Venous Thrombo prophylaxis in Pregnancy and Puerperium: the Saudi Algorithm
Arab H1*, Abduljabbar H2, Sabr Y3, Bondogji N2, Mosali F4, Alhazmi J5, Bajouh O2, Nasrat H6 and Wahbah E7
1Dr Arab Medical Center, Saudi Arabia
2King Abdulaziz University Hospital, Saudi Arabia
3King Saud University Hospital, Saudi Arabia
4Alyamamah Maternity & Children Hospital, Saudi Arabia
5Almadinah Maternity & Children Hospital, Saudi Arabia
6Alola Polyclinic, Saudi Arabia
7Prince Sultan Medical City, Saudi Arabia
Submission: January 28, 2017; Published: February 14, 2017
*Correspondence Address: Hisham Arab, Women and Fetal Health Program, Dr Arab Medical Center SSOG-Jeddah, Saudi Arabia, Email: arab123@gmail.com
How to cite this article: Arab H, Abduljabbar H, Sabr Y, Bondogji N, Mosali F, et al. Venous Thrombo prophylaxis in Pregnancy and Puerperium: the Saudi Algorithm. J Gynecol Women's Health. 2017; 2(3): 555590. DOI: 10.19080/JGWH.2017.02.555590
Abstract
Adequate use of venous thromboembolism (VTE) prophylaxis is considered an indicator of quality of care in any medical institution. However, like in many other countries, we found out that in Saudi Arabia around 50% of Obstetric patients failed to get thromboprophylaxis when it was indicated, and the main reason was physicians overlooked performing the thromboembolism risk assessment. Accordingly, many measures took place in our health system over the past few years, including increasing doctors and patients' awareness, improving admission protocols, and simplifying the risk assessment procedure for a busy practitioner. 9 experts from around the country reviewed all published international guidelines on this subject, and came up with an algorithmic approach with the recommended prophylactic measure for any patient at a glance. By excluding the past history of thromboembolism and/or Thrombophilia which is very rare among our pregnant patients at booking (<1%), attention will be directed to one page algorithm that covers all other possibilities whether antenatal or postnatal, with the recommended measure of thromboprophylaxis. A word of caution, complicated cases or conditions that require the use of anticoagulant other than low molecular weight heparin (LMWH) should consult a thrombosis specialist or refer to major guidelines.
Keywords: Venous thromboembolism prophylaxis; Guidelines; Antenatal; Puerperium; LMWH; Algorithm; Pregnancy
Abbreviations: VTE: Venous Thromboembolism; LMWH: Low Molecular Weight Heparin; CASP: Critical Appraisal Skills Programme; PGM: Prothrombin Gene Mutation; UH: Unfactionated Heparin; ACOG: The American Congress of Obstetricians and Gynecologists; SOGC: The Society of Obstetrician and Gynecologist of Canada; RCOG: The Royal College of Obstetricians and Gynecologists; ACCP: American College of Chest Physician
Introduction
Venous thromboembolism (VTE) considered one of the leading causes of maternal morbidity and mortality all over the world. The reported incidence of VTE from most developed countries ranging between 1-2 cases per 1000 pregnancies [1]. The risk of deep venous thrombosis is five times higher comparing to non pregnant women [2]. The risk of VTE is greater in postpartum than during the antenatal period. Deepvein thrombosisin pregnant women occurs more frequently in the left leg (85%, vs. 55%) among nonpregnant [3]. Searching in the literature, there is very limited data about the incidence ofVTE in pregnancy and puerperium in Saudi Arabia. The reported study from Saudi Arabia showed that the incidence of VTE is 1.25 cases per 1,000 deliveries (95% CI 0.89-1.16) [4]. The Maternal mortality rate was 0.02 5 case per 1,000 deliveries. Exploring the risk factors to develop VET that was described in different guidelines showed that the majority of obstetrical population in our society is considered as high risk. These risk factors included but not limited to, multiparity, obesity, advanced maternal age repeated cesarean section and consanguinity marriages which may increase the risk of inherited thrombophilia thereby increasing risks of VTE. The above mentioned fact made it essential to develop tailored guidance to our high risk obstetrical population compared to the developed countries.
