Relationship of Severe Maternal Morbidity and Maternal Mortality to Serum Lipids in Hypertensive Disorders of Pregnancy
Chhabra S*, Tembhare A and Agrawal V
Department of Obstetrics Gynecology, Mahatma Gandhi Institute of Medical Sciences, India
Submission: October 07, 2016; Published: December 06, 2016
*Corresponding author: Chhabra S, Mahatma Gandhi Institute of Medical Sciences, Sevagram-442 102, Wardha, Maharashtra, India. Email:chhabra_s@rediffmail.com;schhabra@mgims.ac.in
How to cite this article: Chhabra S, Tembhare A, Agrawal V. Relationship of Severe Maternal Morbidity and Maternal Mortality to Serum Lipids in Hypertensive Disorders of Pregnancy. 2016; 1(5): 555575. DOI: 10.19080/JGWH.2016.01.555575
Abstract
Background: Hypertensive disorders of pregnancy (HDP) are associated with a large burden of maternal morbidity and mortality. Whether lipids affect the maternal outcome in these cases is not well known. Research goes on.
Objective: Study was conducted to know relationship between lipid abnormalities and severe morbidity, mortality in women with hypertensive disorders of pregnancy.
Methodology: In the present prospective study of women with singleton pregnancy of gestational 28 weeks and beyond with hypertensive disorders ,fasting serum lipids, cholesterol, triglycerides, high density lipoproteins (HDL), low density lipoproteins (LDL) and very low density lipoproteins (VLDL) were estimated at entry and repeated every 7 days. Cases were observed for placental abruption, renal failure, pulmonary edema, HELLP (Hemolysis, Elevated liver enzymes, and low platelets), partial HELLP or post partum haemorrhage (PPH) and followed up till 7 days postpartum.
Results: Occurrence of maternal complications was significantly less (p0.001) in women who had mild or severe PIH with normal serum lipid levels (p0.001). In women with mild and , severe preeclampsia with normal lipid levels the occurrence of the complications was significantly less (p0.001). Only 2 women with eclampsia had normal lipid levels, hence comparison was not possible. Also there was linear relationship between period of gestation, serum lipid abnormalities and severity of maternal morbidities. Between the gestation period from 28-34 weeks, out of 292 women, 167(57.19%) women had lipid abnormalities compared to 222(99.10%) of 224 women between gestational age 34-37 weeks. Of the 964 women with hypertensive disease, four (0.41%) women died. Maternal Mortality Ratio (MMR) was 465.65 amongst HDP, 28.6% of the overall maternal mortality during the study period. Overall MMR during the study period was 200.54. MMR without hypertensive disorders was 163.34. Four women who died had deranged lipids
Conclusion: As observed dyslipidemia and severity of hypertensive disorders of pregnancy and complications are closely associated. Further research is needed to uncover these associations.
Keywords: Hypertensive disorders; Lipid derangement; Severe maternal morbidity and mortality.
Introduction
Hypertensive disorders of pregnancy (HDP) are associated with a large burden of maternal morbidity and mortality Roberts [1], Dekkar [2], Farrokh-Eslamlou et al. [3], Bauserman et al. [4], Soni-Trinidad et al. [5] due to the disease itself or the consequences and interventions. In spite of being common disorders, HDP are not very well understood as to why some mothers do well and others do not. Among many causative factors, serum lipids are believed to play an active role in the etiopathogenesis of hypertensive disorders of pregnancy Boden [6], Wakatsuki et al. [7], Herrera [8], Lain et al. [9], Herrera et al. [10], Nickens et al. [11] and may be affect the outcome too. HDP are known to be associated with dyslipidemia, placental dysfunction and endothelial cell activation. Dukić et al. [12], Stocks [13], Szpera-Gozdziewicz et al. [14] whether lipids affect the maternal outcome in these cases is not well known and research goes on.
Objective
Study was conducted to know the relationship between lipid abnormalities and maternal morbidity and mortality in women with hypertensive disorders of pregnancy.
Material and Methods
The present prospective study of women with singleton pregnancy of gestational age 28 weeks and beyond with pregnancy induced hypertension (PIH- hypertension systolic blood pressure 140 , diastolic 90 or more) or preeclampsia [with proteinuria of more than 300mg/l in 24 hrs or 30 mg/dl (1+ dipstick) detected in random urine] or eclampsia (hypertension associated with seizures non-attributable to any other cause) was carried out over a period of two years at a rural institute after taking informed consent.
