- Research Article
- Abstract
- Introduction
- Background and Relevance of GSR in Forensic Investigation
- Gunpowder and Gunshot Residue
- Discussion
- Gunshot Residue Collection and Sample Preparation
- GSR Analysis Methods
- Evolution of Methods of Detecting Gunshot Residue
- Capillary Electrophoresis (CE)
- Electrochemistry
- Determination of Firing Distance Methods
- Development of Rapid and Portable/On-Site Detection
- Integration of Machine Learning/Artificial Intelligence into GSR Analysis
- Conclusion
- References
Gunshot Residues-Forensic Spectrometric and Electrochemical Detection Approaches
Dwight Kelly Souder, Veera Shakar Pulusu and Howard D Dewald*
Department of Chemistry and Biochemistry, Ohio University, USA
Submission: May 17, 2024;Published: June 18, 2024
*Corresponding author: Howard D Dewald, Department of Chemistry and Biochemistry, Ohio University, Athens, OH 45701, USA, Email: dewald@ohio.edu
How to cite this article: Souder DK, Pulusu VS, Dewald HD. Gunshot Residues-Forensic Spectrometric and Electrochemical Detection Approaches. J Forensic Sci & Criminal Inves. 2024; 18(3): 555990. DOI:10.19080/JFSCI.2024.18.555990.
- Research Article
- Abstract
- Introduction
- Background and Relevance of GSR in Forensic Investigation
- Gunpowder and Gunshot Residue
- Discussion
- Gunshot Residue Collection and Sample Preparation
- GSR Analysis Methods
- Evolution of Methods of Detecting Gunshot Residue
- Capillary Electrophoresis (CE)
- Electrochemistry
- Determination of Firing Distance Methods
- Development of Rapid and Portable/On-Site Detection
- Integration of Machine Learning/Artificial Intelligence into GSR Analysis
- Conclusion
- References
Abstract
Gunshot residue (GSR) is a key component in forensic chemistry, and many changes have occurred in ammunition and modern chemical warfare. In a crime investigation, GSR is important evidence to determine whether suicide occurred or not, firing distance, and many other factors. Several methods are available to collect GSR samples from crime scenes, and different techniques are available to qualify and quantify the complex samples. GSR can be analyzed by color-based tests and electrochemical, spectroscopic, and microscopic procedures. Color or spot test analysis will give faster results but lacks accuracy, poor sensitivity and cannot identify interference; these color tests are not preferred nowadays. Electrochemical and spectroscopic procedures can be combined with liquid and gas chromatographic separations. Further, capillary electrophoresis methods or scanning electron microscopy can provide accurate results with low detection limits. Even integration of machine learning/artificial intelligence to GSR in analysis is being used. The development of portable instrumentation for on-site analysis is important, too. However, as discussed in this review, every methodology has some limitations and advantages.
Keywords: Gunshot residue analysis; Forensic; Colorimetry; Spectrometric; Electrochemical
Abbreviations: GSR: Gunshot Residue; IGSR: Inorganic Gunshot Residue; OGSR: Organic Gunshot Residue; SG: Smokeless Gunpowder; NC: Nitrocellulose; NG: Nitroglycerine; NQ: Nitroquanidine; SEM: Scanning Electron Microscope; EDX: Energy Dispersive X-ray; SPME: Solid-Phase Microextraction; HSSE: Headspace Sorptive Extraction; GC-MS: Gas Chromatography-Mass Spectrometry; IR: Infrared; UV: Ultraviolet; AAS: Atomic Absorption Spectroscopy; ICP: Inductively Coupled Plasma; AES: Atomic Emission Spectroscopy; EM: Electromagnetic; LIBS: Laser-Induced Breakdown Spectroscopy; IMS: Ion Mobility Spectrometry; PPB: Parts-Per-Billion; DPA: Diphenylamine; EC: Ethyl Centralite; MS: Mass Spectrometry; TEA: Thermal Energy Analysis; ECD: Electron Capture Detector; FID: Flame Ionization Detector; HPLC: High Performance Liquid Chromatography; RI: Refractive Index; LC-MS: Liquid Chromatography-Mass Spectrometry; CDTA: Diaminocyclohexane Tetraacetic Acid; SDS: Sodium Dodecyl Sulfate; CV: Cyclic Voltammetry; SPCE: Screen Printed Carbon Electrode; SWV: Square-Wave Voltammetry; NTA: Nontoxic Ammunition; ASV: Anodic Stripping Voltammetry; HMDE: Hanging Mercury Drop Electrode; MFE: Mercury Film Electrode; GCE: Glassy Carbon Electrode; ISE: Ion-Selective Electrode; PVC: Polyvinyl Chloride; MGT: Modified Griess Test; NAA: Neutron Activation Analysis; AbrSV: Abrasive Stripping Voltammetry; ML: Machine Learning; NN: Neural Network; AI: Artificial Intelligence; LR: Likelihood Ratio
- Research Article
- Abstract
- Introduction
- Background and Relevance of GSR in Forensic Investigation
- Gunpowder and Gunshot Residue
- Discussion
- Gunshot Residue Collection and Sample Preparation
- GSR Analysis Methods
- Evolution of Methods of Detecting Gunshot Residue
- Capillary Electrophoresis (CE)
- Electrochemistry
- Determination of Firing Distance Methods
- Development of Rapid and Portable/On-Site Detection
- Integration of Machine Learning/Artificial Intelligence into GSR Analysis
- Conclusion
- References
Introduction
Since 2020, gun-related incidents have emerged as the primary cause of mortality in the United States. There was a total of 45,222 fatalities, up 15% from the previous year. Of those total fatalities, 24,292 were from suicide, 19,384 were from homicide, 535 were accidental, 611 were legal intervention, and 400 were from undetermined intent [1]. In all situations where firearms were used, gunshot residue (GSR) was left behind. Forensic analysis of the GSR is instrumental in allowing investigators to determine the cause of death and intent (homicide, suicide, or accidental) and to link the weapon to the suspect and possible witnesses [2]. Over the years, many techniques have been developed to glean information about the firearm and the suspect who fired the weapon. As far back as 1858, forensic researchers tested for GSR using various chemical tests, where a positive result would produce a colorimetric indicator to verify the presence of multiple substances often found in ammunition [3].
As the world of forensic science evolves, the scope of gunshot residue (GSR) analysis is expanding, and methods that have been used, such as colorimetry, electrochemistry, and other advanced detection approaches, will be adapted to help determine GSR analysis. A closer look at this field reveals a realm where analytical chemistry meets criminal justice, offering insights crucial in forensic investigations and transforming the field. Ammunitions and explosives are made with different kinds of metals and are composed of various ratios. The purpose of analyzing GSR in criminal investigations is to determine whether a suspect fired a gun, firing distance estimation, and identifying the size of bullet holes [4]. Detection methods for GSR depend on analyzing chemical substances, such as organic compounds and inorganic compounds deposited on the shooter's hair, hands, face, and clothes, etc. after discharge [5]. It is vital to determine whether a gunshot death is a suicide or homicide in forensics or crime scene investigation by measuring the distance between the fired gun and the victim [6]. A gunshot residue is composed of several different components. These include partially burned propellant powders, unburned propellant powders, primer particles, smoke, lubricants, organic and inorganic metal residues from the cartridge, and small metal parts from the weapon [4,7]. Sampling of gunshot residue, techniques, and storage procedures are key roles in the investigation process. There is concern about cross-contamination within a forensics lab [8]. As many advances are happening in forensic science, there are still limitations in the detection of gunshot residue, which are being addressed.
- Research Article
- Abstract
- Introduction
- Background and Relevance of GSR in Forensic Investigation
- Gunpowder and Gunshot Residue
- Discussion
- Gunshot Residue Collection and Sample Preparation
- GSR Analysis Methods
- Evolution of Methods of Detecting Gunshot Residue
- Capillary Electrophoresis (CE)
- Electrochemistry
- Determination of Firing Distance Methods
- Development of Rapid and Portable/On-Site Detection
- Integration of Machine Learning/Artificial Intelligence into GSR Analysis
- Conclusion
- References
Background and Relevance of GSR in Forensic Investigation
The study of GSR is crucial and one of the valuable pieces of evidence in forensic investigation for several reasons. The evidence collected from GSR can include partially burnt and burnt remains of primers, propellants, cartridge casings, and firearms. The GSR is valuable evidence for forensic analysis, and it could be found on the hair, hands, and clothes of the shooter, as well as their surroundings [9]. As for the source of the components, most organic substances come from propellants and lubricants, whereas inorganic substances come from primers, propellants, cases, rings of jackets, and ammunition barrels [8]. The analytical techniques provide high sensitivity, precision, selectivity, and the ability to separate and identify complex organic and inorganic mixtures, making them crucial for GSR analysis [9]. It is becoming increasingly important to detect organic compounds in GSR that come from a firearm propellant. Detecting organic residues on firearms can help determine the nature of ammunition used and help identify the weapon [3].
- Research Article
- Abstract
- Introduction
- Background and Relevance of GSR in Forensic Investigation
- Gunpowder and Gunshot Residue
- Discussion
- Gunshot Residue Collection and Sample Preparation
- GSR Analysis Methods
- Evolution of Methods of Detecting Gunshot Residue
- Capillary Electrophoresis (CE)
- Electrochemistry
- Determination of Firing Distance Methods
- Development of Rapid and Portable/On-Site Detection
- Integration of Machine Learning/Artificial Intelligence into GSR Analysis
- Conclusion
- References
Gunpowder and Gunshot Residue
Gunpowder is also known as black powder. Nitrocellulose and nitroglycerin are key components in gunpowder. Apart from these chemicals, gunpowder consists of organic additives, stabilizers, plasticizers, flash inhibitors, and lubricants. The higher properties of modern propellants, including higher burn rates, reduced smoke production, and greater stability, have largely replaced black powder [5]. During firing, GSR is generated by the explosive reaction inside the barrel [10].
Components of gunpowder and gunshot residue
Gunshot residues (GSR) or firearm residue discharges have gained increased interest among forensic investigators due to advancements in analytical instrumentation and understanding of analyte formation after combustion events [11]. Inorganic gunshot residue (IGSR), commonly known as primer residue, has been the focus of analysts until recently [12]. Other components are known as organic gunshot residue (OGSR), which originates from propellant and lubricants [13]. The variations of firearms and ammunition are an immense topic beyond this paper’s scope. As a general reference, the approach of this paper will discuss the typical single-use ammunition fired from a standard handgun, revolver, or rifle. As the trigger is squeezed, a series of mechanical movements occur, resulting in the primer being impacted by a firing pin [9]. As the name implies, the primer is the first substance that initiates the reaction that will eventually propel the projectile out of the gun barrel. The primer is contained at the ammunition’s base, either along the outer rim or the ammunition’s center. The primer consists of highly reactive explosives, typically made from lead styphnate, with barium nitrate being the oxidizer [9]. Although many ammunition manufacturers also use other compounds, many contain heavy metals such as lead azide, and antimony trisulfide [14]. As will be discussed further in this paper, these heavy metal compounds within the primer often play a crucial role in determining if a firearm has been discharged [15]. The primer then ignites the propellant.
Most propellants are smokeless gunpowder (SG). These propellants are often made up of nitrocellulose (NC), or a mixture of NC and nitroglycerine (NG), or in military grade ammunition a mixture of NC, NG, and nitroguanidine (NQ) [9]. These propellants, among many other ingredients within the propellant, leave behind OGSR. When the primer ignites these substances, the propellant deflagrates [9]. During deflagration, a large amount of heated gas is produced, significantly increasing the pressure within the casing. This pressure pushes against the projectile, which is held within the casing by friction. As the projectile is forced forward down the gun barrel, there is a release of GSR. A cut-away diagram of the two common types of ammunition, a rimfire and a centerfire, is shown in Figure 1. Upon the firing of the ammunition, the temperature and pressure within the casing reach intensely high levels, enough to cause some of the metals within the ammunition to become molten. As these particles escape the gun, the particles rapidly cool, forming mostly spheroid microparticles ranging from 1-10m [16].