Admitting that the incidence and the mortality rate are under estimated in the face of lacking postmortem autopsy to confirm the diagnosis of suspected death related to VTE and that we need more work to determine our own incidence and risk factors. This algorithm was developed based on the best available data and international guidelines to date. The aim of this algorithm is to help the health care providers across the Kingdom of Saudi Arabia in implementing systematic and simplified approach towards the prevention and management of VTE during pregnancy, labor and puerperium.
Methodology
The development group for these guidelines consists of a panel of Maternal-Fetal Medicine experts in different sectors in the medical care system in the kingdom of Saudi Arabia including National Guard, military, universities, ministry of health and private sector.
Literature search was carried out at the following electronic databases: The Cochrane Library (including the Cochrane Database of Systematic Reviews, the Cochrane Central Register of Controlled Trials and the Database of Abstracts of Reviews of Effects [DARE]), EMBASE, the ACP Journal Club, PUBMED, Medline, (CDSR), Journal full text via OVID search engine and Science Direct.
The databases were searched using the relevant Medical Subject Headings (Mesh) terms, including all subheadings. The principal search terms used were: 'venous thromboembolism', 'thrombosis', 'pregnancy', 'postpartum', 'puerperium', 'antenatal', 'prenatal'. The search was limited to humans and the English language.
In addition, the reference lists of studies selected for inclusion were scanned for relevant studies. Guidelines on venous thromboprophylaxis in pregnancy and puerperium from different well recognized health care institutions and organization including: The American College of Obstetricians and Gynecologists (ACOG) [5,6], The Society of Obstetrician and Gynecologist of Canada (SOGC) [7], The Royal College of Obstetricians and Gynecologists (RCOG) [8,9], American College of Chest Physician (ACCP) [10], The Australian [11] and The Irish [12] were reviewed.
Due to the lack of evidence in thromboembolic events among pregnant women in Saudi Arabia a pilot data collection study performed in the city of Medina to compare the prevalence and the risk factors of VTE among pregnant Saudi women with data reported in the literature.
The targeted clinical questions were divided into tasks, which were distributed among the committee members; each member was assigned individual topics. On completing the task the assigned member was asked to present the results of his work to the group to be appraised by workgroup members using Critical Appraisal Skills Programmed (CASP) checklist [13].
This working group has concluded that they are in agreement with the classification of evidence level and the grade of recommendation presented in the Green-top Guideline No. 37a April 2015 of the RCOG. Hence, for this purpose readers are referred to this guideline.
A comprehensive summary of this group's recommendations for VTE thromboprophylaxis in pregnancy and puerperium is the mainstay of this document followed by an appendix of quick reference algorithm to simplify this task for the busy clinician.
Risk Assessment and Thromboprophylaxis Recommendations
There are certain factors that determine the magnitude for the risk of VTE in association with the pregnancy or the puerperium. These are called risk factors that have been identified after an extensive literature search and presented recently in 2 major guidelines published from Canada (2014) and United Kingdom (2015).
After establishing the characteristics of the Saudi reproductive performance and their VTE risk factors, the reviewers critically analyzed those international guidelines, and developed their expert opinion of the best applicable thromboprophylactic recommendations for this population as outlined in this review.
Risk Assessment is a dynamic process that should be carried out on the first pregnancy visit and repeated whenever there was any development or admission during the course of pregnancy, during labor, and immediately after delivery.
The Algorithm
We have identified 2 major personal risk factors and they are:
- Personal history of previous VTE
- Personal history thrombophilia
The incidence of these 2 personal risk factors is <1%. Hence, it would be much easier during risk assessment process to exclude these 2 conditions from the beginning by using Algorithm 1 first (Figure 1). On this algorithm, the health care provider will find the recommended Thromboprophylaxis measure according to the patient situation.
Regardless of any risk factor, the personal history of previous VTE or thrombophilia is an independent factor that dictates its prophylactic measures in a pre-conceptional counseling assessment or anytime the patient found to be pregnant.
Situation 1
Previous history of VTE due to thrombophilia: Whether it was a heritable (antithrombin deficiency) or an acquired (antiphospholipid syndrome) thrombophilia, these patients are often on long term oral anticoagulants and a hematologist should be involved in their thromboprophylaxis management. High dose of LMWH (50-75% of the therapeutic dose) should be recommended throughout the antenatal period and for 6 weeks postpartum. Then, they should go back to their pre-pregnancy treatment modality.
