The Effect of Administration of Metformin on BMI and Insulin Resistance in Patients with Polycystic Ovary Syndrome
Afroz M1*, Khanom A1, Anwar BR2 and Laila R3
1Assistant professor Gynae oncology, National Institute of Cancer Research and Hospital, Bangladesh
2Associate professor Gynae oncology, National Institute of Cancer Research and Hospital, Bangladesh
3Gynaecologist, BIRDEM, Bangladesh
Submission: July 18, 2017; Published: July 31, 2017
*Corresponding author: Afroz M, Assistant professor Gynae oncology, National Institute of Cancer Research and Hospital, Bangladesh, Email: afrozmahenaz@yahoo.com
How to cite this article: Khanom A, Afroz M, Laila R, Anwar B. The Effect of Administration of Metformin on BMI and Insulin Resistance in Patients with Polycystic Ovary Syndrome. J Gynecol Women's Health 2017; 6(3): 555686. DOI: 10.19080/JGWH.2017.06.555686.
Abstract
Objective: Polycystic ovary syndrome (PCOS) is a common endocrine condition that affects women of reproductive age, is known to be associated with insulin resistance. The aim of the study was to evaluate the effects of metformin on insulin resistance, body mass index (BMI) and waist hip ratio (WHR) in patients affected by PCO Syndrome.
Materials and Method: Blood samples of eighty age-matched PCOS women, diagnosed by standard criteria, were taken to test FBS and Insulin before treatment. Patients were then divided randomly into two groups. In treated group (n=40), Metformin was prescribed three times a day (1500 mg daily) and in control group w ithout treatment. After three months, sample of blood was taken again in order to test the variance of the above mentioned parameters to compare with those amounts before test.
Results: BMI was 27.33±3.52 and 27.14±3.89Kg/m2 before treatment in case and control groups respectively. This ratio changed after treatment to 27.23±3.46 and 26.97±3.91Kg/m2 in case and control groups respectively (P value>0.84) and no significant change in WHR also. Insulin/glucose ratio was 3.45(1.04-9.21) and 3.11(1.02-15.79) while it changed after treatment to 2.52(0.79-6.86) and 2.61(0.87-10.0) in case and control group respectively . The change was significant in case group (P-value=0.004). The treated group presented significantly lower level of fasting Insulin ((17.30(5.7-66.3)vs10.90(3.4-50,0)) (p=0.001) and increased insulin sensitivity (HOMA S) (38.85(10.1-11.7) vs 61.75(12.09-199.5)) (p=0.001)) after treatment with Metformin.
Conclusion: Treatment with Metformin during three months causes decreases in insulin resistance though no effect on BMR
Introduction
Polycystic ovary syndrome is one of the most common metabolism and endocrine disorders among women with the prevalence of about 5%- 10% worldwide [1]. Although the disease was first described by Stein and Leventhal in 1935, the criteria for diagnosis of the syndrome has changed across the decades [2,3].
In 2003, a consensus workshop at a conference of the European Society for Human Reproduction and Embryology and American Society for Reproductive Medicine determined polycystic ovary syndrome to be present if two out of the three following criteria are met: anovulation or oligoovulation, clinical or laboratory excess androgen activity, or an ultrasound image of polycystic ovaries (if other endocrine disorders are excluded) [4,5].
Currently, it is believed that insulin resistance and hyperinsulinemia cause characteristic clinical symptoms and hormonal abnormalities in polycystic ovary syndrome.
PCO patients are resistance to insulin and it is not related to obesity [1], while different studies have shown that insulin has a main role in pathogenesis of this syndrome and has direct effect on ovarian steroidogenes is and consequently, it stimulates the synthesis of androgens in theca cells and reduces steroid hormone binding globulin (SHGC) in liver and increases the level of androgen [6]. Infertility is also a common issue faced by women with PCOS, most often attributed to anovulation. In addition, other factors may be operant in PCOS to lower these Women's fertility, including reduced oocyte quality, defects in endometrial development, and implantation abnormalities [7].
Insulin resistance associated with hyperinsulinaemia affects approximately 50% of PCOS patients, both obese and slim [8-10]. This results from the resistance of peripheral tissues to insulin and from decreased hepatic degradation of insulin. Resistance to insulin is most likely caused by tissue insulin receptor defects [11-13].