PIH and preeclampsia were further subdivided into mild (diastolic Blood Pressure 90-110 mm Hg or less), and severe (diastolic BP >110 mm Hg). Fasting serum lipids, cholesterol, triglycerides, high density lipoproteins (HDL), low density lipoproteins (LDL) and very low density lipoproteins (VLDL) were estimated at entry and repeated every 7 days.
Cases were observed for placental abruption, renal failure, pulmonary edema, HELLP (Hemolysis, Elevated liver enzymes, and low platelets), partial HELLP or post partum haemorrhage (PPH) and followed up till 7 days postpartum.
Results
A total of 7233 births took place during the study period of which, 964 (13.32%) women had hypertensive disorders. 609 (63.17%) had Mild PIH, 83 (8.60%) severe PIH, 108 (11.20%) mild preeclampsia, 115, (11.92%) severe preeclampsia and 49 (5.08%) eclampsia. Of the 652 women with mild PIH, 325 had elevated cholesterol, 3 (0.92%) of them developed placental abruption, 10 (3.07%) PPH, and 3 (0.92%) had multiple problems (placental abruption, partial HELLP and PPH). Five hundred and ninety nine had elevated triglycerides, 3 (0.53%) of them developed placental abruption, one (0.17%) had partial HELLP, 9 (1.61%) had PPH and one (0.17%) had multiple problems. Seventy two women had elevated LDL, 3 (4.16%) of them had placental abruption, one (1.38%) partial HELLP and 5(6.94%) had PPH. One hundred fifty four women had elevated VLDL, of which 2 (1.29%) developed partial HELLP, 7(4.54%) PPH and one (0.64%) had multiple problems. Fourth four women had low serum HDL, 2 (4.54%) of them had placental abruption and 8 (18.18%) PPH. Overall of mild PIH cases, 7 (1.07%) had partial HELLP, 11 (1.68%) had placental abruption, 39(5.98%) had PPH, and 5 (0.76%) had multiple problems (including PPH, partial HELLP). The occurrence of maternal complications was significantly less (p<0.001) in the women who had mild PIH with normal serum lipid levels.
Amongst the 67 women with severe PIH, 61 had elevated cholesterol, 6 (9.83%) of them had placental abruption, 3(4.91%) partial HELLP, 2(3.27%) HELLP, 5 (8.19%) PPH and 3 (4.91%) had multiple problems. Sixty one women had elevated triglycerides, ten (16.39%) of them had placental abruption, 2 (3.27%) partial HELLP, 2(3.27%) HELLP, 9 (14.75%) PPH and 6(9.83%) had multiple problems. Fifty nine women had elevated VLDL, of which 4 (6.77%) had placental abruption, one (1.69%) HELLP, 4 (6.77%) partial HELLP, 7 (11.86%) PPH and 2(3.38%) had multiple problems. Thirty two women had elevated LDL, of which, 9 (28.12%) had placental abruption, 11 (34.37%) partial HELLP, 2 (6.25%) HELLP, 6 (18.75%) PPH and 5 (15.62%) had multiple problems. All of them had normal HDL. Overall of 67 women with severe PIH, 29 (43.28%) had placental abruption, 20 (29.85%) partial HELLP, 7 (10.44%) HELLP, 27 (40.29%) had PPH and 16 (23.88%) had multiple problems. In the women with severe HDP with normal lipid levels, morbidities were significantly less (p<0.001).
Among 91 women with mild preeclampsia, 89 had elevated cholesterol; seven of them (7.86%) had placental abruption, one (1.12%) PPH and 2 (2.24%) had multiple problems. Eighty three had elevated triglycerides, of which 7(8.43%) had placental abruption, 4 (4.81%) PPH and 4 (4.81%) had multiple problems. Thirty four had elevated LDL of which 4 (11.36%) had placental abruption, 3 (8.82%) PPH and one (2.94%) had multiple problems. Eighty three women had elevated VLDL of which 6 (7.22%) had placental abruption and 3 (3.61%) had PPH. Nine had lower serum HDL of which 3 (33.33%) had placental abruption, one (11.11%) partial HELLP, one (11.11%) HELLP, one (11.11%) renal failure and 3 (33.33%) had PPH. Overall 91 women with mild PE with abnormal lipid levels, 27 (29.67%) had placental abruption, one (1.09%) each had partial HELLP, HELLP and renal failure, 14 (15.38%) women had PPH and 7 (7.69%) women had multiple problems. In the women with mild preeclampsia and normal lipid levels, the occurrence of these complications was significantly less (p<0.001).