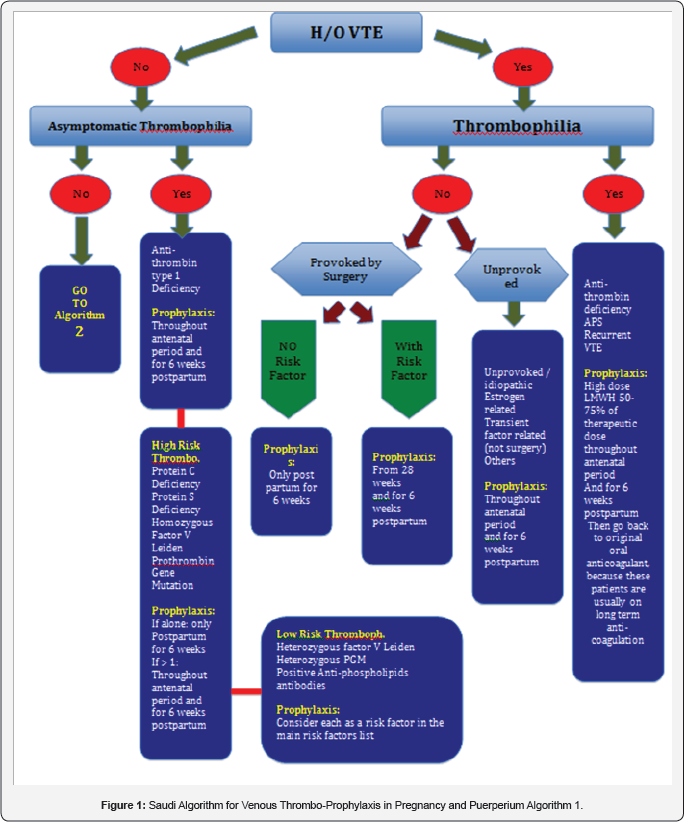
Situation 2
Previous history of VTE without thrombophiliaIf the VTE was unprovoked, i.e. idiopathic, estrogen related, or due to transient factor not related to surgery, patient should have thromboprophylaxis throughout the antenatal period and for 6 weeks postpartum. If the VTE was provoked by surgery, then Risk Factors from (Table 1 & 2) should be explored: in the absence of risk factors then prophylaxis is limited to the 6 weeks postpartum period. However, the presence of any risk factorin this situation would require thromboprophylaxis commenced at 28 weeks gestation and continued for 6 weeks postpartum.
Situation 3
Asymptomatic thrombophilia is present but no previous history of VTE: Thromboprophylaxis depends on the type of the thrombophilia Thromboprophylaxis is recommended throughout the antenatal period and for 6 weeks postpartum.
Anti-thrombin type I deficiency: Thromboprophylaxis is recommended throughout the antenatal period and for 6 weeks postpartum.
High risk thrombophilia: such as protein C deficiency, protein S deficiency, Homozygous Factor V Leiden and Prothrombin Gene Mutation (PGM):
- If more than one are co-existing, the prophylaxis will be similar to antithrombin deficiency
- However, if only one exists, the recommended prophylaxis will be limited to the 6 weeks postpartum period.
Low risk thrombophilia: such as heterozygous factor V Leiden, Heterozygous PGM, or positive anti-phospholipids antibodies: Thromboprophylactic recommendation is to consider each one of the above as a risk factor added to Table 1 & 2 and act according to recommended management of using such tables.