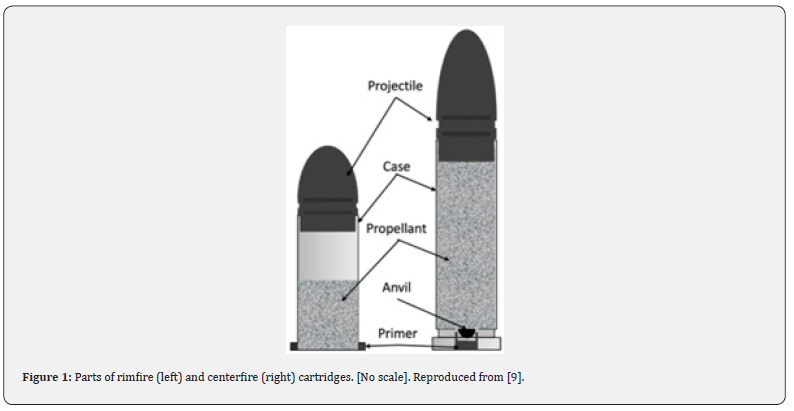
Inorganic gunshot residue: Gunshot residues are primarily composed of inorganic and organic elements (Table 1). In GSR, most of the organic components are derived from propellant and lubricant materials, while most of the inorganic ingredients come from the primer, propellant, case, core, and barrels of the projectiles [17]. Most of the inorganic compounds found in gunshot residue (IGSR) originate from the primer, which commonly contains three complexes: a primary explosive or initiator like lead styphnate (C6HN3O8Pb), an oxidizer like barium nitrate (Ba(NO3)2), and fuel-like antimony trisulfide (Sb2S3). Each manufacturer prepares the inorganic components of the primer mixture differently, and many of these components are made up of heavy metals [14]. Even though these primers are still used most commonly nowadays, they contain toxic elements such as lead, barium, and antimony, which are released into the environment when discharged [18]. Indoor shooting activities and environmental concerns are particularly at risk from such toxic elements. The heavy metals present in ammunition are toxic to humans. To make these explosives less toxic, lead-free (sometimes called “green”) ammunition is becoming more popular. Less toxic elements are utilized, for example, titanium chloride (pyrotechnic system), diazole (primary explosive), and zinc peroxide as opposed to lead, barium, and antimony [19]. Replacing the lead with safer substitute compounds changes the chemical makeup, microparticle size and shape, and surface characteristics [20]. This has led forensic researchers to adopt new criteria to interpret the data from lead-free ammunition [21]. Primers have significantly evolved or changed in composition because of the firing process and metals used. In the beginning, mercury fulminate, potassium chlorate, and antimony trisulfide were used as the primary explosive, along with glass powder (the friction agent) and potassium chlorate (the oxidant) [22]. Due to the use of black powder in ammunition, GSR traditionally contained a variety of compounds. In the early 1950s, for the U. S. military to avoid firearms corrosion, these primers were replaced by the Sinoxid®-type primer, barium nitrate, antimony sulfide (pyrotechnic system), and a mixture of lead styphnate (primary explosive). In addition to being corrosion resistant, Sinoxid® type primers are also chemically stable [23].
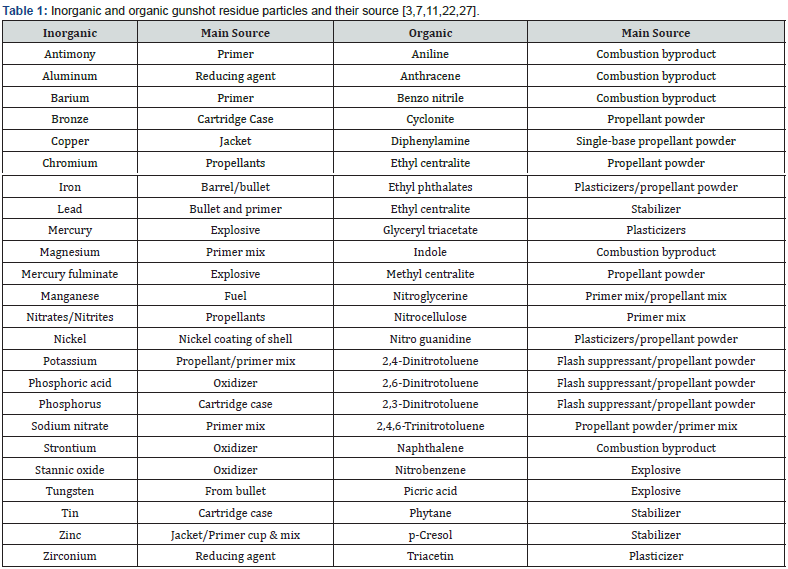
Organic gunshot residue: The propellant powder and primer mixture are the most common sources of organic compounds in ammunition, but they can also be found in every part of the weapon. Black powder generally contains 75% potassium nitrate, 15% sulfur, and 10% charcoal [5]. Organic gunshot residues typically comprise a smokeless powder containing primary explosives, sensitizers, flash inhibitors, stabilizers, plasticizers, and gelatinizers [24]. These stabilizers, moderators, flash inhibitors, coolants, anti-wear, plasticizers, and surface lubricants are added to smokeless powders to preserve and prolong their shelf life [25]. Organic components in GSR are derived from smokeless powder propellants with varying compositions. A single-based smokeless powder is made from nitrocellulose as an explosive, a double-based smokeless powder is made from nitrocellulose and nitroglycerin with nitroglycerin increasing the potential energy potential of gunpowder, and a triple-based smokeless powder is made from nitrocellulose, nitroglycerin, and nitroguanidine [5,25]. There is the possibility of a reaction between ammunition stabilizers and sensitizers while it is in storage [26]. Due to incomplete ignition, vaporization, and condensation of smokeless powder, degradation products and unburnt components can be found in GSR after a firearm has been discharged. The discharge process also produces combustion products such as pyrolysis, pyrosynthesis, and stoichiometric combustion. Many combustion products in spent cartridges and firearm muzzles can be detected, including benzonitrile and polycyclic aromatic hydrocarbons [27]. (Table 1)
GSR distribution
Many factors affect how GSR is distributed, such as the type of gun, type of ammunition, variations of the chemicals used to make the ammunition, where the gun was fired (inside or outside), distance to the target, and environmental conditions. Due to the several types of guns, the construction of the gun and how the casing is removed from the gun are essential in how the GSR is released and where it settles. Most of the GSR exits the gun barrel, but as it exits, the microparticles near the edge of the plume tend to curl back (vortex) onto itself. In contrast, the microparticles near the middle of the plume tend to move toward the projectile’s target. Depending on the barrel’s length and the projectile's velocity will depend upon how much of the plume of GSR will settle on the shooter. Shorter barrels, such as handguns, tend to have more GSR settling on the hands of the shooter than longer-barreled guns like rifles. Also, slower-moving projectiles generally produce more significant plumes of GSR near the end of the barrel than higher-velocity projectiles [28]. The other source of the GSR plume is where the casing is removed or ejected from the chamber of the gun or, in the case of revolver-style guns, along the gap within the ammunition barrel. The plume from these areas of the gun can settle on the hands, head, and shoulders and even be inhaled [28]. Knowing how the GSR plume is dispersed can give forensic researchers a better idea of the type of gun used, the type of ammunition, and the shooter’s proximity to the target. If a gun is fired within a car (as in a drive-by shooting), the GSR tends to collect in the car’s headliner, the window frame from which it was fired, the dashboard, and the seats [29].
Challenges of gunshot residue identification
The complex composition of GSR and the varied factors affecting its detection and analysis make identifying gunshot residue extremely challenging. Due to variation in GSR composition, it can differ depending on the type of ammunition, environmental factors, and firearms [3]. In most cases, GSR is spread from the firearm to the body, hair, clothes, and surrounding environment of the person who fired the gun. As a result, finding out the timeline of a shooting incident can be challenging due to the persistence of residue [3]. Upon contact with contaminated surfaces or objects, GSR may be transferred by secondary transmission. As a result of this, it can be challenging to interpret locations and residue patterns [14]. In complex matrices, detecting trace amounts of GSR can be challenging. Limits of detection and quantification of analytical techniques play a crucial role in accurately identifying GSR components [14]. GSR tests can be false positive or inaccurate due to dust, dirt, other debris, and environmental contaminants [14].
- Research Article
- Abstract
- Introduction
- Background and Relevance of GSR in Forensic Investigation
- Gunpowder and Gunshot Residue
- Discussion
- Gunshot Residue Collection and Sample Preparation
- GSR Analysis Methods
- Evolution of Methods of Detecting Gunshot Residue
- Capillary Electrophoresis (CE)
- Electrochemistry
- Determination of Firing Distance Methods
- Development of Rapid and Portable/On-Site Detection
- Integration of Machine Learning/Artificial Intelligence into GSR Analysis
- Conclusion
- References
Gunshot Residue Collection and Sample Preparation
Hand washing
Washing is typically done to the hands and the nearby surfaces near the shooter. Water or dilute acid, typically hydrochloric acid, and nitric acid is applied to the hands or surface. The rinse is then collected and stored in a plastic bottle for later analysis. Washing typically produces high yields but may cause skin irritation and interfere with some of the inorganic GSR detection methods [7].
Tape lifts
Tape lifting is one of the more common ways of collecting GSR, primarily IGSR [4]. An adhesive strip is repeatedly placed on the surface of skin, hair, and other materials. It is less effective on fabrics as it also collects fibers from the clothing [7]. Using the tape lifting method has the advantage of not being so harsh and irritating when applied to the skin, compared to doing a mild acid wash of the hands. The tape is glued to a plastic polyethylene base, which is then soaked in hydrochloric acid and chloroform. This process dissolves the glue and releases the trapped GSR contents to be analyzed [30]. The best-performing medium for collecting IGSR particles was double-sided tape when compared to various adhesives [4].
Vacuum lifts
One of the most effective instruments is a vacuum for the GSR collection from the clothes where the firing has occurred. Inorganic and organic residues from clothing were collected successfully using vacuum collection techniques [31]. Few types of vacuum filters are used to collect OGSR, polytetrafluoroethylene, fiberglass, and Teflon with 0.5 µm particle size, and the results show that there was a greater collection efficiency with Teflon filters [32]. An effective method for collecting IGSR was to use tape lifts on clothing, followed by vacuum lifting for organic residues [33].
Swabbing
Gunshot residue or explosive samples can be collected using dry or wet swabs. Depending on the type of the target material, several types of solvents are often applied to the swabbing surface. Typically, swabbing is performed on the hands and solid surfaces to capture GSR [7]. Several organic and inorganic solvents have been tested for their efficiency in removing nitroglycerin from hands. Contact with the skin for a prolonged time is not recommended with acetone and acetonitrile [32,34]. By measuring the amount of nitroglycerin removed from the hands, the solvent efficiency was determined, how much-interfering material was removed from the hands, and how stable nitroglycerin was in the swabbing solvent. Many chances are there that the solvent used in the swabbing procedure could dissolve other compounds and cause interference with the experiment. A complete, stable, and consistent recovery was achieved with ethanol [32,34].
Glue lifts
The collection of GSR from hands has been achieved using glue lifts, which is an efficient technique. To get accurate results, sticky glues were applied to the sample, allowed to dry, and removed from the surface containing much evidence. Because this method is less sticky than tape lift, it is more effective [35].
