abdomen correlated negatively with the years of severe dysmenorrhea [11].
One condition that closely mimics the issues related to visceral pelvic pain is that of the post-cholecystectomy syndrome where there is pain that is similar to when the diseased gall bladder was present [12]. The original observation of allodynia and hyperalgesia in the right upper quadrant of the abdomen was made by Mackenzie in 1913. In addition to the emergence of allodynia as a consequence of biliary colic, he also noted a tender area within the allodynia corresponding to an anterior cutaneous nerve [13].
Pain Testing at the Bedside
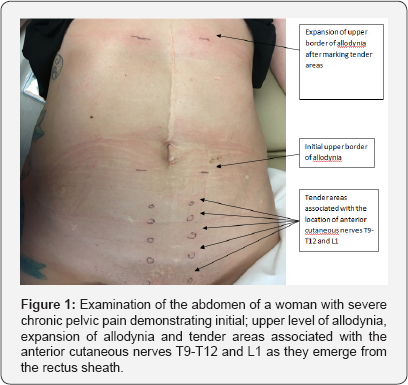
The detection of allodynia can be accomplished by drawing a cotton-tipped applicator down the mid-clavicular line along the imaginary lateral border of the rectus muscle bilaterally toward the region where the T12 and L1 nerves would emerge from the anterior rectus sheath [14]. In some instances, the allodynia must be provoked by placing pressure on the areas where the nerves emerge [15,16]. Also, the sensation in some instances is not initially painful but appears to be a paresthesia initially. However repetitive testing will enhance the pain experience a process called summation and commonly results in a dramatic increase in the area of allodynia a process called expansion presumably from increasing neuronal activity in the corresponding segments of the spinal cord [17]. The identification of these tender areas and expansion of allodynia with repeated testing are demonstrated in Figure 1.
Within the area of allodynia in the region of the T12 and L1 nerves, pain pressure thresholds can be tested using an algometer to detect hyperalgesia - defined as excessive pain. Reduced pressure pain thresholds permit further objective confirmation of the central sensitization. Notably however there are complaints that women with allodynia commonly make such as an inability to wear tight jeans and lie on their stomachs with comfort [18].
What are the Practical Implications of Pain Sensitization?
There are practical benefits to the identification of pain sensitization both when there is a visible lesion and where none is identified. With a visible lesion, the presence of sensitization is a clinical marker for objective improvement from the removal of the nociceptive focus. Preoperative sensitization has been shown to predict improvement postoperatively, presumably from the removal of the initiating pathology [19]. When no visible lesion is identified at laparoscopy the identification of sensitization validates the woman's complaint and reduces stress associated with the comment that nothing was found. It may also lead to the recognition of alternative causes for sensitization, most commonly severe dysmenorrhea but may also include irritable bowel disease and interstitial cystitis.
Summary
The benefit of quantitative sensory tests permits an appreciation of severity and allows an objective means to follow the effectiveness of treatment. The value of these clinical tests, the ease of bedside testing and the ability to provide an alternative explanation for the pelvic pain can contribute to the diagnostic accuracy of women having surgery for non-acute pelvic pain.
References
- Howard FM (1993) The role of laparoscopy in chronic pelvic pain: promise and pitfalls. Obstet Gynecol Surv 48(6): 357-387.
- Schliep K, Stanford JB, Chen Z, Zhang B, Dorais JK, et al. (2012) Interrater and intrarater reliabillity in the diagnosis and staging of endometriosis. Obstet Gynecol 120(1): 104-112.
- Milingos S, Protopapas A, Drakakis P, Liapi A, Loutradis D, et al. (2003) Laparoscopic management of patients with endometriosis and chronic pelvic pain. Ann N Y Acad Sci 997: 269-273.
- Almeida FDP, Oliveira LJ, Amaral VF (2008) Accuracy of laparoscopy for assessing patients with endometriosis. Sao Paulo Med J 126(6): 305308.
- Petersen KK, Simonsen O, Laursen MB, Nielsen TA, Rasmussen S, et al. (2015) Chronic postoperative pain after primary and revision total knee arthroplasty. Clin J Pain 31(1): 1-6.
- Skou ST, Graven-NT, Rasmussen S, Simonsen OH, Laursen MB, et al. (2013) Wide spread sensitization in patients with chronic pain after revision total knee arthroplasty. Pain 154(9): 1588-1594.
- IASP Task Force on Taxonomy (1994) Pain Terms. In: Merskey H, Bogduk N (Eds.), A Current List with Definitions and Notes on Usage (2nd edn), IASP Press, USA, pp. 209-214.
- Woolf CJ (2011) Central sensitization: implications for the diagnosis and treatment of pain. Pain 152(Suppl 3): S2- S15.
- Bajaj P, Madsen H, Arendt-NL (2003) Endometriosis is associated with central sensitization: a psychophysical controlled study. J Pain 4(7): 372-380.
- Stratton P, Khachikyan I, Sinaii N, Ortiz R, Shaw J (2015) Association of chronic pelvic pain and endometriosis with signs of sensitization and myofascial pain. Obstet Gynecol 125(3): 719-728.
- Jarrell J, Arendt-NL (2016) Allodynia and Dysmenorrhea. J Obstet Gynaecol Can 38(3): 270-274.
- Giamberardino MA, Affaitati G, Lerza R, Lapenna D, Costantini R, et al. (2005) Relationship between pain symptoms and referred sensory and trophic changes in patients with gallbladder pathology. Pain 114(1-2): 239-249.
- Mackenzie J (1913) Symptoms and Their Interpretation. (2nd edn), USA.
- Jarrell J, Malekzadeh L, Yang H, Arendt-NL (2015) The Significance of Cutaneous Allodynia in a Woman With Chronic Pelvic Pain. J Obstet Gynaecol Can 37(7): 628-632.
- Jarrell J, Giamberardino MA, Robert M, Nasr-EM (2011) Bedside testing for chronic pelvic pain: discriminating visceral from somatic pain. Pain Res Treat 2011(2011): 6.
- Nasr-EM, Jarrell J (2013) Cotton-tipped applicator test: validity and reliability in chronic pelvic pain. Am J Obstet Gynecol 208(1): 52-55.
- Jarrell J, Arendt-NL (2013) Quantitative sensory testing in gynaecology: improving preoperative and postoperative pain diagnosis. J Obstet Gynaecol Can 35(6): 531-535.
- Jarrell J, Malekzadeh L, Arendt-NL (2014) Diagnostic properties of a questionnaire to detect abdominal and perineal allodynia in women with chronic pelvic pain: WIP-0278. 14(7): 79.
- Jarrell J, Ross S, Robert M, Wood S, Tang S, et al. (2014) Prediction of postoperative pain after gynecologic laparoscopy for nonacute pelvic pain. Am J Obstet Gynecol 211(4): 360-368.






























