Dual Recovery of DNA and Fingerprints using Minitapes
Salem K Alketbi1,2*and Alsoofi Saif2
1University of Central Lancashire, UK
2General Department of Forensic Science and Criminology, UAE
Submission:June 01, 2022;Published:June 16, 2022
*Corresponding author:Salem K Alketbi, General Department of Forensic Science and Criminology, University of Central Lancashire, Preston, UK, Dubai Police, UAE
How to cite this article:Salem K Alketbi, Alsoofi Saif. Dual Recovery of DNA and Fingerprints using Minitapes. J Forensic Sci & Criminal Inves. 2022; 16(1): 555929 DOI:10.19080/JFSCI.2022.16.555929.
Abstract
Touch DNA and fingerprints are usually found on many items at crime scenes; thus, they are a powerful type of evidence to help investigators to link suspects to crimes committed. Dual recovery of Touch DNA and fingerprints has been previously investigated and proven to be challenging, therefore this study examined the possibility of collecting Touch DNA before fingerprint collection using Scene Safe FAST minitapes. The recovery of Touch DNA from the deposited fingerprints with minitapes (MT) was successful with all the collected samples producing full DNA profiles. Furthermore, the recovery of fingerprints was 90% successful from glass and 100% from stainless steel, with fingerprint recovery not impacted by the collection of DNA or the number of minitape lifts (p < 0.05).
Keywords: Forensic science; Trace DNA; Touch DNA; Fingerprint; DNA recovery; SceneSafe FAST minitape; DNA extraction; PrepFiler express BTA; AutoMate express; Quantifiler™ human DNA quantification kit; GlobalFiler™ PCR amplification kit
Introduction
Touch DNA and fingerprints are typically left at crime scenes and are useful to link the suspects to their crimes. Touch DNA usually affected by many variables [1-9], and often both can be collected from touched items such as tools, mobile phones, door or window handles, weapons etc, but it is challenging to collect both trace DNA and fingerprints when they are found on the same surface because the DNA collected from the fingerprints is often found in very small quantities [10]. Furthermore, the powder used to collect fingerprints can interfere and damage Touch DNA, thereby inhibiting DNA profiling. Similarly, fingerprint samples can also be destroyed with DNA recovery methods, such as swabbing or tape-lifting if they are done prior to fingerprinting visualisation. Dual recovery of Touch DNA and fingerprints have been previously investigated by swabbing after lifting fingerprints [11-12], therefore this study examined the possibility of collecting Touch DNA before fingerprint collection using minitapes.
Materials & Methods
Experimental setup & deposition
A participant, previously confirmed as a good shedder, was asked to clean their hands with antibacterial soap, avoid any type of activity for 5 minutes, then charge the fingers of both hands with eccrine sweat from behind their ears to load the fingers with enough DNA [2]. After a further 5 minutes, the participant was asked to touch the surfaces using their index, middle and ring fingers, then thumb by applying medium pressure on two types of surfaces for one minute. The same process was repeated for all the depositions. The test surfaces were non-porous stainless steel (SS; 7.5 x 5cm) and glass (G; 7.5 x 2.5cm) and they were sterilised before use with 2% Virkon (viricidal disinfectant) and ultraviolet radiation (UV) for 20 minutes.
DNA recovery & extraction
DNA samples were collected using SceneSafe FAST™ minitape (1-Tape Kit) (MT) and fingerprints were collected using Black Fingerprint Powder (EVIDENT). Touch DNA was collected in five different ways: one minitape lift (1L), three minitape lifts (3L), six minitape lifts (6L), nine minitape lifts (9L) and fifteen minitape lifts (15L). Fingerprints were collected after the different minitape lifts to determine whether the number of lifts can damage the fingerprint. Next, the lower sticky part of the minitapes was cut directly into extraction tubes and extracted using the PrepFiler Express BTA™ kit with AutoMate Express (using 460μl of lysis buffer instead of 230μl) following the manufacturer’s instructions, with a final elution of 50μl.