The objective of this study was to find out any effect of metformin on BMI, Waist /Hip ratio (WHR) and also to explore the effect of Metformin on insulin sensitivity in patients with Polycystic Ovary Syndrome.
Materials and Methods
Diagnosed cases of PCOS, attending the OPD of BSMMU and BIRDEM from January 2005 to December 2005 were included in the study. PCOS was diagnosed by oligomenorrhea or amenorrhea, obesity, hirsutism, altered LH/FSH ratio and polycystic ovary by USG finding. The total number of patients was 80. All were within reproductive age (Range: 18-40 years) and were not taking any medications known to affect carbohydrate or sex hormone metabolism. An informed consent was obtained from the subjects before the study.
The study subjects were randomly assigned into two groups. (Group-I and Group-II) using a computer-generated table of random number Group-I was Treated group (40 patients) who received Tab. Metformin 500mg T.D.S. consecutively for 3 months. Group-II was Control group (40 patients).Patients having PCOS with co-existing disease (i.e. Diabetes Mellitus, Heart Disease), diagnosed case of Congenital Adrenal Hyperplasia and acute kidney and liver diseases were excluded from the study. PCOS women with pregnancy and getting treatment with clomifen citrate were not included.
Height was measured in centimeter (cm), Weight in kilogram (kg) with light clothing and without shoes. Body mass index (BMI) was calculated as weight in kg divided by height in square meter. Waist-hip ratio was calculated as measurement of waist in cm divided by maximum measurement of hip in cm.
After selection of the subjects an appointment was given and advised to come on the appointed day in fasting condition. Fasting blood samples from each subject were collected. Fasting samples were used for glucose and insulin assay by Homeostatic Model Assessment (HOMA) method (Table 1).

Results are expressed as Mean±SD and Median (Min-Max) where applicable, Mann-Whitney U test was done for the analysis, *= Bonferroni T test was done for the analysis
For this purpose 5ml blood sample was drawn from the ante-cubital vein with all aseptic precautions. Blood sample was allowed to clot and centrifuged for 10 minutes at a rate of 3000 rpm. Serum separated was allequoted and preserved in the freezer at -70 °C for analysis of fasting insulin and glucose. At the end of study period i.e. after 3 months again blood was reflected from all the subjects to evaluate the study parameters. The fasting plasma glucose concentration was analyzed by the glucose oxidase method (Randox Laboratories Ltd., UK). Plasma insulin was assayed by chemiluminescence-based USA technique (Tiffany, 1999) using automated analyzer (IMMULITE, USA) with a commercial kit (Diagnostic Products USA).
Insulin secretory capacity (ac assessed by insulin secretion HOMA 93) and insulin resistance (ac Assessed by insulin sensitivity HOMA 93) were calculated from fasting blood glucose (mmol/1) and fasting plasma insulin (pmol/1) values by homeostasis model assessment (HOMA) using HOMA-CIGMA software Levy, Mathews and Sawans 1998.
Results
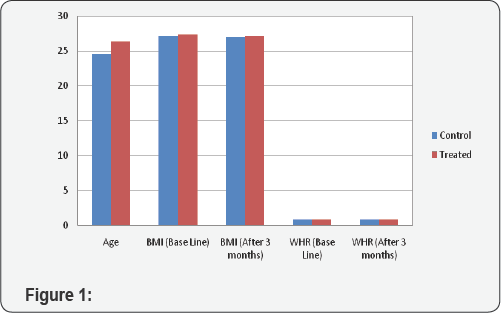
All the studied PCOS women of control and treated groups were within the reproductive age (18 - 40 years). Results are expressed as M±SD years. The mean age of 40 control PCOS women (24.55±4.36) and 40 treated PCOS women (26.30±5.26) were matched (Figure 1).
In basal conditions, there was no difference between BMI in control group (M±SD; 27.14±3.89) & treated group (27.33±3.52). During the 3 months pharmacological treatment with metformin the mean BMI did not change in any group (control 26.97±3.91 vs treated 27.13±3.46, p= 0.848) (although there was a slight reduction in BMI of both the groups). No significant changes in the Waist/hip ratio values were observed between the two groups (0.82±0.0478 basal control vs 0.82±0.0542 control 3 months later and 0.82±0.0465 basal treated vs 0.81±0.0542 treated 3 months later, p=NS). Bar chart showing effect on BMI and WHR in treatment and control group.