Of all the 115 women with severe preeclampsia, 110 had elevated cholesterol, triglycerides and VLDL, 9 (8.18%) of them had placental abruption, 5 (4.54%) had partial HELLP, 6 (5.45%) HELLP, 10(9.09%) PPH, 3 (2.72%) pulmonary edema and one (1.36%) had renal failure. Thirty four women had elevated LDL, of which 4 (11.76%) had placental abruption, 5 (14.70%) had HELLP, 13 (38.23%) partial HELLP, 3 (8.82%) had PPH and 2 (5.88%) had renal failure. Twenty two women had low HDL 3 (12.5%) of them had HELLP, one (4.16%) placental abruption, 4 (16.66%) had PPH, one (4.16%) had pulmonary edema and 2(8.32%) had renal failure. Overall of 115 women with severe PE with lipid abnormalities, 14 (12.17%) had placental abruption, 18(15.65%) had partial HELLP, 14(12.17%) had HELLP, 17(14.78%) had PPH, 4 (3.47%) had pulmonary edema and 5 (4.34%) had renal failure. On comparing women having severe preeclampsia and normal lipid levels, complications were significantly less (p<0.001). (Table 1& 2).
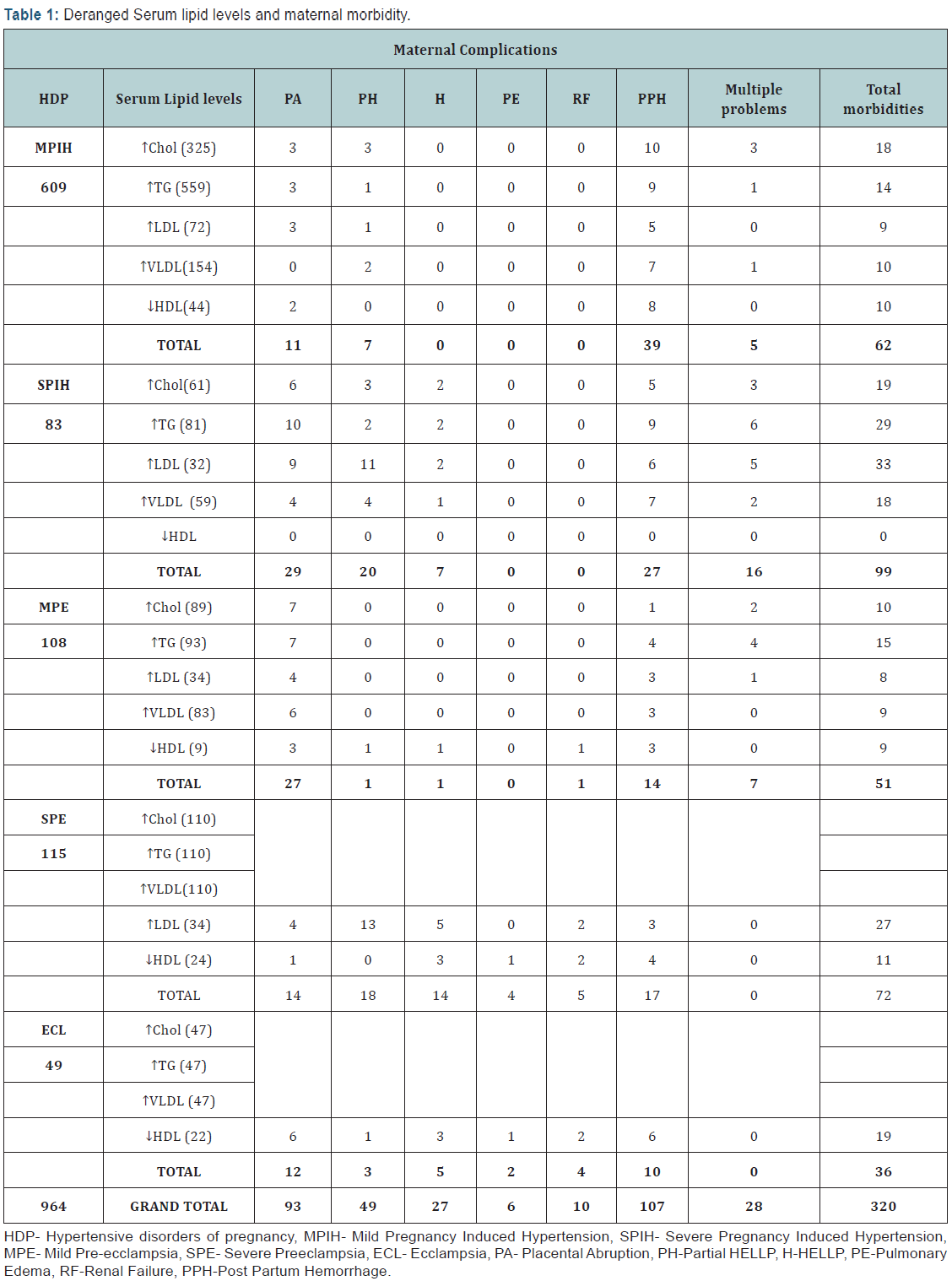

Fourth seven women with eclampsia had elevated cholesterol, elevated triglycerides and elevated VLDL. 6 (12.76%) of them had placental abruption, 2 (4.25%) had partial HELLP, 2 had (4.25%) HELLP, 4 (8.51%) had PPH, one (2.12%) had pulmonary edema and 2 (4.25%) had renal failure. Twenty two women with eclampcia had low HDL 6 (27.27%) of them had placental abruption, 3 (13.63%) had HELLP, one (4.54%) had partial HELLP, 6 (27.27%) had PPH, one (4.54%) had pulmonary edema and 2 (9.09%) had renal failure. Of 47 women with eclampsia with deranged lipids, 12 (25.53%) had placental abruption, 3 (6.38%) had partial HELLP, 5 (10.63%) had HELLP, 10 (21.27%) had PPH, 2 (4.25%) had pulmonary edema and 4 (8.5%) women had renal failure. Only 2 women with eclampsia had normal lipid levels, hence comparison was not possible.