Paraffin wax
Using paraffin wax is another method of collecting GSR. Wax is poured over the hands, allowed to harden, and then peeled off. GSR is collected within the wax and is analyzed. Although it is effective at trapping a large amount of GSR, it is also prone to give false positive test results [7].
Nasal collection
After a gun is fired, a plume of GSR lingers in the air near the shooter. During this time, people in the near vicinity would inhale some of the GSR, where it would collect in the nasal hairs. One method of collecting the GSR is to have the person blow their nose into a sterile tissue. The dispelled mucus and proteins within the nose can capture GSR for about 48 hours [7,36]. Another method is to use a small metal disc with a handle called a “Nasal Stub” with a graphite adhesive attached to the flat portions of the disk placed inside the nose. The adhesive side of the disc is brushed up against the nasal hairs, being careful not to touch the nose so as not to collect any mucus. The lab is then able to analyze the Nasal Stub with a SEM. Through tests, it was found that even after 20 hours, GSR could still be collected from nasal hair [37].
Hair recovery
The hair is another place that GSR typically gathers. One way to collect GSR from the hair is to comb the hair with a fine-toothed comb. Using a comb to collect GSR proved complicated for those with curly hair [7].
Clothing collection
Organic GSR tends to last longer on clothing than on the skin [38]. Clothing becomes an effective source of information. The presence of GSR would imply that the individual was in the area where a gun was fired. Typically, the GSR is removed by swabbing, tape lifting, or vacuuming. After which, the GSR that had been lifted is analyzed by a scanning electron microscopy-energy dispersive X-ray analysis (SEM/EDX) [7]. Often, SEM is used to analyze the GSR and any patterns on the clothing because chemical tests may destroy such evidence [39]. If the victim or shooter wears dark clothing, finding the GSR becomes challenging. It was found that using light between 445 nm to 495 nm while using a filter allowed the GSR to stand out and allowed it to be photographed [39].
Contamination
In general, the contribution or source of contamination is air, temperature, and humidity when dealing with metal particles. A critical issue of sample contamination can significantly affect the accuracy and reliability of forensic results in a GSR analysis. The collection, handling, and analysis of GSR samples can result in accidental contamination. During the GSR sample collection, handling procedure, and analysis process, there is a possibility that it could lead to accidental contamination [11].
Sample extraction
In crime scenes or where the explosives take place, gunshot residues will spill all over, and it is not easy to collect and separate them. Once forensics experts collect the samples from different surfaces, those samples may undergo a sample extraction procedure if necessary. Sample preparation procedures ensure that target compounds are extracted from complex matrices or that interferences are removed from the target analyte. Many sample extraction procedures are available nowadays to make the analysis effective. As far as organic gunshot residue and smokeless gunpowder samples are concerned, solvent extraction, solid-phase microextraction (SPME), and headspace sorptive extraction (HSSE) are the most common methods for sample preparation [40].
Sample preparation
The initial collection of GSR has just been discussed. These include swabbing, vacuuming, tape lifting, hand washing, clothing recovery, paraffin wax, nasal recovery, and hair recovery. After collection, the GSR may have to be shipped to a forensics lab and analyzed later. The preservation of the material is critical. Therefore, the collected GSR is stored in an airtight container to prevent evaporation, and to preserve the OGSR, it is stored at temperatures of 0°C or less [41]. When GSR is extracted from a surface and onto a collection medium, such as swabs or tape, typically, the GSR needs to be separated from its extraction medium. One technique of separating swabs from the metallic GSR is burning off the cotton swab, in a process called dry ashing. The disadvantage of dry ashing the cotton swab is that there are significant losses of antimony, one of the three main metallic components often used to identify GSR [30]. Another process of separating lead GSR from cotton swabs is soaking the swabs in EDTA, placing it in vials of nitric acid, or finally placing them in an ultrasonic bath. This preparation method demonstrated good lead collection results [30]. After the collection of the GSR from its lifting material, the extracted material is placed in a centrifuge tube. Acetonitrile is added to the centrifuge tube, transferred to another centrifuge tube, dried under a nitrogen atmosphere, and reconstituted before analysis [38]. A similar process is also used in preparing OGSR, such as NC, for electrochemical detection methods. A small fabric sample is placed in a centrifuge tube with acetonitrile and heated at 60°C for 30 minutes in an ultrasonic bath. An organic solvent is added, which may interfere with the possibility of extracting IGSR. Using a syringe filter, the solution is filtered through a 0.22 μm filter. Depending on the concentration of the GSR, diluting the solution with the appropriate solvent or buffer solution may be necessary to bring the analyte’s concentration within the readable range of the electrochemical detection method [42]. Using carbon tape as a lifting medium for the GSR has several advantages. First, without any further modification, it can be used in SEM equipment to investigate the morphology of the GSR [43]. The sample on the carbon tape can also be used in square wave voltammetry detecting lead, antimony, and copper. Even after voltammetry has been conducted on the sample, carbon tape effectively retains the GSR components [43].
Limitations due to environmental conditions and human’s actions
Not all shootings occur indoors. Outdoor environmental factors, such as wind and temperature, offer additional challenges to analyzing GSR. When a gun is fired, the amount of GSR present depends on the type of ammunition and the type of gun being fired. Most of the GSR exits out of the end of the barrel, while some of the GSR exits where the cartridge is ejected [28]. Both wind speed and direction play an important role in how the GSR is distributed. In calm conditions, such as being indoors, lighter GSR tends to stay suspended in the air longer than heavier GSR. If GSR is abut 1 μm to 5 μm, it will remain suspended for about 10 minutes, 1 μm GSR for about 90 minutes, and 0.3 μm for 630 minutes. Higher wind speeds can carry larger GSR particles farther than calmer winds [44]. Wind direction, especially at higher wind speeds, can affect the amount of GSR that hits its target. In a headwind, less GSR reaches the target, which can give a false reading that the shooter is further away from the target. In a tailwind, more GSR reaches the target, giving a false reading that the shooter is closer to the target [45]. Hot temperatures due to air temperature or the individual’s body heat can significantly degrade the compounds, especially for OGSR, but have little effect on IGSR [46]. As mentioned earlier, this is why OGSR is stored in an air-tight container at temperatures below 0°C [41]. Not only do environmental temperatures affect the OGSR degradation rate, but so do the various analytical methods. Due to the hot temperatures during gas chromatography-mass spectrometry (GC-MS), organic compounds like NG became undetectable during the analytical process [47]. After firing a gun, the shooter’s actions can also affect the GSR distribution and concentration. OGSR, due to being more volatile and soluble in oils, tends to dissolve into the shooter’s skin or evaporate [46]. Therefore, OGSR is less likely to be transferred to other surfaces where the shooter may come into contact. Even if the shooter runs shortly after shooting, it has been shown to have little effect on lowering the concentration of the OGSR that has been absorbed into the skin. Even physical rubbing of the hands has little effect on the concentration of OGSR. Hand washing, especially with rubbing alcohol-based hand sanitizer, has shown a significant drop of OGSR within the skin. IGSR, on the other hand, tends to settle onto the skin and clothing of the shooter and, as a result, quickly falls or is washed off during physical movement or rubbing of the hands [21].
Even if a gun is fired within a vehicle, the GSR disperses throughout the car, with higher concentrations of GSR in the seats and dashboard where the firing occurred. If a long period separates between the firing of the gun and when the car is tested for GSR, it was observed that the concentration of GSR was significantly lower on the seats. It is hypothesized that this could be due to people entering and exiting the vehicle, carrying away the GSR on their clothing and skin. In such situations, where there is a long separation between the firing of the gun and GSR testing, surfaces such as the dashboard are a better candidate to test for GSR [29].
One of the significant limitations when analyzing GSR is that no single detection method can simultaneously analyze the major components of GSR, especially OGSR and IGSR [44]. One of the significant indicators that a sample is GSR and no residue from other sources is the presence of lead, antimony, and barium together. The environment is filled with lead, barium, and antimony with non-GSR sources Table 2.
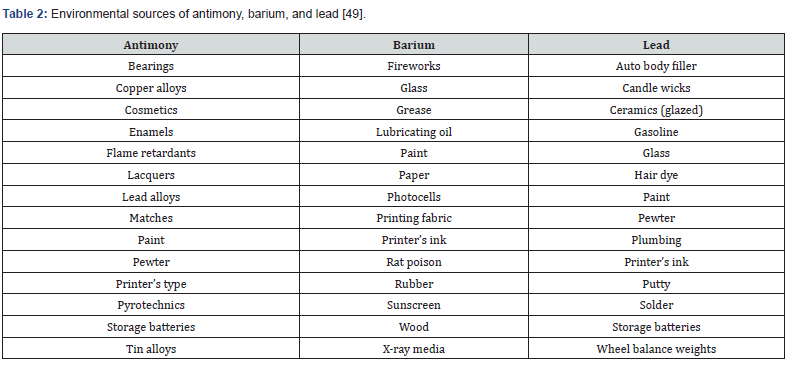
As seen in Table 2 individuals can come into contact with lead, barium, and antimony from non-GSR sources, which could give a false-positive to various detection methods. Another limitation is the GSR sample’s concentration not being within the linear range. GSR samples can be diluted if they are too concentrated or solvent evaporated if the GSR is too diluted [48].
- Research Article
- Abstract
- Introduction
- Background and Relevance of GSR in Forensic Investigation
- Gunpowder and Gunshot Residue
- Discussion
- Gunshot Residue Collection and Sample Preparation
- GSR Analysis Methods
- Evolution of Methods of Detecting Gunshot Residue
- Capillary Electrophoresis (CE)
- Electrochemistry
- Determination of Firing Distance Methods
- Development of Rapid and Portable/On-Site Detection
- Integration of Machine Learning/Artificial Intelligence into GSR Analysis
- Conclusion
- References
GSR Analysis Methods
In earlier days, as soon as gunshot residue samples arrived at a lab, samples were inspected visually under a microscope. Since the early 19th century, numerous color tests have been developed for GSR detection. However, these color spot tests degrade, destroy samples, contaminate with surrounding particles, and require an excess quantity of samples with less accurate results [49]. But these tests are easy to perform and give quick results [9,25]. In more recent years, gunshot residue detection has been performed using a combination of traditional techniques such as colorimetry and sophisticated instrumental analysis [14]. A quantitative and qualitative assessment of the composition of GSR in microgram or nanogram quantities can be performed using these techniques.
Colorimetric/chemical test procedure
A variety of colorimetric/chemical tests for GSR have been used in several forensic laboratories, including the dermal nitrate/paraffin test, Walker test, Marshall and Tewari test, modified Griess test, Harrison and Gilroy test, sodium rhodizonate test, Lunge test, and the di-thio-oxamide test.
Dermal nitrate or paraffin test: To test for nitrates from partially and unburned propellants, the dermal nitrate test was developed by Gonzalez in 1933. Initially, 0.25% solution of N, N1-diphenylbenzidine in strong sulphuric acid solution of sprayed over the crime scene but eventually replaced by diphenylamine was dissolved in a concentrated sulphuric acid solution. This test resulted in a deep blue color from partially burned or unburned propellant particles. Other commonly used materials also showed positive results with this paraffin test. So, due to the inaccurate results, this test could not be applied further [50,51].
Griess test or walkers test: A method for nitrite testing is the Griess test, developed by Griess in 1858. In this test, a bromide filter paper was treated with 2-napthylamine and disulphonic acid, then placed on a GSR cloth and applied with a hot electric iron. A dark red color spot identifies GSR [4,13,17,20,52].