Fasting glucose and insulin and glucose to insulin ratio before and at the end of the study period are reported in Table 2. At baseline there were no differences in any parameters between PCOS controls and PCOS treated group. None of the studied PCOS women were known to be diabetic. Baseline fasting glucose (mmol/1) levels of the control and treated groups were 4.88±1.78 and 4.93±0.84 respectively. No significant difference was found in fasting glucose in either control or treated group during the treatment (serum glucose mmol/1, control 4.79±0.82 vs treated 4.69±0.78, p=0.588). At baseline, fasting insulin (mlU/ml) levels was similar in control (15.45(5.4-67)) and treated (17.3(5.7- 66.3)) groups. At the end of the study period fasting insulin of the control group decreased slightly, although the decrease was not statistically significant [control before 15.45(5.4- 67.0) vs control after 11.35(5.5- 42.3)) p=1.101. On the contrary insulin significantly decreased in the treated group [Treated before 17.3(5.7-66.3) vs Treated after 10.9(3.4-50)] p=0.001. No significant change in the insulin to glucose ratio was found among the control group before and after 3 months 3.11(1.02- 15.79) vs 2.61(0.87 - 10), p=0.214. On the other hand, there was a significant change in Insulin to glucose ratio in the metformin treated group after 3 months (Treated before 3.45(1.05- 9.21) vs Treated after 2.52(0.79 - 6.86)), p=0.004.
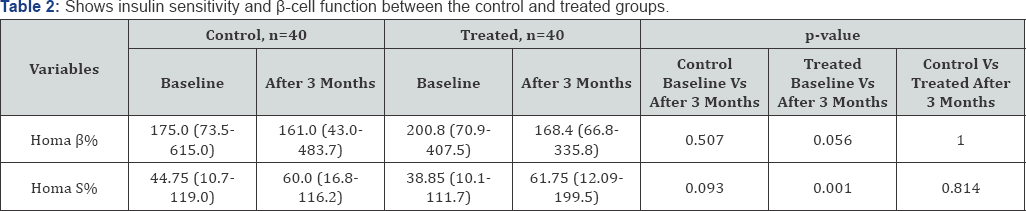
Insulin secretory capacity (Homa B%) of the control group at the basal and after 3 months of study period were 175(73.5- 615) and 161(43-483.7) respectively, p=0.507. p-cell secretory capacity tended to be lower although not significantly, in the group treated with metformin [Treated group before 200.8(70.9- 407.5) vs Treated 3 months after 168.4(66.8- 335.8) p=0.056). There were no statistically significant improvements found in the insulin sensitivity (HomaS%) in the control group during the study period before 44.75(10.7-119) vs after 60(16.8-116.2), p= 0.093. On the contrary, Metformin had a highly significant effect in improving Insulin sensitivity in the treated group of PCOS women [before 38.85(10.1-111.7) vs 3 months after 61.75(12.9- 199.5) ], p=0.001 including that insulin resistance is improved in treated PCOS women with metformin.
Discussion
Failure of the target cells to respond to normal or ordinary levels of insulin is regarded as insulin resistance (IR) [14]. The presence of IR, however, leads to a compensatory increased production of insulin by the pancreatic beta cells to control the hyperglycaemia which ultimately fails leading to T2DM. In PCOS, hyperinsulinaemia has been thought to increase hyperandrogenaemia via a central role [15] or by decreasing the circulating levels of sex hormone binding globulin. IR is not considered a diagnostic criterion in PCOS [16]. However, it is recognized by many as a common feature in PCOS independent of obesity [17].
An estimated prevalence of IR among PCOS patients of 60-70% has been reported. However, being overweight or obese is common among PCOS women, affecting up to 88% of these women, therefore casting doubt on the role IR plays in the pathogenesis of PCOS [17]. Further, clinical quantification of IR is not accurate enough [17] to enable a better understanding of the role of IR in PCOS pathogenesis or to incorporate it into the work up programme of PCOS patients. However, it is generally acceptable that IR plays a significant role in PCOS either directly or through obesity and represents a clinical concern to physicians and patients [17].