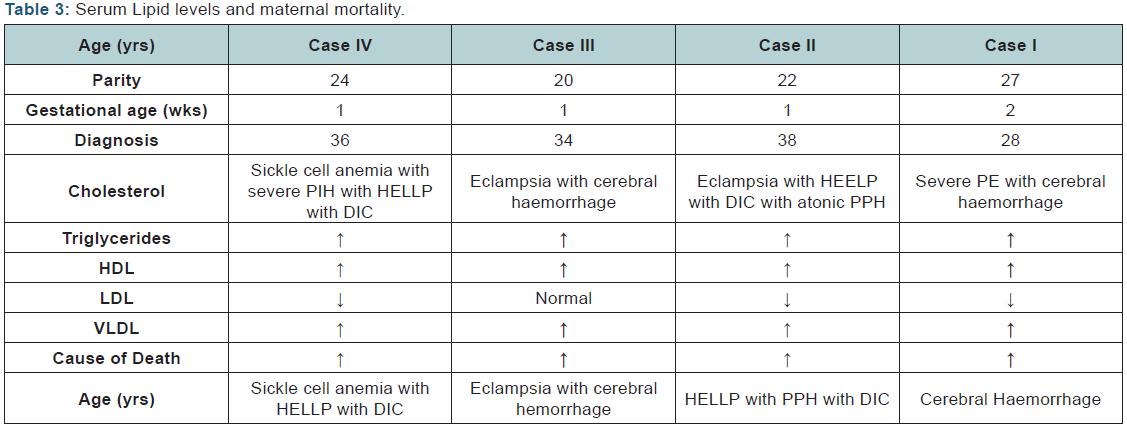
There was linear relationship between period of gestation, serum lipid abnormalities and severity of maternal morbidities. Between the gestation period from 28-34 weeks, out of 292 women, 167(57.19%) women had lipid abnormalities, compared to 222(99.10%) of 224 women between gestational age 34-37 and 432(68.03%) of 635women of gestation >37 weeks. Of the 964 women with hypertensive disease four (0.41%) women died. Amongst HDP cases. Maternal Mortality Ratio (MMR) was 465.65, 28.6% of the overall maternal mortality during the study period. Overall MMR during the study period was 200.54. MMR without these disorders was 163.34. All of the 4 women who died had deranged lipids; one was a 27 year old second para with severe preeclampsia at 28 weeks. She presented in coma and was diagnosed to have cerebral hemorrhage. She had elevated cholesterol, triglycerides. LDL, VLDL below normal HDL levels. The second woman was a 22 years primipara who had post partum eclampsia with HELLP, DIC and atonic PPH. She had elevated cholesterol, triglycerides, and LDL, VLDL and below normal HDL. The third case, a 20 years primipara with eclampsia, died due to intracranial hemorrhage and had elevated cholesterol, triglycerides, LDL, VLDL and normal HDL levels. Fourth woman was 24 years primipara with severe PIH with HELLP with DIC. She had elevated cholesterol, triglycerides, and LDL, VLDL below normal HDL levels. All the eclamptic women (2 of 4 who died) had severely deranged serum lipid levels. (Table 3)
Discussion
In spite of being a common entity, known since ages, with dangerous implications for the mother and the baby, hypertensive disorders of pregnancy still remain an unsolved problem. They affect pregnancy in many ways, but not all pregnancies are affected in the same way or to similar extent and research continues.