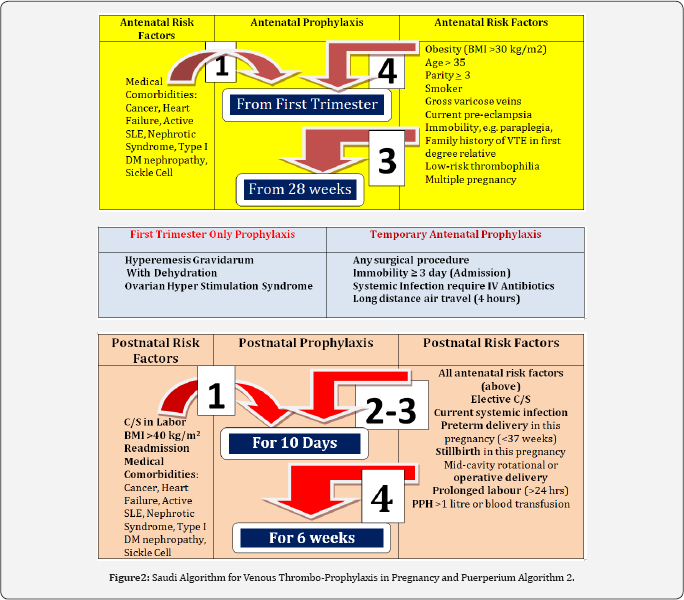
Situation 4
thrombophilia: This constitutes the vast majority of our patients and their thromboprophylactic management depends on risk factors that are grouped in ( Tables 3), namely: Antenatal, postnatal and transient. According to the number of risk factors present from any of the tables the recommended thromboprophylactic measure is stated at the end of the arrow. This Tables 3 are grouped in one page and collectively called Algorithm 2 ( Figure 2).
Antenatal thromboprophylaxis ((Table 1)
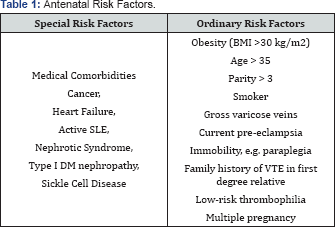
- In the presence of any Special antenatal risk factor, prophylaxis should be started from the first trimester.
- In the presence of four (4) Ordinary risk factors, prophylaxis should also be started from the first trimester.
- In the presence of three (3) Ordinary factors, prophylaxis should be started from 28 weeks.
- In the presence of one or two (1 or 2) Ordinary risk factors, no antenatal thromboprophylaxis is needed.
Postnatal thromboprophylaxis: (Table 2)
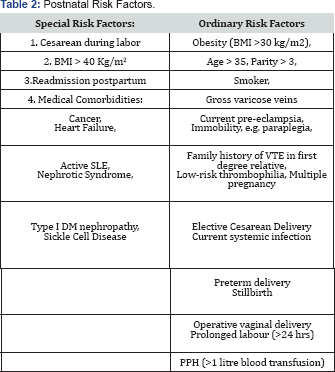
- In the presence of any Special antenatal risk factor, prophylaxis should be started from the first trimester.
- In the presence of four (4) Ordinary risk factors, prophylaxis should also be started from the first trimester.
- In the presence of three (3) Ordinary factors, prophylaxis should be started from 28 weeks.
- In the presence of one or two (1 or 2) Ordinary risk factors, no antenatal
Transient (Temporary) thromboprophylaxis: (Table 3)
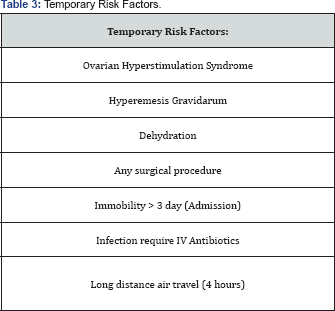
- Any case of ovarian hyperstimulation syndrome that got pregnant should be receiving antenatal prophylaxis throughout the first trimester only
- Temporary thromboprophylaxis is recommended in pregnancy when it is complicated by any of the following: Hyperemesis Gravidarum with dehydration, admission for a surgical procedure, immobility for more than 3 days, systemic infection requiring IV antibiotics, and long distance air travel (> 4 hours).
Thromboprophylaxis during labor and delivery
Women receiving thromboprophylaxis during the antenatal period should:
- Stop further doses if developed vaginal bleeding or labor started.
- Omit morning dose if going for Cesarean section.
- Not have regional analgesia till 12 hours after the last prophylactic dose.
Women who just delivered should receive a VTE prophylaxis:
- Immediately after a normal delivery
- 4 hours after removal of the epidural catheter
- 6 hours after a Cesarean delivery
Choice of Anticoagulant and Dosing
Unfractionated Heparin (UH) and LMWH do not cross the placenta and do not cause teratogenicity or fetal bleeding. Due to its lower side-effects profile and ease of dosing and administration LMWH is recommended over UH for use in pregnancy. The purpose of this algorithmic guidance is to be simple and direct and hence LMWH is the only anticoagulant agent referred to for thromboprophylaxis recommendations in this document. Table 4 is showing different dosing schemes based on the patient's pre-pregnancy weight.