DNA quantification, amplification, and analysis
Extracted samples were quantified using the Quantifiler® Trio DNA Quantification Kit, QuantStudio 5 Real-Time PCR (qPCR) and HID Real-Time PCR analysis software v1.3 (Thermo Fisher Scientific) according to the manufacturer’s instructions. DNA amplification was performed using a GlobalFiler™ PCR Amplification Kit on an ABI GeneAmp® 9700 PCR System (Life Technologies, Foster City, CA) for 29 cycles. The PCR products were size-separated and detected on an ABI 3500 Genetic Analyzer (Life Technologies) using 1μl PCR product, 9.6μl Hi-Di™ formamide, and 0.4μl GeneScan™ 600 LIZ® Size Standard v2.0 (Thermo Fisher Scientific). Statistical analysis was performed with RStudio using factorial analysis of variance (ANOVA) and Microsoft Excel. Blanks were taken from surfaces after sterilisation and negative controls for the collection and extraction methods, all of which were DNA free when quantified and amplified.
Results & Discussion
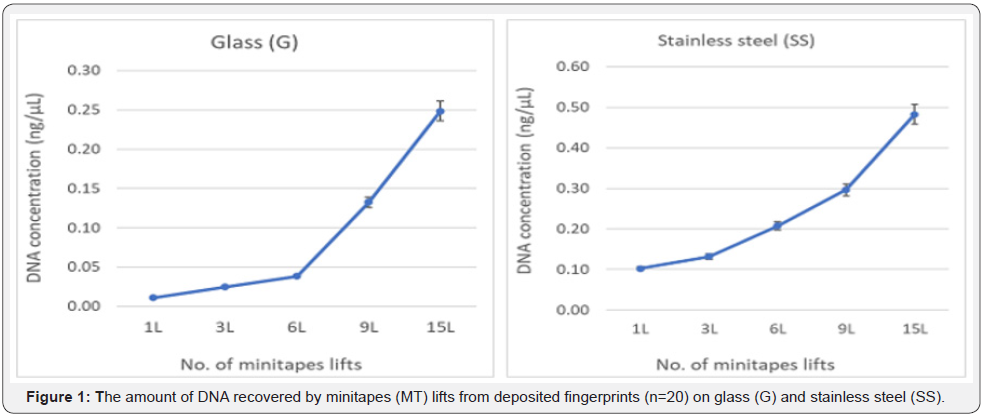
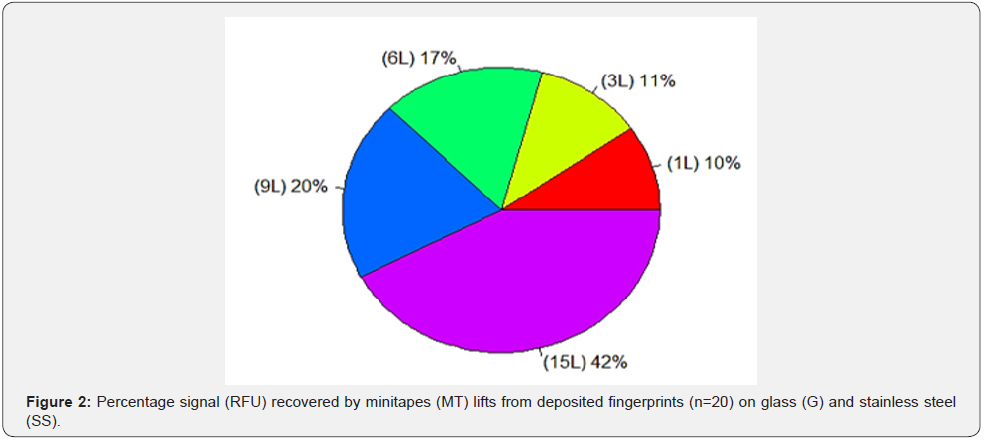
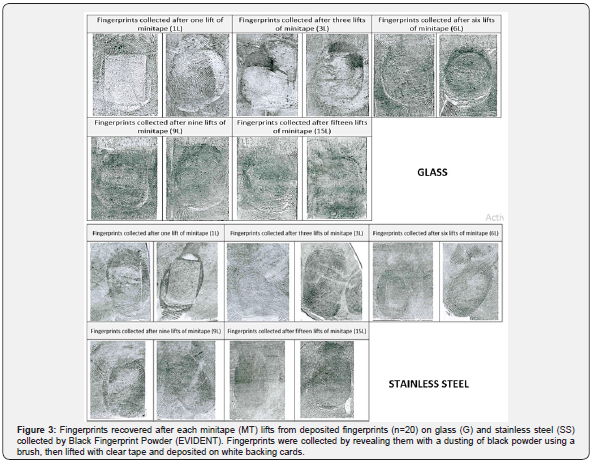
The recovery of Touch DNA from the deposited fingerprints with minitapes (MT) was 100% successful as all the collected samples produced full DNA profiles. The amount of Touch DNA collected was dependent on the surface area (p<0.05), with more DNA collected from the 7.5 x 5cm SS than the 7.5 x 2.5cm G surface (mean SS = 0.24ng/μl vs. G = 0.09ng/μl). Likewise, the amount of Touch DNA collected was affected by the number of minitapes (MT) lifts (p < 0.01), with the amount of collected DNA increasing with more MT lifts (Figure 1). Similarly, the average signal (RFU) was affected by the surface area (p<0.01) and the number of minitapes (MT) lifts (p < 0.01), with a higher average peak height for SS than G (RFU mean SS = 4788 vs. G = 2623). Also, the average peak height increased with the number of MT lifts (Figure 2). The recovery of fingerprints was 90% successful from the G and 100% from the SS surface (Figure 3). Fingerprints were not impacted by the collection of DNA or the number of minitape lifts (p > 0.05) when they were deposited by a participant considered to be a good shedder. Recognisable patterns and features were observed in most fingerprints for database comparison.
Conclusion