In the present study it was found that there was significant relationship between lipid derangements and severity of hypertensive disorders and its impact on severity of morbidity it causes. Among 964 hypertensive women, the most pathological, serum triglycerides were elevated in 890 (92.32%) women. Other lipids deranged were elevated cholesterol in 637 (66.07%) % women, 453 (46.99%) women had elevated VLDL, 181 (18.77%) women had elevated LDL and 99 (10.26%) women had low serum HDL levels. Among all the lipids, LDL correlated best with the risk of complications, placental abruption, PPH, HELLP, partial HELLP, renal failure and pulmonary edema and more research needs to be done.
In one of our earlier studies Chhabra et al. [15], presence of HELLP/partial HELLP was found to be associated with the adverse pregnancy even in cases of mild hypertensive disorders also as reported by some other researchers Ray et al. [16], Ekhator and Ebomoyi [17], Vigil-De Gracia P [18] This suggests that something else is also responsible in the pathogenesis of complications in hypertensive disoeders during pregnancy. It has been reported that the lipids are deranged in all these subjects Ray et al. [16], Ekhator and Ebomoyi [19]. All women who died had severe hypertensive disorders and elevated cholesterol, triglycerides, LDL, VLDL levels while, and 3 had below normal HDL levels.
Mudd et al. [19] have reported that high TG, LDL and Cholesterol are modestly associated with the risk of hypertensive disorders of pregnancy and maternal and fetal/neonatal complications. Scifres et al. [20] found that maternal serum fatty acid binding protein 4 (FABP4) levels are elevated before the clinical onset of preeclampsia which was indirect indication of dyslipidemia having a causative relationship with hypertensive disorders of pregnancy and its complications. Ghio et al. [21] have suggested that increased TG during pregnancy appear to increase risk of preeclampsia and preterm birth. Authors suggested that maternal hypertriglyceridemia had a role in increasing cardiovascular risk later in life and increasing future morbidity. So may be women who have lipid derangements and hypertensive disorders have more chances of cardiovascular disease [22-28].
Conclusion
As observed dyslipidemia and severity of hypertensive disorders of pregnancy and complications are closely associated. Further research is needed to uncover these associations.
References
- Roberts JM (1998) Pregnancy-related hypertension. In: Creasy K & Resnik R (Eds.), Maternal-fetal medicine. W B Saunders Company, Philadelphia, USA, pp. 833-872.
- Dekker GA (2014) Management of preeclampsia. Pregnancy Hypertens 4(3): 246-247.
- Farrokh-Eslamlou H, Aghlmand S, Oshnouei S (2014) Persistence of Hemorrhage and Hypertensive Disorders of Pregnancy (HDP) as the Main Causes of Maternal Mortality: Emergence of Medical Errors in Iranian Healthcare System. Iran J Public Health 43(10): 1395-1404.
- Bauserman M, Lokangaka A, Thorsten V, Tshefu A, Goudar SS, et al. (2015) Risk factors for maternal death and trends in maternal mortality in low- and middle-income countries: a prospective longitudinal cohort analysis. Reprod Health 12(Suppl 2): S5.
- Soni-Trinidad C, Gutiérrez A, Santa Rosa-Moreno FJ, Reyes-Aguilar A (2015) Maternal morbidity and mortality and risk factors related to an obstetric emergency. Ginecol Obstet Mex 83(2): 96-103.
