The Impact of Collection Method on Touch DNA Collected from Fabric
Salem K Alketbi1,2*
1University of Central Lancashire, Preston, UK
2General Department of Forensic Science and Criminology, Dubai Police, Dubai, UAE
Submission:March 04, 2022;Published:April 08, 2022
*Corresponding author: Salem K Alketbi, University of Central Lancashire, Preston, UK and General Department of Forensic Science and Criminology, Dubai Police, Dubai, UAE
How to cite this article: Salem K A. The Impact of Collection Method on Touch DNA Collected from Fabric. J Forensic Sci & Criminal Inves. 2022; 15(5): 555922. DOI: 10.19080/JFSCI.2022.15.555923.
Abstract
Trace DNA, widely known as Touch DNA, is a type of DNA evidence commonly found at crime scenes, and it is frequently used to link suspects to crimes committed but it is a more challenging type of DNA evidence compared to other biological samples. This study investigated the influence of collection method and extraction type on Touch DNA collected from fabric. The amount of collected Touch DNA from the fabric was significantly affected by collection type (p < 0.05), with Scene Safe Fast™ minitape (K545) being more efficient than Copan cotton swab (150C) and Copan nylon flocked swab (4N6FLOQSwabs®) on recovering touch DNA from fabric.
Keywords: Forensic Science; Trace DNA; Touch DNA; DNA Recovery; Cotton Swab; Nylon Swab; Scene Safe Fast Minitape; DNA Extraction; QIAamp DNA Investigator Kit; Prep Filer Express BTA; Automate Express; Quantifiler™ Human DNA Quantification Kit; Global Filer™ PCR Amplification Kit
Abbreviations: DNA: Deoxyribonucleic Acid; UV: Ultraviolet Radiation; CS: Cotton Swab; NS: Nylon Swab; MT: Mini Tapes
Introduction
Trace DNA, widely known as Touch DNA, is a type of DNA evidence commonly found at crime scenes, and it is frequently used to link suspects to crimes. Often it is collected from many commonly used surfaces such as tools, door handles, clothes, etc. [1-3] but it is a more challenging type of DNA evidence compared to other biological samples as the surface type [4], environmental factors [5,6], collection methods [2,4], collection techniques [7,8] and extraction kits [4] can influence the amount of collected Touch DNA. Collection methods such as a cotton swab, nylon swab and tapes are often used to collect Touch DNA and previous studies showed that different types of surfaces require different collection methods [1,4]. Therefore, this study investigated the influence of the collection method and extraction type on Touch DNA collected from fabric.
Materials and methods
Experimental setup and deposition
A fabric composed of 65% polyester and 35% cotton fabric was selected for this study as it is a popular synthetic material used in the fashion industry [9] (Figure 1). The fabric was cut into 5 x 7 cm pieces for easier DNA deposition and collection. For the DNA deposition, a participant was requested to wash their hands with antibacterial soap, cease from activity for 10 minutes, then rub a fabric piece (5 x 7 cm) for 1 min between both hands. This procedure was repeated for each deposition. The fabric surfaces were washed at 50°C, dried and sterilized before use with ultraviolet radiation (UV) for 25 minutes.
DNA recovery and extraction
Samples were collected using a Copan cotton swab (150C) (CS) moistened with 100μL of sterile distilled water applied using a plastic spray bottle technique [7], Copan nylon flocked swab (4N6FLOQSwabs®) (NS) moistened with 30μL of sterile distilled applied by pipette as recommended by the manufacturer, and Scene Safe Fast™ minitape (K545) (MT). No water was added to the MT but to increase the amount of Touch DNA collected, each minitape was applied 16 times to the area [4]. Samples collected with CS, NS and MT were cut directly into extraction tubes for extraction using the Prep Filer Express BTA™ kit with Automate Express (using 460μL of lysis buffer instead 230μL) (EXT1) according to the manufacturer’s instructions and manually using the QIAamp® DNA Investigator Kit (Qiagen) (EXT2) as per the manufacturer’s protocol. For the extraction, full swab heads were used for CS and NS, and the lower sticky part of the minitape, with a final elution volume of 50μL.