Modified griess test: As an improvement of the Griess test to test for nitrites, a Modified Griess test was developed. This test uses a desensitized photographic paper treated with sulfanilic acid in distilled water, along with α-naphthol in methanol [39]. The photographic paper is placed over the suspected GSR area and steam-ironed with diluted acetic acid. If nitrites are present, orange-colored specks appear on the photographic paper. Especially if the GSR is difficult to see due to dark clothing, this test can allow the area of the GSR to be visualized. This can be beneficial in allowing investigators to see how far away a victim was from the shooter based on the spread of the GSR [4,17,52].
Harrison and gilroy test: In 1959, Harrison and Gilroy developed a test to detect the three common metals found in GSR, antimony, lead, and barium. Diluted hydrochloric acid moistened a swab. After sample collection, the swab must be dried and treated with tri-phenylarsonium iodide before adding sodium rhodizonate. The swab would change colors based on the type of metal that may be present. The swab would turn orange for antimony, red for both lead and barium, purple for lead after it was treated with dilute hydrochloric acid, and colorless for barium. The disadvantages of this test were that it was prone to unstable color changes, did not provide quantifiable results, and the GSR was not suitable for additional tests [7,53,54].
Marshall and tewari test: These tests are used to estimate the range of firing. For the Marshall test, in a diluted sulphanilic acid, desensitized photographic paper is soaked for a few minutes. After drying this paper, treated with N-α naphthyl-ethylenediamine hydrochloride in methanol and then diluted acetic acid. After applying heat to the photographic paper, nitrites give a purple color. In the Tewari test, 1 gm of antazoline hydrochloride is dissolved in water, and then concentrated HCl was added until the white precipitate dissolves, then filter paper soaked in acetone is placed over the sample, air-dried and a deep yellow spot indicates the presence of nitrites [3,4,55].
Di-thio-oxamide Test: A moistened cotton swab or paper containing diluted ammonium hydroxide is applied to the bullet hole. To a dry swab are add a few drops of di-thio-oxamide dissolved in ethanol solvent. Copper gives a green, nickel gives a violet, and cobalt gives a brown color [3,25,56].
Sodium rhodizonate method: This test detects Pb efficiently. In this test, colored complexes formed through the reaction between sodium rhodizonate and metallic divalent ions. pH determines the color of the solution, which can range from blue to violet. At a neutral pH, it will give a blue-violet color; at an acidic pH, it will give a dark red color [57-60].
- Research Article
- Abstract
- Introduction
- Background and Relevance of GSR in Forensic Investigation
- Gunpowder and Gunshot Residue
- Discussion
- Gunshot Residue Collection and Sample Preparation
- GSR Analysis Methods
- Evolution of Methods of Detecting Gunshot Residue
- Capillary Electrophoresis (CE)
- Electrochemistry
- Determination of Firing Distance Methods
- Development of Rapid and Portable/On-Site Detection
- Integration of Machine Learning/Artificial Intelligence into GSR Analysis
- Conclusion
- References
Evolution of Methods of Detecting Gunshot Residue
There are limitations associated with color tests, even though they can be performed at a lower cost, require few skills, and have no complicated procedures involved [25]. False-positive results can result when environmental factors are misinterpreted, a lack of consistency and specificity is present, and foreign materials are present at crime scenes, all of which can interfere with the accuracy of the tests [25]. These tests are insufficient for analysis due to the possibility of destroying the sample and interfering with other environmental constituents. These color tests were continuously used in several laboratories between the 1960s and the 1970s but are not specific to GSR, so they are less frequently used today. As a result, instrumental-based techniques replaced the color spot test to quantify the total amount of elements present in a GSR sample [3].
Scanning electron microscopy
As previously mentioned, the chemical/color tests for GSR can interfere with the integrity of the samples to be further investigated. When dealing with forensics, it is crucial to maintain the integrity of the evidence if future tests need to be performed. An essential tool that can be found in a forensics lab is a scanning electron microscope (SEM). As in traditional light microscopes, visible light’s longer wavelengths limit their resolution, magnification, and depth of field. Electrons have a much shorter wavelength than visible light, thus allowing for the material to be investigated in greater detail [61]. SEM can resolve GSR that is 0.5 to 10 μm in size [3] and reveal the spherical morphology of the GSR [14]. Along with the SEM, using an energy-dispersive X-ray (EDX) can determine the elemental composition of the GSR in a non-destructive manner [47]. This ability of SEM to investigate the form, structure, and composition of GSR makes it a valuable tool in forensic labs [43]. Some of the drawbacks of SEM are the size of the instrument, the need for trained personnel, and the amount of time to operate it [43]. OGSR can degrade within 4 to 24 hours. Getting the GSR collected, sent to a lab, and prepared and analyzed can exceed the critical time to record the necessary evidence [62].
Spectral methods
In many labs, spectroscopy is a common technique to determine the composition and structure of a material by its interaction with various electromagnetic radiation, such as visible light, infrared (IR), ultraviolet (UV), X-rays, or radio waves. Spectroscopy also has the advantage in that the sample is not compromised, as it often is with the chemical/color detection methods mentioned earlier. Compared to SEM, there is often less sample preparation needed before being analyzed [63].
Atomic absorption spectroscopy: Atomic absorption spectroscopy (AAS) has long been known as one of the most powerful, sensitive, and convenient instruments. This instrument is used for quantitatively determining trace elements and metal content in gunshot residue. The principle behind this technique is free atoms in a gaseous state absorb radiation at a specific frequency in an atomizer [3]. The two most common atomization methods used in AAS are flame atomization and electrothermal atomization (graphite furnace). Atomic absorption spectrometers consist of a radiation source, atomizer, monochromator, detector, and readout device [64]. Atomic absorption spectroscopy alone cannot determine barium and antimony, but flameless or electrochemical atomizers can help determine these elements [64]. Lower-energy state atoms absorb energy from a radiation source to produce atomic absorption spectra. On returning to the lower-energy or ground state, neutral atoms in an excited state emit energy, producing atomic absorption spectra [64]. AAS provides information on whether a person fired based on the amount of metal or element constituents, and it is estimated that 90% of cases of success could be achieved using these techniques. This technique can accurately detect lead metal. Fluid nebulization had a lower detection limit and was more sensitive than conventional AAS [3].
Inductively coupled plasma-atomic (optical) emission spectroscopy (ICP-AES): Examples of Inductively Coupled Plasma-Atomic (Optical) Emission Spectroscopy (ICP-AES) use include quantitative determination in GSR analysis and predictions of the number of shots fired [65–67]. With the ICP a sample solution is nebulized to form a mist and introduced directly into an argon plasma torch where it is atomized and the subsequent emission spectra are detected, often with a charge coupled device.
Infrared spectroscopy: Infrared (IR) spectrometers utilize the infrared section of the electromagnetic (EM) spectrum to analyze various chemical structures. When the IR shines upon various compound regions, various groups will absorb specific wavelengths of the IR and exhibit symmetric, asymmetric, or scissoring oscillations [68]. IR spectroscopy is typically used with other tests, such as SEM, to verify findings. The results of IR spectroscopy allow for the identification of various components within the GSR, and by combining the results from Raman spectroscopy, it can also be used to help determine the shooter’s distance and the ammunition's caliber [69].
Raman spectroscopy: Raman spectroscopy utilizes an incident monochromatic light (laser) to excite the particles within the sample. Most scattering photons do not change their energy (Rayleigh scattering). Still, some of the photons will experience a shift in their energy due to inelastic interactions with the material. These photons may have more or less energy than the incident light. These shifts correspond to the vibration and rotation of the bonds within the molecule (like IR spectroscopy and NMR) [70]. Raman spectroscopy is typically used to analyze the chemical composition of GSR, which includes lead, barium, and strontium. Part of the challenge is identifying GSR from lead-free ammunition. Combining the results from Raman spectroscopy and SEM-EDX can be an effective tool in correctly identifying GSR [20]. There is also research on using Raman spectroscopy to identify the caliber size from the GSR [69].
X-ray spectroscopy: As indicated previously, Energy-Dispersive X-ray Spectroscopy (EDX) is often integrated with SEM. The SEM provides the beam of electrons onto the sample. The inner electrons become excited, and X-rays are emitted as they return to the ground state. An X-ray detector detects the X-rays recording the energy being emitted. By combining the SEM with the EDX, they can simultaneously examine both the elemental composition of the objects and the GSR morphology [71,72]. The SEM-EDX is ideal for analyzing GSR’s primer residue, which produces some of the most identifying features within the GSR [73].
Laser-induced breakdown spectroscopy (LIBS): Laser-induced breakdown spectroscopy (LIBS) is a technique that can be applied in the forensic analysis of IGSR. The process involves focusing a high-energy laser pulse onto a GSR sample, causing heating and vaporization of the material. The heated vapor emits specific wavelengths, where a spectrometer analyzes and identifies the composition of the GSR samples [74]. One of the advantages of LIBS is that with minimal sample preparation, it produces results in less than a minute of multiple elements simultaneously. Even if the GSR is from environmentally friendly/lead-free ammunition, LIBS can identify the lead-free IGSR components of the ammunition. During analysis, there is limited destruction of the sample, allowing the sample to be further examined by SEM-EDX or electrochemical detection [74]. Portable LIBS can be used for on-site analysis of GSR, enabling spatial analysis. By scanning the hands of the shooter and the surrounding area, the spatial analysis can provide information into the deposition pattern of the GSR, aiding in the collection of the GSR for further analysis and potentially reconstructing the scenario during the shooting [74].
Ion mobility spectrometry: Ion Mobility Spectrometry (IMS) is a widely recognized method used for many years to identify gunshot residues and a variety of explosives [75]. It is an atmospheric pressure approach that uses an electric field to separate the reactant and product ions after they react with the sample. This technique is widely used due to its high sensitivity and small size (field-portable), and it can detect in the range of parts per billion (ppb) [75]. Gunpowder extracted from different cartridges was studied to determine positive and negative ions, nitro, and nitroso derivatives produced from gunpowder stabilizers such as diphenylamine (DPA) and ethyl centralite (EC). The EC peak was more intense than the DPA peak; according to the plasma-grams acquired in the positive and negative ion mode, DPA and EC produced no significant number of anions [75]. The IMS instruments, such as benchtop and portable models, produced similar findings regarding the presence of gunshot residue on hand swabs [76]. In an IMS analysis, stability studies revealed that gunshot residue components are degraded when stored at room temperature. Owing to this reason, analysis is required fast and accurate to overcome this problem [76]. IMS is not a suitable technique to determine the limit of detection of organic gunshot residue, although it is a semi-quantitative method [76].
Mass spectrometry: Mass spectroscopy (MS) is a standard analytical tool used to determine the molecular mass by measuring its mass-to-charge ratio (m/z). The unknown sample is ionically charged and then electrostatically pulled along a pathway. The ions are separated depending on the mass-to-charge (m/z), and then a detector registers the signals [77]. For GSR to be analyzed using MS, typically, the GSR is collected by swabbing or tape lifting from the hands and surrounding surfaces [3]. The GSR is removed from its lifting material by dissolving the lifting material or soaking it. Then, an ultrasonic bath (sonicator) may remove the GSR from its holding surface [44,78]. MS has the advantage of detecting and measuring the various metals (lead, barium, and antimony), along with the IGSR and OGSR components. Since each substance has its unique m/z ratios, MS can play a crucial role in the forensic analysis of GSR [79]. Forensic tools such as SEM are crucial in helping to identify GSR. However, Raman spectroscopy, IR spectroscopy, EDX spectroscopy, and MS spectroscopy provide support and confirmation from the SEM results [11].