- Boden G (1996) Fuel metabolism in pregnancy and in gestational diabetes mellitus. Obstet Gynecol Clin North Am 23(1): 1-10.
- Wakatsuki A, Ikenoue N, Okatani Y (2000) Lipoprotein particles in preeclampsia: Susceptibility to oxidative modification.Obstet Gynaecol 96(1): 55-59.
- Herrera E (2002) Lipid metabolism in pregnancy and its consequences in the fetus and newborn. Endocrine 19(1): 43-55.
- Lain KY, Catalano PM (2007) Metabolic changes in pregnancy. Clin Obstet Gynecol 50(4): 938-948.
- Herrera E, Ortega-Senovilla H (2010) Disturbances in lipid metabolism in diabetic pregnancy - Are these the cause of the problem? Best Pract Res Clin Endocrinol Metab 24(4): 515-525.
- Nickens MA, Long RC, Geraci SA (2013) cardiovascular disease in pregnancy: (women’s health series). South Med J 106(11): 624-630.
- Dukić A, Zivancević-Simonović S, Varjacić M,Dukić S (2009) Hyperlipidemia and pregnancy. Med Pregl 62(Suppl 3): 80-84.
- Stocks G (2014) Preeclampsia: pathophysiology, old and new strategies for management: An educational review. Eur JAnaesthesiol 31(4): 183- 189.
- Szpera-Gozdziewicz A, Breborowicz GH (2014) Endothelial dysfunction in the pathogenesis of pre-eclampsia. Front Biosci (Landmark Ed) 19: 734-746.
- Chhabra S, Kakani A (2007) Maternal mortality due to eclamptic and not eclamptic hypertensive disorders: a challenge. Journal of ObstetricsGynaecology 27(1): 25-29.
- Ray JG, Diamond P, Singh G, Bell CM (2006) Brief overview of maternal triglycerides as a risk factor for pre-eclampsia. An International Journal of Obstetrics and Gynaecology 113: 1-8.
- Ekhator CN, Ebomoyi MI (2012) Blood glucose and serum lipid profiles during pregnancy. African Journal of Diabetes Medicine 20(1): 16-19.
- Vigil-De Gracia P (2015) HELLP syndrome. Ginecol Obstet Mex 83(11): 48-57.
- Mudd LM, Holzman CB, Catov JM, Senagore PK, Evans RW (2012) Maternal lipids at mid-pregnancy and the risk of preterm delivery. Acta Obstet Gynecol Scand 91(6): 726-735.
- Scifres CM, Catov JM, Simhan H (2012) Maternal serum fatty acid binding protein 4 (FABP4) and the development of preeclampsia. J Clin Endocrinol Metab 97(3): E349-E356.
- Ghio A, Bertolotto A, Resi V, Volpe L, Di Cianni G (2011) Triglyceride metabolism in pregnancy. Adv Clin Chem 55: 133-153.
- Irgens HU, Reisaeter L, Irgens LM, Lie RT (2001) Long term mortality of mothers and fathers after pre-eclampsia: population based cohort study. BMJ 323(7323): 1213-1217.
- Chesley LC, Annitto JE, Cosgrove RA (1968) The familial factor in toxemia of pregnancy. Obstetrics& Gynecology 32(3): 303-311.
- Sapre S, Joshi V, Sharma P, Sharma AK (1996) Critical evaluation of provocative tests in predicting Pre-eclampsia.Journal of Obstetrics and Gynaecology of India 46(1): 16-20.
- Vatten JL, Skjaerven R (2004) Is preeclampsia more than a disease? BJOG 111(4): 298-302.
- Sharami SH, Tangestani A, Faraji R, Zahiri Z, Amiri A (2012) Role of dyslipidemia in preeclamptic overweight pregnant women. Iran J Reprod Med 10(2): 105-112.
- Basu A, Alaupovic P, Wu M, Jenkins AJ, Yu Y, et al. (2012) Plasma lipoproteins and preeclampsia in women with type 1 diabetes: a prospective study. J Clin Endocrinol Metab 97(5): 1752-1762.
- Murai JT, Muzykanskiy E, Taylor RN (1997) Maternal and fetal modulators of lipid metabolism correlate with the development of preeclampsia. Metabolism 46(8): 963-967.