Dual recovery of Touch DNA and fingerprints is successful from distinct fingerprints deposited by good shedders on nonporous surfaces with the use of minitapes and Black Fingerprint Powder. The number of minitape lifts to collect Touch DNA does not affect the quality of the fingerprint if performed carefully and using low-medium pressure to avoid smearing the fingerprint. Further studies should be performed on other types of Touch DNA shedders to confirm these results.
Acknowledgement
This study was approved by the General Department of Forensic Science and Criminology in Dubai Police and ethical approval was granted by the University of Central Lancashire’s Research Ethics Committee (ref. no. STEMH 912).
References
- Alketbi SK (2018) The affecting factors of Touch DNA. Journal of Forensic Research 9: 424.
- Alketbi SK, Goodwin W (2019) The effect of surface type, collection, and extraction methods on Touch DNA. Forensic Science International. Genetics Supplement Series 7(1): 704-706.
- Alketbi SK, Goodwin W (2019) The effect of time and environmental conditions on Touch DNA. Forensic Science International. Genetics Supplement Series 7(1): 701-703.
- Alketbi SK, Goodwin W (2019) The effect of sandy surfaces on Touch DNA. Journal of Forensic, Legal & Investigative Sciences 5: 034.
- Alketbi SK, Goodwin W (2019) Validating Touch DNA collection techniques using cotton swabs. Journal of Forensic Research 10: 445.
- Alketbi SK (2020) Collection of Touch DNA from Rotten Banana Skin. International Journal of Forensic Sciences 5(4): 000204.
- Alketbi SK, Goodwin W (2021) Touch DNA collection techniques for non-porous surfaces using cotton and nylon swabs. Journal of Scientific & Technical Research 36(3): 28608-28612.
- Alketbi SK (2022) The Impact of Collection Method on Touch DNA Collected from Fabric. Journal of Forensic Sciences & Criminal Investigation 15(5): 555922.
- Alketbi SK, Goodwin W (2022) The Impact of Area Size and Fabric Type on Touch DNA Collected from Fabric. Journal of Forensic Sciences & Criminal Investigation 16(1): 555926
- Raymond JJ, van Oorschotc RAH, Gunn PR, Simon JW, Rouxa C (2009) Trace DNA success rates relating to volume crime offences. Forensic Science International: Genetics Supplement Series 2(1): 136-137.
- Alem L, Valentin ESB, Cunha MA, Santos OCL, Nogueira TLS, et al. (2017) Efficiency of DNA recovery from fingerprints enhanced with black and magnetic powders. Forensic Science International: Genetics Supplement Series 6: e490-e491.
- Alexander Sinelnikov, Karl Reich (2017) Materials and methods that allow fingerprint analysis and DNA profiling from the same latent evidence. Forensic Science International: Genetics Supplement Series 6: e40-e42.






