Inductively coupled plasma-mass spectrometry (ICP-MS): ICP-MS was initially demonstrated in 1980 for determining elemental concentrations [80]. In this technique, the sample was ionized by high-temperature plasma, and ions were detected and separated by mass spectra based on the mass-to-charge ratio (m/z) [3]. The ICP-MS method is widely used in environmental, forensic, industrial, nuclear, medical, and other diverse applications for tracing and ultra-tracing liquid, gaseous, and solid samples [81]. Most commercial ICP-MS equipment has quadrupole mass analyzers with a resolution of about unit mass numbers, allowing the user to perform rapid full mass scanning and multiple element analyses [81]. For trace element analysis, ICP-MS has gained wide acceptance for its high sensitivity, low detection limits, linear dynamic ranges, low interference, and speed of analysis and has been used extensively in forensics [17,81,82]. This technique will improve precision and recovery if preventive measures are taken [81]. ICP-MS was used to determine the total element concentrations of Sb, Ba, and Pb using the isotopes Sb, Ba, and Pb [3]. When analytes are present at positive GSR levels, using internal standards and large dilutions will reduce nonspecific interference problems (matrix effects) to a minimum [81].
Gas chromatography-mass spectrometry (GC-MS): In the gas chromatographic (GC) technique, a sample was injected into the injection port and passed through a column with a carrier gas, and components were separated based on their boiling point [3]. Pyrolysis gas chromatography is a more advanced approach than gas chromatography. In this approach, small solid forensic samples are heated at high temperatures to decompose or convert them into gaseous molecules. These molecules will be separated by gas chromatography and detected by mass spectrometry and different detectors [83]. Thermal energy analysis (TEA), mass spectrometry (MS), electron capture detector (ECD), flame ionization detector (FID), and various detectors are used with GC to detect efficiently organic residues in firearm smokeless powder. However, better results are achieved by combining GC with thermal energy analysis and mass spectrometry [84]. Gas chromatography is not optimal for GSR analysis due to the incompatibility of some compounds, such as nitrate esters [84].
High-performance liquid chromatography-mass spectrometry (LC-MS): In HPLC, the mobile phase (liquid) passes through a column filled with solid (silica with different particle sizes) particles, and the mobile phase carries the sample so that it can be eluted at different retention times depending on the nature of the sample. HPLC can operate at different temperatures based on the target analyte. HPLC can be used in both normal phase and reversed phase, and it is connected to UV-visible, refractive index (RI), and mass detectors. There are different kinds of mass analyzers are available based on the requirements. Many forensics labs use liquid chromatography coupled to tandem mass spectrometry (LC-MS/MS) to detect GSR due to its high sensitivity and selectivity, and it has become a widely used instrument [26]. Additional sample preparations are necessary due to the IGSR particle structure to qualify and quantify samples correctly using LC-MS/MS [21]. LC-MS/MS is commonly used to identify analytes using multiple reaction monitoring, and quantifier and qualifier ions are crucial in this process. In this technique, ionized molecules and atoms are separated based on the differences in charge-to-mass ratios. In OGSR detection, LC-MS methods have the most accurate detection limits and appear to be one of the most effective techniques for detecting OGSR [85].
- Research Article
- Abstract
- Introduction
- Background and Relevance of GSR in Forensic Investigation
- Gunpowder and Gunshot Residue
- Discussion
- Gunshot Residue Collection and Sample Preparation
- GSR Analysis Methods
- Evolution of Methods of Detecting Gunshot Residue
- Capillary Electrophoresis (CE)
- Electrochemistry
- Determination of Firing Distance Methods
- Development of Rapid and Portable/On-Site Detection
- Integration of Machine Learning/Artificial Intelligence into GSR Analysis
- Conclusion
- References
Capillary Electrophoresis (CE)
A wide range of forensic samples has been analyzed using capillary electrophoresis, including explosive compounds and gunshot residues [86]. Compared to current techniques, capillary electrophoresis analyses organic and inorganic gunshot residues simultaneously, faster, and cheaper [7]. Diaminocyclohexane tetraacetic acid (CDTA) was used as a complexing agent in the pre-capillary complexation process to separate inorganic residues and efficiently separate aminopolicarboxilic acids for lanthanoid elements [87]. All the metals must be complexed in pre-capillary complexation. To prevent complexes from dissociating inside the capillary during pre-capillary complexation, a low concentration of CDTA is added to the background electrolyte to prevent the complexes from dissociating inside the capillary [88]. Micellar phases were added to the background electrolyte to separate organic gunshot residues since most lack acid-base properties [7]. Counter-electroosmotic conditions separate inorganic residues, and simultaneous inorganic and organic residues were analyzed using sodium dodecyl sulfate (SDS) micelles. Separation of organic gunshot residues was achieved by increasing the concentration of SDS [7]. Adding SDS to the metal–CDTA complexes did not affect selectivity or separation, enabling the simultaneous separation of organic and inorganic gunshot residues [7].
- Research Article
- Abstract
- Introduction
- Background and Relevance of GSR in Forensic Investigation
- Gunpowder and Gunshot Residue
- Discussion
- Gunshot Residue Collection and Sample Preparation
- GSR Analysis Methods
- Evolution of Methods of Detecting Gunshot Residue
- Capillary Electrophoresis (CE)
- Electrochemistry
- Determination of Firing Distance Methods
- Development of Rapid and Portable/On-Site Detection
- Integration of Machine Learning/Artificial Intelligence into GSR Analysis
- Conclusion
- References
Electrochemistry
Electrochemical detection methods involve using various electrochemical sensors to identify and analyze various chemical substances. The electrochemical sensors usually consist of electrodes where the reaction occurs, a reference electrode, and an electrolyte solution. As the electrodes are placed in a solution of the substance being analyzed, an electrical process occurs at the surface of the working electrode producing a small electrochemical signal that is recorded by the instrument.
Voltametric methods Voltammetry is one of the electrochemical methods used to identify and quantify the presence of specific substances within the GSR. Voltammetry measures the current produced through an oxidation or reduction reaction of the sample substance as an electrical potential difference is applied the electrodes [89].
Cyclic Voltammetry (CV): In cyclic voltammetry (CV), an inert electrode material is used to control the energy of the electrons by modulating it with a potentiostat [90]. During this process, a voltammogram shows the relationship between the applied potential and current response [91]. The potential is varied linearly at a specific scan rate. The potential may be first swept negatively from the starting potential to the switching potential, referred to as the cathodic trace. The scan direction is reversed, and the potential is swept positively back to the starting point, referred to as the anodic trace [90]. CV uses three electrodes, a working electrode, a reference electrode, and an auxiliary electrode. In this type of setup, a small number of current flows through the reference electrode, allowing the electrode potential to remain stable. Also, having three electrodes minimizes voltage errors from ohmic losses in the solution by having the reference electrode near the working electrode [92]. A thin diffusion layer develops near the electrode’s surface where the mass transport processes occur [92]. An application of CV is to analyze the presence of copper in GSR. This has shown to be a quick and reliable procedure for quantifying copper, from the projectile, at a crime scene. A shooter’s hands and arms are swabbed with a gold-coated screen-printed carbon electrode (SPCE). The GSR is analyzed by CV after 20 μL of acetate buffer is placed on the SPCE. The CV is scanned at a rate of 100 mV/s, scanning from −100 mV to 100 mV. The oxidation of Cu0 to Cu2+ is detectable at low concentrations. Since the diffusion rate of Cu2+ increases linearly at the electrode’s surface as the Cu2+ increases, the peak current of the cyclic voltammogram can be used to determine the concentration of Cu2+ [93].
Square-Wave Voltammetry (SWV): Square Wave Voltammetry (SWV) is an electrochemical technique that provides greater performance, compared to cyclic voltammetry. SWV utilizes computer software to achieve greater sensitivity and the ability to reduce background currents. Using a computer, a staircase waveform is combined with a large amplitude square wave modulation. This technique helps analyze reversible electrode reactions. It measures the current response to a normalized current function, which depends on the potential sequence applied to the electrode. The current function is independent of time and can be used to analyze the data obtained from SWV tests [94]. One of the applications of SWV is to detect the existence of an inorganic chemical marker made up of a luminescent complex [(Eu2Zr)(btc)3(Hbtc)5.6H2O]. This is advantageous when working with GSR from nontoxic ammunition (NTA), which lacks the typical metals of barium, lead, and strontium [95]. A supporting electrolyte solution of 0.1 M KCl and a carbon paste electrode surface were used. With the incorporated luminescent marker, the carbon paste electrode presented two anodic peak currents at the 0.4 V region (vs. Ag/AgCl) and at the 0.75 V (vs Ag/AgCl). Plus, a single peak is at 0.4 V (vs Ag/AgCl) at the cathodic [95]. Even though SEM-EDX can detect GSR from both standard and nontoxic ammunition, the SWV is advantageous due to its lower cost and inexpensive supplies and equipment for forensic laboratories [95]. In addition to detecting trace levels, square-wave voltammetry is used in high-performance liquid chromatography (HPLC) with electrochemical detection [42].
Anodic Stripping Voltammetry (ASV): Anodic Stripping Voltammetry (ASV) is a sensitive electrochemical technique that detects common metals in GSR, such as lead and antimony. The detection of barium is more challenging as it requires such a high negative potential that water reduction occurs [30]. The GSR is collected by adhesion or swabbing. Then, the GSR is dissolved in an electrolyte solution such as hydrochloric acid, sulfuric acid, or nitric acid. The analysis of GSR with ASV usually consists of a three-electrode setup using a hanging mercury drop electrode (HMDE) or a thin mercury film electrode (MFE) acting as the working electrode [96]. A deposition step accumulates the metallic ions in the mercury working electrode. At a negative potential value, metallic ions are reduced, forming an amalgam on the electrode’s surface. Depending on the target analytes’ concentration, the deposition duration may vary [97]. For lead and antimony, deposition times range from 180 to 280 s within GSR, with lead peaks at -0.50 V and antimony at -0.24 V [30].
Amperometry
Amperometry is another electrochemical detection method with applications in analytical chemistry, biosensing, and chemical environmental monitoring. Some of the earlier and popular applications are monitoring dissolved oxygen or glucose within blood [98]. Amperometry is performed by placing a working electrode into a solution that contains the analyte that is being investigated. The electrode’s potential is set to a specific value, and the current is measured as it flows through the electrode. A redox reaction occurs between the analyte and the electrode’s surface as a potential is applied to the electrode. The current between the working and auxiliary electrodes is measured due to the flow of electrons from the redox reaction. The concentration of the analyte can be determined since the concentration is directly proportional to the current [98]. By using various membranes and electrodes, amperometry sensors can be made sensitive to the substance of interest and reduce interference with other substances. Many of these sensors can produce fast results with minimal preparation. The sensitivity of these sensors can detect concentrations in a micromolar range [98].
Nitrite amperometric sensor: One application of amperometry used in GSR analysis is the nitrite amperometric sensor. Nitrites (NO2−) are inorganic anions originating from the primer [3]. A glassy carbon electrode (GCE) that is used to detect the nitrites is coated with palladium which has good conductivity and catalytic properties. The sensitivity towards nitrites can be further enhanced by including carbon nanoparticles [3]. As the electrode is placed into the sample, the nitrites undergo a redox reaction at the electrode’s surface. The redox reaction generates an electric current proportional to the concentration of the nitrite ions, which produces a linear range of 0.10 μmol/L to 4 mmol/L, with the lowest detection limit being 0.030 μmol/L [3].