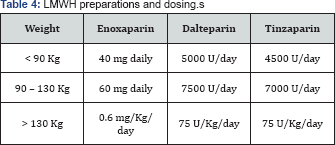
Readers are referred to other guidelines for usage of other anticoagulants. Aspirin should not be used as a VTE thromboprophylactic agent. Anti-embolism stockings can only be used in conjunction with anticoagulant prophylaxis.
Conclusion
In order to ensure that all women at risk of VTE are identified and received the appropriate thromboprophylaxis this quick and easily glanced algorithm was developed to be utilized by Saudi health care providers in hospitals and clinics as well as rural areas. By excluding the past history of thromboembolism and/or Thrombophilia which is very rare among our pregnant patients at booking (<1%), attention will be directed to one page algorithm that covers all other possibilities whether antenatal or postnatal, with the recommended measure of thromboprophylaxis. A word of caution, complicated cases or conditions that require the use of anticoagulant other than LMWH should consult a thrombosis specialist or refer to major guidelines.
Authors’ contributions
The authors had 4 meetings over a one year period to write the above statements based on published data and to bring their expertise in the thromboprophylactic management in pregnancy and puerperium. All authors contributed to the writing and integration of the different segments of the manuscript. All authors read and approved the final manuscript.
References
- Kane EV, Calderwood C, Dobbie R, Morris CA, Roman E, (2013) A population-based study of venous thrombosis in pregnancy in Scotland 1980-2005. Eur J Obstet Gynecol Reprod Biol 169(2): 223-229.
- Heit JA, Kobbervig CE, James AH, Petterson TM, Bailey KR, et al. (2005) Trends in the incidence of venous thromboembolism during pregnancy or postpartum: a 30-year population-based study. Ann Intern Med 143(10): 697-706.
- Greer IA (2012) Thrombosis in pregnancy: updates in diagnosis and management. Hematology Am Soc Hematol Educ Program 203-207.
- Soomro RM, Bucur IJ, Noorani S (2002) Cumulative Incidence of Venous Thromboembolism During Pregnancy and Puerperium: A Hospital- Based Study. Angiology 53: 429-434.
- James A, Committee on Practice Bulletins-Obstetrics (2011) Practice Bulletin no. 123: thromboembolism in pregnancy. Obstet Gynecol 118(3): 718-729.
- Branch DW, Holmgren C, Goldberg JD (2012) Practice Bulletin no 132: antiphospholipid antibody syndrome. Obstet Gynecol 120(6): 15141521.
- Chan WS, Kent NE, Rey E, Corbett T, David M, et al. (2014) Venous thromboembolism and antithrombotic therapy in pregnancy. J Obstet Gynaecol Can 36(6): 527-553.
- https:// www.rcog.org.uk/en/guidelines-research-services/guidelines/ gtg37a/
- Royal College of Obstetricians and Gynaecologists (2015) Green-top Guideline No. 37b. Thromboembolic disease in pregnancy and the puerperium: acute management.
- Bates SM, Greer IA, Middeldorp S, Veenstra DL, Prabulos AM, et al. (2012) American College of Chest Physicians (2012) VTE, thrombophilia, antithrombotic therapy, and pregnancy: Antithrombotic Therapy and Prevention of Thrombosis, (9th edn), American College of Chest Physicians Evidence-Based Clinical Practice Guidelines. Chest 141(2 Suppl): e691S-e736S
- Mclintock C, Brighton T, Chunilal S, Dekker G, McDonnell N, et al.(2012) Recommendations for the diagnosis and treatment of deep venous thrombosis and pulmonary embolism in pregnancy and the postpartum period. Anzjog 52(1): 14-22.
- Clinical Practice Guideline, Venous Thromboprophylaxis In Pregnancy:(2013) Institute of Obstetricians and Gynaecologists, Royal College of Physicians of Ireland And HSE Clinical Care Programme in Obstetrics and Gynaecology and Irish Haematology Society. Version 1.0, Publication Date: November; Guideline No. 20.
- Critical Appraisal Skills Programme (CASP) (2013) Qualitative Research Checklist. Secondary: Critical Appraisal Skills Programme (CASP) Qualitative Research C






