Metformin was the first insulin sensitising drug (ISD) to be used in PCOS to investigate the role of insulin resistance in the pathogenesis of the syndrome [18]. Insulin resistance (IR) and secondary hyperinsulinemia affect approximately 65-70% of women with PCOS.19 Many of these women are also obese, which further exacerbates their IR. Insulin stimulates ovarian theca cell androgen production and secretion, and suppresses the hepatic production of sex hormone-binding globulin. The increased intraovarian androgens then disrupt folliculogenesis. Hyperinsulinemia may also directly cause premature follicular atresia and antral follicle arrest [19]. The resulting anovulation also leads to unopposed estrogen production and endometrial proliferation in women with PCOS, leading to an increased risk of endometrial hyperplasia.
Metformin is currently the most widely used drug worldwide for the treatment for type 2 DM. Its primary action appears to be an inhibition of hepatic glucose production and an increase in peripheral insulin sensitivity. The benefits of metformin on insulin sensitivity have been demonstrated in non-DM women with PCOS [20]. The use of metformin is associated with increased menstrual cyclicity, improved ovulation, and a reduction in circulating androgen levels [21]. Metabolic benefits are enhanced in the presence of weight loss, and weight loss itself may be enhanced in the presence of metformin [22].
Metformin (1,1-dimethylbuguanide hydrochloride) is a biguanide currently used as an oral antihyperglycemic agent. Its primary clinical action is to inhibit hepatic glucose production, although it also decreases intestinal glucose uptake and increases insulin\sensitivity in peripheral tissues. Metformin has antilipolytic effects, lowering circulating free fatty acid concentrations, which ultimately aids in reducing gluconeogenesis [17].
Metformin works by improving the sensitivity of peripheral tissues to insulin which results in a reduction of circulating insulin levels [17]. Metformin inhibits hepatic gluconeogenesis and it also increases the glucose uptake by peripheral tissues and reduces fatty acid oxidation [17]. Metformin has a positive effect on the endothelium and adipose tissue independent of its action on insulin and glucose levels [17].
Metformin likely plays its role in improving ovulation induction in women with PCOS through a variety of actions, including reducing insulin levels and altering the effect of insulin on ovarian androgen biosynthesis, theca cell proliferation, and endometrial growth. Also, potentially through a direct effect, it inhibits ovarian gluconeogenesis and thus reduces ovarian androgen production [19].
Metformin has useful effects in reduction ofthe cardiovascular risk factors and it is the early available medication against hyperglycemia which decreases macrovascular complications in diabetic patients [23]. In our study the control and treatment group, insulin resistance was evaluated based on the fasting glucose, fasting insulin and insulin to glucose ratio.
To date, a single, adequate marker of insulin resistance that would be both highly sensitive and specific is lacking. The gold standard worldwide is the hyperinsulinemic-euglicemic clamp technique; however, this method is time-consuming and thus more applicable to research than to daily clinical practice. 24 Measurement of the fasting insulin concentration (I0) is an easy marker to obtain, and values equal to 20 or higher indicate the presence of insulin resistance. Santana et al. [24] favoured the QUICKI, HOMA-IR, and fasting insulin to fasting glucose ratio as the most useful indexes in the evaluation of resistance to insulin. We have used both HOMA and fasting glucose insulin ratio in this study. In our study the average fasting insulin value was similar between control and treatment group; in control group it is 15.45 (5.4-67) and in treatment group 17.30(5.7-66.3). But there is significant decrease in plasma insulin in treatment group after treatment with metformin.
It was shown that treatment with 1500 mg of metformin for 8 weeks decreases the level of serum insulin Metformin increases the sensitivity to insulin that decreases the chance of diabetes 23.Our results showed that sensitivity to insulin (FBS/insulin ratio) has increased in the treated group when compared before and after three month.
In a study designed to control the effect of body weight, the administration of metformin was without effect on insulin resistance in extremely overweight women with polycystic ovaries. In another-well designed study, metformin again had no effect on insulin resistance when body weights remained unchanged, and baseline weights and hyperinsulinemia were only modestly increased [23].