Potentiometric Ion-Selective Electrodes (ISE)
Ion-selective electrodes (ISE) are a type of electrode chemical sensor that selectively detects and measures the concentration of a specific ion while excluding other ions. The ion-selective electrodes fundamentally differ from the other electrochemical detection methods in that a redox reaction does not occur at the electrode. At the base of the electrode, a thin selective membrane is attached to the end of the electrode that is capable of binding to a specific ion [99]. Although ISEs tend to be used more for biological or environmental monitoring applications, detecting specific ions in GSR, such as lead or nitrate, is feasible. The electrode available for detecting and measuring lead uses a polycrystalline membrane made from a 1 to 2 mm thin pellet of a mixture of Ag2S and PbS. The pellet is sealed in a nonconductive plastic cylinder with an internal solution of the lead analyte and a reference electrode. The lead ions carry the charge across the membrane. A potential forms across the membrane due to the difference between the activities of the lead ions [98].
Nitrates are detectable by liquid-based ISE which uses a porous polyvinyl chloride (PVC) membrane saturated with tetradodecyl ammonium nitrate. Due to the difference in the equilibrium position between the nitrate ions and the tetradodecyl ammonium nitrate a potential form across the membrane. One of the advantages of liquid-based ISE is their ability to be stored and re-used quickly. If care is taken by keeping the PVC membrane moist by keeping a wet gauze over the membrane while in storage, the longevity of the electrode can be significantly extended. Prior to use, the electrode needs to be soaked for 0.5 to 1 hour in an analyte solution [98].
- Research Article
- Abstract
- Introduction
- Background and Relevance of GSR in Forensic Investigation
- Gunpowder and Gunshot Residue
- Discussion
- Gunshot Residue Collection and Sample Preparation
- GSR Analysis Methods
- Evolution of Methods of Detecting Gunshot Residue
- Capillary Electrophoresis (CE)
- Electrochemistry
- Determination of Firing Distance Methods
- Development of Rapid and Portable/On-Site Detection
- Integration of Machine Learning/Artificial Intelligence into GSR Analysis
- Conclusion
- References
Determination of Firing Distance Methods
An important piece of information in the ballistic reconstruction of a shooting incident is the distance between the firing gun and the victim, as it may help determine whether a gunshot death was a suicide or homicide [4,100]. Numerous approaches are involved in estimating firing distance, such as visual comparison, instrumental techniques, chemical tests, and evaluation with existing standard procedures. GSR analysis data can estimate shooting distances, time since firearm discharges, ammunition types, and injury patterns and connect the shooter with their firearm use [4]. At the first instance in the crime scene, the GSR pattern was examined by visualization methods, and the investigators relied on color tests for immediate results [101]. These methods are not always accurate. In addition to being destructive, such color tests are also insensitive [102]. These color tests also can not give a clear GSR pattern for lead-free or nontoxic ammunition [103]. Due to these reasons, non-destructive methods must be developed for accurate measurement and classification of GSR. Several techniques based on chromatography coupled with a diode array detector (DAD) and mass spectrometry have been employed in GSR analysis with reliable outcomes [104,105].
For better results, shooting distances are estimated by analyzing gunshot residues on targets, such as by using an ICP-AES instrument [65], or by visualizing their distribution patterns, such as by using X-ray fluorescence [106], atomic absorption spectroscopy (AAS) [107], and inductively coupled plasma-mass spectrometry (ICP-MS) [108]. The colorimetric method is one of the methods to perform a GSR analysis for shooting distance estimation. This colorimetric method depends on the formation of colors, and organic and inorganic GSR particles can be detected [109]. This method is still in use, but it has some limitations. For example, the sodium rhodizonate test was used for the GSR sample collection on the skin to determine the difference between close and far-range shots. Since sodium rhodizonate reagents also produced positive results by keratinous hair follicle structures in this case, skin samples may increase the possibility of false positives [110]. This technique was improved with small changes and is used to transfer GSR particles to clothing for shooting distance estimation [111]. In addition, the firing distance range was estimated using the modified Griess test (MGT) based on the GSR distribution pattern on the victim's clothes. When the potassium hydroxide solution and the MGT reagent react with GSR, the MGT test detects nitrites. The MGT is an effective method, but it would be preferable if the GSR pattern could be revealed without reagents or chemicals [112]. In spectroscopy, electromagnetic radiation interacts with the GSR sample, and it will estimate the firing distance [55]. Additionally, Neutron Activation Analysis (NAA) [113] was used to evaluate the concentration and pattern of Sb deposited around bullet entrance holes to determine the firing distance [114].
- Research Article
- Abstract
- Introduction
- Background and Relevance of GSR in Forensic Investigation
- Gunpowder and Gunshot Residue
- Discussion
- Gunshot Residue Collection and Sample Preparation
- GSR Analysis Methods
- Evolution of Methods of Detecting Gunshot Residue
- Capillary Electrophoresis (CE)
- Electrochemistry
- Determination of Firing Distance Methods
- Development of Rapid and Portable/On-Site Detection
- Integration of Machine Learning/Artificial Intelligence into GSR Analysis
- Conclusion
- References
Development of Rapid and Portable/On-Site Detection
Once a gun is fired, collecting evidence as soon as possible is crucial as the evidence begins to degrade and disperse. The development of rapid and portable detectors to analyze gunshot residue has been significant in the advancement of forensic science. Forensic laboratories can take weeks to months to analyze GSR samples. Therefore, having portable instruments to analyze the evidence increases the likelihood of getting accurate results. Due to technological advancements, what was once done in a laboratory by a trained expert can now be fast, inexpensive, portable, and performed by someone with some basic training. Electrochemical, LIBS, and MS devices have been developed that can analyze IGSR, OGSR, or both [38]. Using abrasive stripping voltammetry (AbrSV), a portable unit can test a potential shooter’s skin for traces of GSR using a “swipe and scan” procedure. A screen-printed sensor strip is rubbed on the suspect’s hand. A few drops of a buffer solution are added to the electrode, and ASV is performed on the sample, testing for lead, antimony, and copper. A digital display shows the positive or negative results of whether GSR is detected [115]. All these developments have improved the efficiency of forensic investigation and increased the ability to gather evidence promptly.
- Research Article
- Abstract
- Introduction
- Background and Relevance of GSR in Forensic Investigation
- Gunpowder and Gunshot Residue
- Discussion
- Gunshot Residue Collection and Sample Preparation
- GSR Analysis Methods
- Evolution of Methods of Detecting Gunshot Residue
- Capillary Electrophoresis (CE)
- Electrochemistry
- Determination of Firing Distance Methods
- Development of Rapid and Portable/On-Site Detection
- Integration of Machine Learning/Artificial Intelligence into GSR Analysis
- Conclusion
- References
Integration of Machine Learning/Artificial Intelligence into GSR Analysis
The integration of machine learning (ML), neural networks (NN), and artificial intelligence (AI) is being developed to aid in the analysis of GSR and improve upon the forensic investigation [116]. The analysis of GSR is performed by an expert who analyzes and evaluates the results based on their knowledge and expertise. Using ML, a likelihood ratio (LR) is being developed. Developing this LR system can help support the claims of the forensic researcher [117]. In one of the research studies, ML algorithms and NN were trained with 204 images of various gunshot wounds from varying distances. The algorithm, with 98% accuracy, was able to determine whether the wound was from a gunshot and the relative distance to the gun [118]. ML can assist in analyzing large datasets of GSR analysis and identifying trends. Combining IGSR and OGSR information with ML generates an LR to determine if an individual is a high risk of being the shooter or a bystander. This capability can support forensic investigative decision-making [46]. The successful implementation of ML, NN, and AI in GSR analysis requires large datasets that have been validated to be accurate. Collaboration between forensic scientists and programmers is crucial in the development and application of reliable forensic analysis.
- Research Article
- Abstract
- Introduction
- Background and Relevance of GSR in Forensic Investigation
- Gunpowder and Gunshot Residue
- Discussion
- Gunshot Residue Collection and Sample Preparation
- GSR Analysis Methods
- Evolution of Methods of Detecting Gunshot Residue
- Capillary Electrophoresis (CE)
- Electrochemistry
- Determination of Firing Distance Methods
- Development of Rapid and Portable/On-Site Detection
- Integration of Machine Learning/Artificial Intelligence into GSR Analysis
- Conclusion
- References
Conclusion
This review emphasizes topics related to gunshot residue, sample collection, and different conventional and instrumental techniques. GSR was classified into two different categories: organic and inorganic components. Inorganic gunshot residue, commonly known as primer residue, and these components are made up of heavy metals [14]. The propellant powder and primer mixture are GSR's most common sources of organic compounds. Organic gunshot residues are typically composed of a smokeless powder containing primary explosives, sensitizers, flash inhibitors, stabilizers, plasticizers, and gelatinizers [24]. A few challenges are involved in GSR identification and sample collection because the composition of GSRs varies depending on ammunition, environmental factors, and firearms [3]. Due to dust, dirt, other debris, and environmental contaminants, GSR tests can be false positive or inaccurate [14]. Different sampling procedures are available to collect GSR and explosive particles. This field has many gaps and scope to develop artificial intelligence-based equipment, scanners, tailor-made electrodes, electrochemical sensors, and instruments for detecting GSR. If the forensic devices are portable and easy to move, then those are beneficial to quick results and immediate response. Future research may focus on an environmentally friendly, fast, accurate, highly sensitive, and inexpensive instruments that may be developed for the forensic community.
- Research Article
- Abstract
- Introduction
- Background and Relevance of GSR in Forensic Investigation
- Gunpowder and Gunshot Residue
- Discussion
- Gunshot Residue Collection and Sample Preparation
- GSR Analysis Methods
- Evolution of Methods of Detecting Gunshot Residue
- Capillary Electrophoresis (CE)
- Electrochemistry
- Determination of Firing Distance Methods
- Development of Rapid and Portable/On-Site Detection
- Integration of Machine Learning/Artificial Intelligence into GSR Analysis
- Conclusion
- References
References
- Davis A, Geller L, Kim R, Villarreal S, McCourt A, et al. (2022) A Year in Review 2020 Gun Deaths in the U.S. John Hopkins Center for Gun Violence Solutions.
- The “Crime Gun Intelligence Center” Model: Case Studies of the Denver, Milwaukee, and Chicago Approaches to Investigating Gun Crime; Police Executive Research Forum (2017).
- Shrivastava P, Jain VK, Nagpal S (2021) Gunshot Residue Detection Technologies—a Review. Egyptian J Forensic Scis 11(1): 11.
- Saverio Romolo F, Margot, P (2001) Identification of Gunshot Residue: A Critical Review. Forensic Science International 119(2): 195-211.
- Meng H, Caddy B (1997) Gunshot Residue Analysis-A Review. J Forensic Scis 42(4): 14167J.
- Wongpakdee T, Buking S, Ratanawimarnwong N, Saetear P, Uraisin K, et al. (2021) Simple Gunshot Residue Analyses for Estimating Firing Distance: Investigation with Four Types of Fabrics. Forensic Science International 32: 111084.
- Bernal Morales E, Revilla Vázquez AL (2004) Simultaneous Determination of Inorganic and Organic Gunshot Residues by Capillary Electrophoresis. Journal of Chromatography A 1061(2): 225-233.
- Brożek‐Mucha Z (2007) Comparison of Cartridge Case and Airborne GSR—A Study of the Elemental Composition and Morphology by Means of SEM‐EDX. X-ray Spectrometry 36(6): 398-407.
- Serol M, Ahmad SM, Quintas A, Família C (2023) Chemical Analysis of Gunpowder and Gunshot Residues. Molecules 28(14): 5550.
- Rodriguez-Pascual JA, Doña-Fernández A, Loarce-Tejada Y, De Andres-Gimeno I, Valtuille-Fernández E, et al. (2023) Assessment of Gunshot Residue Detection on a Large Variety of Surfaces by Portable LIBS System for Crime Scene Application. Forensic Science International 353: 111886.