The first observational study on metformin in PCOS reported weight loss during metformin therapy 18. In their metaanalysis of all of the earlier small studies, Lord and colleagues reported that metformin had no significant effect on body weight or waist:hip ratio [25]. In a RCT designed specifically to investigate the effect of metformin on body weight, Harborne and colleagues reported a significant decrease in BMI in obese and morbidly obese women independent of lifestyle modification [26]. Others reported no effect of metformin on body weight over and above that induced by lifestyle modification alone [27]. Tang and co-workers ) have indicated that there were no significant changes in insulin sensitivity or lipid profiles after treatment with metformin. Also, in this study, metformin did not improve weight loss or menstrual frequency in patients with PCOS [23]. In contrast to some of the studies that metformin has a good effect on the reduction of weight and BMI [28,29], our investigation showed that there was no significant difference in weight and BMI after treatment with metformin. Aruna's study (2004) indicated that significant improvement menstrual cyclicity, ovulation and pregnancy rates were noted after treatment with metformin [30]. Santana et al. 2004 have shown that there were no changes in BMI after metformin treatment [31,32]. Our results were the same as this study.
Conclusione
Hyperinsulinemia and hyperandrogenism increase the risk of diabetes in PCO patients 1. Women with anovulation, hyperandrogenism and hyperinsulinemia are more exposed to the risk of diabetes mellitus independent from insulin 1. Different studies have shown that continuous anovulation made the patients three times more susceptible to endometrial cancer 23. On the other hand, the risk of breast cancer increases in patients with chronic anovulation three or four times. Metformin increases the sensitivity to insulin that decreases the chance of diabetes [23].
However, treatment with metformin in PCO patients reduce the insulin resistance there by also reduce the complication related to hyperinsulinaemia.
References
- Speroff (2005) Clinical Gynecologic endocrinology and infertility. lippincott Williams 12: 485-513.
- Stein IF, Leventhal ML (1935) Amenorrhea associated with bilateral polycysticovaries. Am J Obstet Gynecol 29: 181-191.
- Adams J, Franks S, Polson DW, Mason HD, Abdulwahid N, et al. (1985) Multi-follicular ovaries: clinical and endocrine features andresponse to pulsatile gonadotropin releasing hormone. Lancet 1985; 8469-8470: 1375-1379.
- Rotterdam ESHRE/ASRM-Sponsored PCOS Consensus Workshop Group (2004) Revised 2003 consensus on diagnostic criteria and longterm health risks related to polycystic ovary syndrome. FertilSteril 81: 19-25.
- Azziz R, Woods KS, Reyna R, Key TJ, Knochenhauer ES, et al. (2004) The prevalence and features of the polycystic ovary syndrome in an unselected population. J Clin Endocrinol Metab 89: 2745-2749.
- Dunaif A (1992) Insulin resistance and ovarian hyperandrogenism. Endocrynologist 2: 248-60
- Legro RS (2007) Pregnancy considerations in women with polycystic ovary syndrome. Clin Obstet Gynecol 50: 295-304.
- Laven JSE, Mulders AGMGJ, van Santbrink EJP, Eijkemans MJC, Fauser BCJM (2005) PCOS: backgrounds, evidence and problems in diagnosing the syndrome. In: Slager E, Fauser B, et al. (Eds.), Gynaecology, obstetrics, and reproductive medicine in daily practice. Proceedings of the 15th Congress of Gynaecology, Obstetrics and Reproductive Medicine. International Congress Series 1279: 10-15.
- Dunaif A, Segal KR, Futterweit W, Dobrjansky A (1989) Profound peripheral insulin resistance, independent of obesity, in polycystic ovary syndrome. Diabetes 1989; 38: 1165-1174.
- Bigos A, Patkowska E, Rosoiowska-Huszcz D (2012) Effect of artificial and natural sweeteners on glucose and insulin in plasma of rats. JPCCR 6(2): 93-97.
- Jakimiuk A,Czajkowski K(2007) Brakowulacjiijajnikipolicystyczne. In: Speroff L, Fritz M (Eds.) Klinicznaendokrynologiaginekologicznainiepiodnosc. Warszawa, Medipage p, 533-573.
- Strączkowski M, Nikoiajuk A, Dzienis-Str^czkowska A (2005) Metodypomiaru insulin oporno sei in-vivo. In: Kinalska I (Eds.) Patofizjologiainast^pstwakliniczneinsulinoopornosci. Warszawa, WIG- Press, p. 215-217.
- Sam S, Dunaif A (2003) Polycystic ovary syndrome: syndrome XX? Trends Endocrinol Metab 14: 365-370.