- Feeney W, Vander Pyl C, Bell S, Trejos T (2020) Trends in Composition, Collection, Persistence, and Analysis of IGSR and OGSR: A Review. Forensic Chemistry 19:100250.
- Blakey LS, Sharples GP, Chana K, Birkett J (2018) Fate and Behavior of Gunshot Residue—A Review. J Forensic Sci 63(1): 9-19.
- Gandy L, Najjar K, Terry M, Bridge CA (2018) Novel Protocol for the Combined Detection of Organic, Inorganic Gunshot Residue. Forensic Chemistry 8: 1-10.
- Brożek-Mucha Z (2017) Trends in Analysis of Gunshot Residue for Forensic Purposes. Anal Bioanal Chem. 409(25): 5803-5811.
- Perez, JJ, Watson DA, Levis RJ (2016) Classification of Gunshot Residue Using Laser Electrospray Mass Spectrometry and Offline Multivariate Statistical Analysis. Analytical Chemistry 88(23): 11390-11398.
- Schwoeble AJ, Exline DL (2000) Current Methods in Forensic Gunshot Residue Analysis, CRC Press: Boca Raton, United States.
- Dalby O, Butler D, Birkett JW (2010) Analysis of Gunshot Residue and Associated Materials—A Review. J Forensic Scis 55(4): 924-943.
- Brede U, Hagel R, Redecker KH, Weuter W (1996) Primer Compositions in the Course of Time: From Black Powder and SINOXID to SINTOX Compositions and SINCO Booster. Propellants Explosives Pyrotechnics 21(3): 113-117.
- Oommen Z, Pierce, SM (2006) Lead‐Free Primer Residues: A Qualitative Characterization of Winchester WinClean, Remington/UMC LeadLess, Federal BallistiClean , and Speer Lawman Clean Fire Handgun Ammunition. J Forensic Sci 51(3): 509-519.
- López-López M, Delgado JJ, García-Ruiz C (2012) Ammunition Identification by Means of the Organic Analysis of Gunshot Residues Using Raman Spectroscopy. Analytical Chemistry 84(8): 3581-3585.
- Feeney W, Menking-Hoggatt K, Vander Pyl C, Ott CE, Bell S, et al. (2021) Detection of Organic and Inorganic Gunshot Residues from Hands Using Complexing Agents and LC-MS/MS. Analytical Methods 1 (27): 3024-3039.
- Minzière VR, Gassner A, Gallidabino M, Roux C, Weyermann C (2023) The Relevance of Gunshot Residues in Forensic Science. WIREs Forensic Science 5(1): e1472.
- Lucas N, Cook M, Wallace J, Kirkbride K P, Kobus H (2016) Quantifying Gunshot Residues in Cases of Suicide: Implications for Evaluation of Suicides and Criminal Shootings. Forensic Sci Int 266: 289-298.
- Chang KH, Jayaprakash PT, Yew CH, Abdullah AFL (2013) Gunshot Residue Analysis and Its Evidential Values: A Review. Australian J Forensic Scis 45(1): 3-23.
- Krishna S, Ahuja P (2023) A Chronological Study of Gunshot Residue (GSR) Detection Techniques: A Narrative Review. Egyptian J Forensic Scis 13(1): 1-21.
- Laza D, Nys B, Kinder JD, Kirsch‐De Mesmaeker A, Moucheron C (2007) Development of a Quantitative LC‐MS/MS Method for the Analysis of Common Propellant Powder Stabilizers in Gunshot Residue. J Forensic Sci 52(4): 842-850.
- Gallidabino MD, Weyermann C (2020) Time since Last Discharge of Firearms and Spent Ammunition Elements: State of the Art and Perspectives. Forensic Science International 311: 110290.
- Ditrich H (2012) Distribution of Gunshot Residues - The Influence of Weapon Type. Forensic Science International 220(1-3): 85-90.
- Burnett BR, Lebiedzik J (2017) Discharge of a Pistol Out a Car Window with the Breech Within the Interior of the Car: Analysis of Gunshot Residue on a Car’s Interior Surfaces. J Forensic Scis 62(3): 768-772.
- O’Mahony AM, Wang J (2013) Electrochemical Detection of Gunshot Residue for Forensic Analysis: A Review. Electroanalysis 25(6): 1341-1358.
- Speers SJ, Doolan K, McQuillan J, Wallace JS (1994) Evaluation of Improved Methods for the Recovery and Detection of Organic and Inorganic Cartridge Discharge Residues. Journal of Chromatography A 674 (1-2): 319-327.
- Goudsmits E, Sharples GP, Birkett JW (2015) Recent Trends in Organic Gunshot Residue Analysis. TrAC Trends in Analytical Chemistry 74: 46-57.
- Zeichner A, Eldar B, Glattstein B, Koffman A, Tamiri T, et al. (2003) Vacuum Collection of Gunpowder Residues from Clothing Worn by Shooting Suspects, and Their Analysis by GC/TEA, IMS, and GC/MS. J Forensic Sci 48(5): 2002390.
- Twibell JD, Home JM, Smalldon KW, Higgs DG, Hayes TS (1982) Assessment of Solvents for the Recovery of Nitroglycerine from Hands Using Cotton Swabs. J Forensic Sci 27(4): 792-800.
- Basu S, Boone CE, Denio DJ, Miazga RA (1997) Fundamental Studies of Gunshot Residue Deposition by Glue-Lift. ASTM J Forensic Sci 42(4): 14168J.
- Schwartz RH, Zona CA (1995) A Recovery Method for Airborne Gunshot Residue Retained in Human Nasal Mucus. ASTM J Forensic Sci 40(4): 13845J.
- Chávez Reyes L, Elgueta López C, Briceño Barrios A, Garrido Soto C, Ibáñez, C, et al. (2018) Development and Application of a New Nose Hairs Sample Collection Device for GSR Particles by Scanning Electron Microscopy with Energy Dispersive X-Ray Spectroscopy (SEM-EDS). Forensic Science International 290: 42-48.
- Dalzell KA, Ott CE, Trejos T, Arroyo LE (2022) Comparison of Portable and Benchtop Electrochemical Instruments for Detection of Inorganic and Organic Gunshot Residues in Authentic Shooter Samples. J Forensic Sci 67(4): 1450-1460.
- Kersh KL, Childers JM, Justice D, Karim G (2014) Detection of Gunshot Residue on Dark‐Colored Clothing Prior to Chemical Analysis. J Forensic Sci 59(3): 754-762.
- Fitsev IM, Blokhin VK, Budnikov GK (2004) Chromatographic Techniques in Forensic Chemical Examinations. Journal of Analytical Chemistry 59(12): 1171-1180.
- Standard Practice for the Collection and Preservation of Organic Gunshot Residue; OSAC 2021-N-0009. Ignitable Liquids, Explosive, and Gunshot Residue Subcommittee Chemistry: Trace Evidence Area Committee; Organization of Scientific Area Committees (OSAC) for Forensic Science, Vers. 1. (2021).
- Profumo A, Capucciati A, Mattino A, Donghi M, Merli D (2024) A Simple Voltammetric Method to Evaluate the Firing Distance through Determination of Nitrocellulose. Talanta 266: 125040.
- O’Mahony AM, Samek IA, Sattayasamitsathit S, Wang J (2014) Orthogonal Identification of Gunshot Residue with Complementary Detection Principles of Voltammetry, Scanning Electron Microscopy, and Energy-Dispersive X-Ray Spectroscopy: Sample, Screen, and Confirm. Analytical Chemistry 86(16): 8031-8036.
- Black O, Smith SC, Roper C (2021) Advances and Limitations in the Determination and Assessment of Gunshot Residue in the Environment. Ecotoxicology and Environmental Safety 208: 111689.
- Charles S, Geusens N, Nys B (2023) Interpol Review of Gunshot Residue 2019 to 2021. Forensic Science International: Synergy 6: 100302.
- Feeney W, Menking-Hoggatt K, Arroyo L, Curran J, Bell S, et al. (2022) Evaluation of Organic and Inorganic Gunshot Residues in Various Populations Using LC-MS/MS. Forensic Chemistry 27: 100389.
- Taudte RV, Beavis A, Blanes L, Cole N, Doble P, Roux C (2014) Detection of Gunshot Residues Using Mass Spectrometry. BioMed Research International 1-16.
- Promsuwan K, Kanatharana P, Thavarungkul P, Limbut W (2020) Nitrite Amperometric Sensor for Gunshot Residue Screening. Electrochimica Acta 331: 135309.
- Thornton JI (1994) The Chemistry of Death by Gunshot. Analytica Chimica Acta 288: 71-81.
- Tagliaro F, Bortolotti F, Manetto G, Pascali VL, Marigo M (2002) Dermal Nitrate: An Old Marker of Firearm Discharge Revisited with Capillary Electrophoresis. Electrophoresis 23(2): 278-282.
- Cowan ME, Purdon PL (1967) A Study of the “Paraffin Test.” J Forensic Sci 12(1): 19-36.
- Muller D, Levy A, Vinokurov A, Ravreby M, Shelef R, et al. (2007) A Novel Method for the Analysis of Discharged Smokeless Powder Residues. J Forensic Sci 52(1): 75-78.
- Price G (1965) Firearms Discharge Residues on Hands. J Forensic Sci Soc. 5(4): 199-200.
- Sharma BR (1976) Firearms in Criminal Investigation and Trials.
- NM Tripathi, NH Abedi M, Bonsu DOM, Badu IK, Afoakwah R, et al. (2021) Spectroscopic (Analytical) Approach to Gunshot Residue Analysis for Shooting Distance Estimation: A Systematic Review. Egyptian J Forensic Scis 11(1): 34.
- Feigl F (1966) Identification of Individual Organic Compounds. In: Spot Tests in Organic Analysis, Elsevier pp.401-566.
- Steinberg M, Leist Y, Goldschmidt P, Tassa M (1984) Spectrophotometric Determination of Nitrites in Gunpowder Residue on Shooters’ Hands. J Forensic Sci 29(2): 464-470.
- Lloyd JBF (1986) Liquid Chromatography of Firearms Propellants Traces. Journal of Energetic Materials 4(1-4): 239-271.
- Bartsch M, Kobus H, Wainwright K (1996) An Update on the Use of the Sodium Rhodizonate Test for the Detection of Lead Originating from Firearm Discharges. J Forensic Sci 41(6): 1046-1051.
- Costa RA, Motta LC, Destefani CA, Rodrigues RRT, Do Espírito Santo KS, et al. (2016) Gunshot Residues (GSR) Analysis of Clean Range Ammunition Using SEM/EDX, Colorimetric Test and ICP-MS: A Comparative Approach between the Analytical Techniques. Microchemical Journal 129: 339-347.
- A Short Introduction to Light and Electron Microscopy (2015) University of Zurich, Europe.
- Trejos T, Vander Pyl C, Menking-Hoggatt K, Alvarado AL, Arroyo LE (2018) Fast Identification of Inorganic and Organic Gunshot Residues by LIBS and Electrochemical Methods. Forensic Chemistry 8: 146-156.
- López-López M, Merk V, García-Ruiz C, Kneipp J (2016) Surface-Enhanced Raman Spectroscopy for the Analysis of Smokeless Gunpowders and Macroscopic Gunshot Residues. Anal Bioanal Chem 408(18): 4965-4973.
- Yeung V, Miller DD, Rutzke MA (2017) Atomic Absorption Spectroscopy, Atomic Emission Spectroscopy, and Inductively Coupled Plasma-Mass Spectrometry. In: Nielsen SS (Ed), Food Analysis in Food Science Text Series, Springer International Publishing, Europe pp.129-150.
- Turillazzi E, Di Peri GP, Nieddu A, Bello S, Monaci F, et al. (2013) Analytical and Quantitative Concentration of Gunshot Residues (Pb, Sb, Ba) to Estimate Entrance Hole and Shooting-Distance Using Confocal Laser Microscopy and Inductively Coupled Plasma Atomic Emission Spectrometer Analysis: An Experimental Study. Forensic Science International 231(1-3): 142-149.
- Koons RD, Haveskost DG, Peters CA (1988) Determination of Barium in Gunshot Residue Collection Swabs Using Inductively Coupled Plasma-Atomic Emission Spectrometry. J Forensic Sci 33(1): 35-41.
- Vanini G, Destefani CA, Merlo BB, Carneiro MTWD, Filgueiras PR, et al. (2015) Forensic Ballistics by Inductively Coupled Plasma-Optical Emission Spectroscopy: Quantification of Gunshot Residues and Prediction of the Number of Shots Using Different Firearms. Microchemical Journal 118: 19-25.
- Theophanides T (2012) Introduction to Infrared Spectroscopy. In: Infrared Spectroscopy – Materials Science, Engineering and Technology, Theophanides T (Ed), In Tech: National Technical University of Athens, Chemical Engineering Department, Europe pp.1-13.
- Bueno J, Sikirzhytski V, Lednev IK (2012) Raman Spectroscopic Analysis of Gunshot Residue Offering Great Potential for Caliber Differentiation. Analytical Chemistry 84(10): 4334-4339.
- Hamann CS, Sonntag MD (2018) Introduction to Raman Spectroscopy in the Undergraduate Curriculum. In: ACS Symposium Series; Sonntag, MD (Editor), American Chemical Society: Washington, United States 1305: 1-11.
- Reed SJB (1995) Introduction to Energy Dispersive X-Ray Spectrometry (EDS).
- Introduction to EDS Analysis - Reference Manual, 2011, Bruker.
- Brożek-Mucha Z (2014) On the Prevalence of Gunshot Residue in Selected Populations – Empirical Study Performed with SEM-EDX Analysis. Forensic Sci Int. 237: 46-52.
- Menking-Hoggatt K, Martinez C, Vander Pyl C, Heller E, Pollock E, et al. (2021) Development of Tailor-Made Inorganic Gunshot Residue (IGSR) Microparticle Standards and Characterization with a Multi-Technique Approach. Talanta 225: 121984.
- West C, Baron G, Minet J-J (2007) Detection of Gunpowder Stabilizers with Ion Mobility Spectrometry. Forensic Science International 166(2-3): 91-101.
- Yeager B, Bustin K, Stewart J, Dross R, Bell S (2015) Evaluation and Validation of Ion Mobility Spectrometry for Presumptive Testing Targeting the Organic Constituents of Firearms Discharge Residue. Analytical Methods 7(22): 9683-9691.
- Siuzdak G (2004) An Introduction to Mass Spectrometry Ionization: An Excerpt from The Expanding Role of Mass Spectrometry in Biotechnology. Journal of the Association for Laboratory Automation 9(2): 50-63.
- Kara İ (2020) The Relationship between Gunshot-Residue Particle Size and Boltzmann Distribution. Forensic Science Research 7(1): 47-52.
- Brünjes R, Schüürman J, Kammer FVD, Hofmann T (2022) Rapid Analysis of Gunshot Residues with Single-Particle Inductively Coupled Plasma Time-of-Flight Mass Spectrometry. Forensic Science International 332: 111202.
- Houk RS, Fassel VA, Flesch GD, Svec HJ, Gray AL, et al. (1980) Inductively Coupled Argon Plasma as an Ion Source for Mass Spectrometric Determination of Trace Elements. Analytical Chemistry 52(14): 2283-2289.
- Koons R (1998) Analysis of Gunshot Primer Residue Collection Swabs by Inductively Coupled Plasma- Mass Spectrometry. J Forensic Sci 43(4): 748-754.
- Ulrich A, Moor C, Vonmont H, Jordi H-R, Lory M (2004) ICP-MS Trace-Element Analysis as a Forensic Tool. Analytical and Bioanalytical Chemistry 378(4): 1059-1068.
- Newlon N, Booker (1979) The Identification of Smokeless Powders and Their Residues by Pyrolysis Gas Chromatography. J Forensic Sci 24(1): 87-91.
- Kirkbride KP, Klass G, Pigou PE (1998) Application of Solid-Phase Microextraction to the Recovery of Organic Explosives. J Forensic Sci 43(1): 76-81.
- Bonnar C, Moule EC, Lucas N, Seyfang KE, Dunsmore RP, et al. (2020) Tandem Detection of Organic and Inorganic Gunshot Residues Using LC-MS and SEM-EDS. Forensic Science International 314: 110389.
- Northrop DM, Martire DE, MacCrehan WA (1991) Separation and Identification of Organic Gunshot and Explosive Constituents by Micellar Electrokinetic Capillary Electrophoresis. Analytical Chemistry 63(10): 1038-1042.
- Timerbaev AR, Semenova OP, Bonn GK (1994) Capillary Zone Electrophoresis of Lanthanoid Elements after Complexation with Aminopolycarboxylic Acids. Analyst 119(12): 2795.
- Timerbaev AR, Shpigun OA (2000) Recent Progress in Capillary Electrophoresis of Metal Ions. Electrophoresis 21(18): 4179-4191.
- McKeever C, Callan S, Warren S, Dempsey E (2022) Magnetic Nanoparticle Modified Electrodes for Voltammetric Determination of Propellant Stabiliser Diphenylamine. Talanta 238: 123039.
- Elgrishi N, Rountree KJ, McCarthy BD, Rountree ES, Eisenhart TT, et al. (2018) A Practical Beginner’s Guide to Cyclic Voltammetry. Journal of Chemical Education 95(2): 197-206.
- Chedid AA, Azevedo LS, Da Silva Galaço ARB, Casagrande TR, Serra OA (2023) Voltammetric Analysis of Luminescent Markers in Gunshot Residues. J Forensic Sci 68(3): 780-789.
- Mabbott GA (1983) An Introduction to Cyclic Voltammetry. Journal of Chemical Education 60(9): 697-702.
- Mohd Hashim NH, Mohd Zain Z, Jaafar MZ (2016) Copper Determination in Gunshot Residue by Cyclic Voltammetric and Inductive Coupled Plasma-Optical Emission Spectroscopy. MATEC Web of Conferences 59: 04005.
- Osteryoung JG, Osteryoung RA (1985) Square Wave Voltammetry. Analytical Chemistry 57(1): 101A-110A.
- Chedid AA, Azevedo LS, Da Silva Galaço ARB, Casagrande TR, Serra OA, et al. (2023) Voltammetric Analysis of Luminescent Markers in Gunshot Residues. J Forensic Scis 68(3): 780-789.
- Liu H, Lin W-F, Taylor L (1980) The Application of Anodic Stripping Voltammetry to Forensic Science I. The Construction of a Low-Cost Polarograph. Forensic Science International 16(1): 43-52.
- Copeland TR, Skogerboe RK (1974) Anodic Stripping Voltammetry. Analytical Chemistry 46(14: 1257A- 1268A.
- Harvey D (2000) Modern Analytical Chemistry, McGraw-Hill: Boston, United States.
- Harris DC, Lucy CA (2016) Quantitative Chemical Analysis (9th Edtn) W.H. Freeman & Company, United States.
- Haag MG, Haag, LC (2011) Some Useful Reagents and Their Application. In: Shooting Incident Reconstruction, Elsevier, pp.67-86.
- López-López M, García-Ruiz C (2014) Recent Non-Chemical Approaches to Estimate the Shooting Distance. Forensic Science International 239: 79-85.
- Martiny A, Campos APC, Sader MS, Pinto MAL (2008) SEM/EDS Analysis and Characterization of Gunshot Residues from Brazilian Lead-Free Ammunition. Forensic Sci Int 177(1): e9-e17.
- Berendes A, Neimke D, Schumacher R, Barth M (2006) A Versatile Technique for the Investigation of Gunshot Residue Patterns on Fabrics and Other Surfaces: m‐XRF. J Forensic Scis 51(5): 1085-1090.
- Latzel S, Neimke D, Schumacher R, Barth M, Niewöhner L (2012) Shooting Distance Determination by M-XRF—Examples on Spectra Interpretation and Range Estimation. Forensic Sci Inter 223: (1-3): 273-278.
- Van Den Hurk RS, Abdulhussain N, Van Beurden ASA, Dekker ME, Hulsbergen A, et al. (2022) Characterization and Comparison of Smokeless Powders by On-Line Two-Dimensional Liquid Chromatography. Journal of Chromatography A 167: 463072.
- Glattstein B, Vinokurov A, Levin N, Zeichner A (2000) Improved Method for Shooting Distance Estimation. Part 1. Bullet Holes in Clothing Items. J Forensic Sci 45(4): 801-806.
- Gagliano‐Candela R, Colucci AP, Napoli S (2008) Determination of Firing Distance. Lead Analysis on the Target by Atomic Absorption Spectroscopy (AAS). J Forensic Sci 53(2): 321-324.
- Freitas JCD, Sarkis JES, Neto ON, Viebig SB (2012) Identification of Gunshot Residues in Fabric Targets Using Sector Field Inductively Coupled Plasma Mass Spectrometry Technique and Ternary Graphs. J Forensic Sci 57(2): 503-508.
- Ananth V, Ahmad UK, Tong SM (2011) Detection of Organic Gunshot Residues for the Estimation of Firing Distance. Malaysian J Forensic Sci 2(1): 36-45.
- Andreola S, Gentile G, Battistini A, Cattaneo C, Zoja R (2011) Forensic Applications of Sodium Rhodizonate and Hydrochloric Acid: A New Histological Technique for Detection of Gunshot Residues: A New Histological Technique for Detection of Gunshot Residues. J Forensic Sci 56(3): 771-774.
- Geusens N, Nys B, Charles S (2019) Implementation and Optimization of the Sodium‐Rhodizonate Method for Chemographic Shooting Distance Estimation. J Forensic Sci 64(4): 1169-1172.
- Atwater CS, Durina ME, Durina JP, Blackledge RD (2006) Visualization of Gunshot Residue Patterns on Dark Clothing. J Forensic Scis 51(5): 1091-1095.
- Krishnan, SS (1967) Determination of Gunshot Firing Distances and Identification of Bullet Holes by Neutron Activation Analysis. J Forensic Sci 12(1): 112-122.
- Capannesi G, Ciavola C, Sedda AF (1993) Determination of Firing Distance and Firing Angle by Neutron Activation Analysis in a Case Involving Gunshot Wounds. Forensic Science International 61(2-3): 75-84.
- O’Mahony AM, Windmiller JR, Samek IA, Bandodkar AJ, Wang J (2012) “Swipe and Scan”: Integration of Sampling and Analysis of Gunshot Metal Residues at Screen-Printed Electrodes. Electrochemistry Communications 23: 52-55.
- Piraianu A-I, Fulga A, Musat CL, Ciobotaru O-R, Poalelungi DG, et al. (2023) Enhancing the Evidence with Algorithms: How Artificial Intelligence Is Transforming Forensic Medicine. Diagnostics 13(18): 2992.
- Matzen T, Kukurin C, Van De Wetering J, Ariëns S, Bosma W, et al. (2022) Objectifying Evidence Evaluation for Gunshot Residue Comparisons Using Machine Learning on Criminal Case Data. Forensic Science International 335: 111293.
- Oura P, Junno A, Junno J-A (2021) Deep Learning in Forensic Gunshot Wound Interpretation—a Proof-of- Concept Study. Int J Legal Med. 135(5): 2101-2106.






