- Le Roith D, Zick Y (2001) Recent advances in our understanding of insulin action and insulin resistance. Diabetes Care 24(3): 588-597.
- Barbieri RL, Makris A, Randall RW, Daniels G, Kistner RW, et al. (1986) Insulin stimulates androgen accumulation in incubations of ovarian stroma obtained from women with hyperandrogenism. J ClinEndocrinolMetab 62(5): 904-910.
- ESHRE/ASRM (2003) Revised 2003 consensus on diagnostic criteria and long-term health risks related to polycystic ovary syndrome (PCOS). Hum Reprod 19: 41-47.
- Lashen H (2010) Role of metformin in the management of polycystic ovary syndrome (Review). Ther Adv Endocrinol Metab 1(3): 117-128
- Velazquez EM, Mendoza S, Hamer T, Sosa F, Glueck CJ (1994) Metformin therapy in polycystic ovary syndrome reduces hyperinsulinemia, insulin resistance, hyperandrogenemia, and systolic blood pressure, while facilitating normal menses and pregnancy. Metabolism 43(5): 647-654.
- Mathur R, Alexander CJ, Yano J, Trivax T, Azziz R (2008) Use of metformin in polycystic ovary syndrome, review. American Journal Of Obstetrics and Gynaecology.
- Jonard S, Dewailly D (2004) The follicular excess in polycystic ovaries, due to intra-ovarian hyperandrogenism, may be the main culprit for the follicular arrest. Hum Reprod Update 10: 107-17.
- Nestler JE (2008) Metformin for the treatment of the polycystic ovary syndrome. N Engl J Med 358(1): 47-54.
- Tan S, Hahn S, Benson S, Dietz T, Lahner H, et al. (2007) Metformin improves polycystic ovary syndrome symptoms irrespective of pretreatment insulin resistance. Eur J Endocrinol 157: 669-676.
- De leo V, La Marca A, Diffo A (1999) Effect of metformin on induced ovulation in women with poly cystic ovary syndrome. FertilSteril 72: 282-285.
- Karimjadeh, Eftekhar M, Taheripanah R, Tayebi N, Sakhavat, et al. (2007) The effect of administration of metformin on lipid profile changes and insulin resistance in patients with polycystic ovary syndrome. Middle east fertility society joutnal 12(3): 174-178.
- Nawrocka-Rutkowska J, Ciecwiez S, Marciniak A, Brodowska A, Wisniewska B, et al. (2013) Insulin resistance assessment in patients with polycystic ovary syndrome using different diagnostic criteria - Impact of metformin treatment. Ann Agric Environ Med 20(3): 528532.
- Lord JM, Flight IH, Norman RJ (2003) Insulin-sensitising drugs (metformin, troglitazone, rosiglitazone, pioglitazone, D-chiro-inositol) for polycystic ovary syndrome. Cochrane Database Syst Rev 3: CD003053.
- Harborne LR, Sattar N, Norman JE, Fleming R (2005) Metformin and weight loss in obese women with polycystic ovary syndrome: comparison of doses. J ClinEndocrinolMetab 90: 4593-4598.
- Tang T, Glanville J, Hayden CJ, White D, Barth JH, et al. (2006) Combined lifestyle modification and metformin in obese patients with polycystic ovary syndrome. A randomized, placebo controlled, double-blind multicentre study. Hum Reprod 21: 80-89.
- Kolodziejczyk B, Duleba AJ, Spaczynski RZ, Pawelczyk L (2000) Metformin therapy decreases hyperandrogenism and hyperinsulinemia in women with polycystic ovary syndrome. Fertil Steril 73(6): 11491154.
- Kazerooni T, Dehghan-Kooshkghazi M (2003) Effects of metformin therapy on hyperandrogenism in women with polycystic ovarian syndrome. GynecolEndocrinol 17(1): 51-56.
- Aruna J, Mittal S, Kumar S, Misra R, Dadhwal V, et al. (2004) Metformin in women with polycystic ovary syndrome. Int J Gynaecol Obstet 2(6): 56.
- Santana LF, de Sa MF, Ferriani RA, de Moura MD, Foss MC, et al. (2004) Effect of metformin on the clinical and metabolic assessment of women with polycystic ovary syndrome. GynecolEndocrinol 19(2): 88-96.






