DNA quantification, amplification, and analysis
Extracted samples were quantified using the Quantifiler® Trio DNA Quantification Kit, Quant Studio 5 Real-Time PCR (qPCR) and HID Real-Time PCR analysis software v1.3 (Thermo Fisher Scientific) according to the manufacturer’s instructions. Amplification of the samples was performed using the Global Filer™ PCR amplification Kit on an ABI GeneAmp® 9700 PCR System (Life Technologies, Foster City, CA) for 30 cycles, following the manufacturer’s recommended conditions. Amplified products were size-separated and detected on an ABI 3500 Genetic Analyzer (Life Technologies) using 1μl PCR product, 9.6μl Hi-Di™ formamide, and 0.4μl Genescan™ 600 LIZ® Size Standard v2.0 (Thermo Fisher Scientific). Statistical analysis was performed with RStudio using factorial analysis of variance (ANOVA) and Microsoft Excel.
Results and Discussion
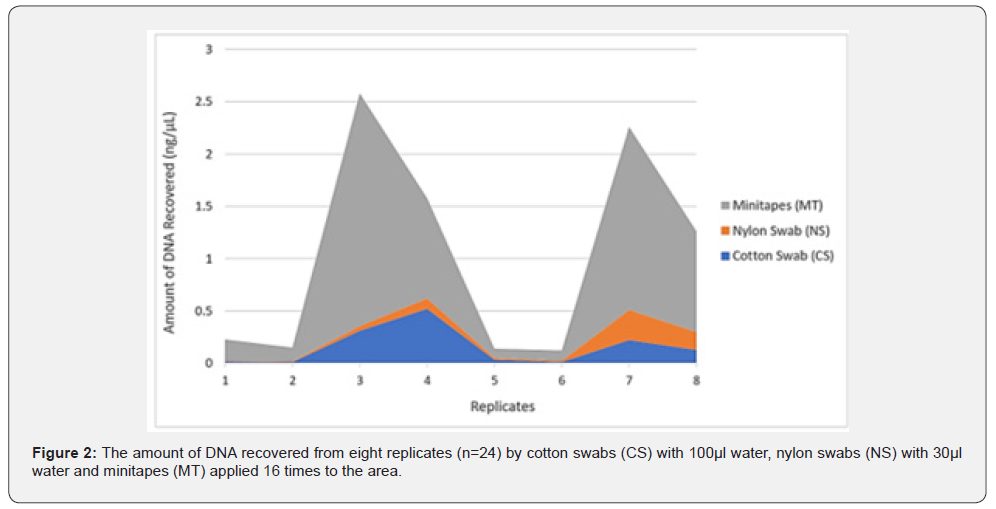
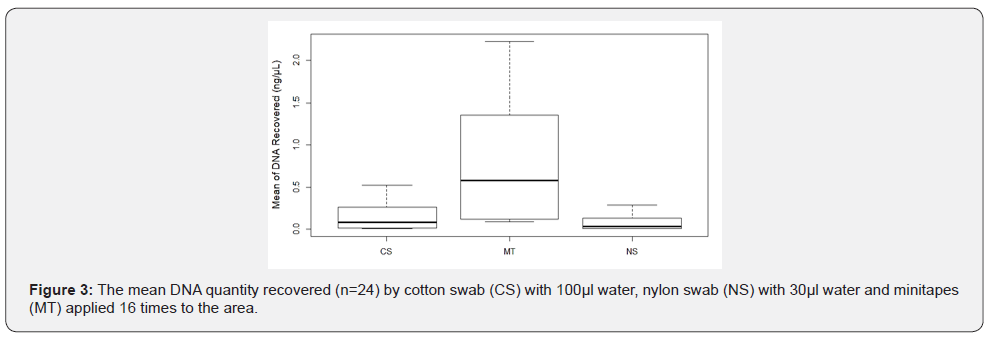
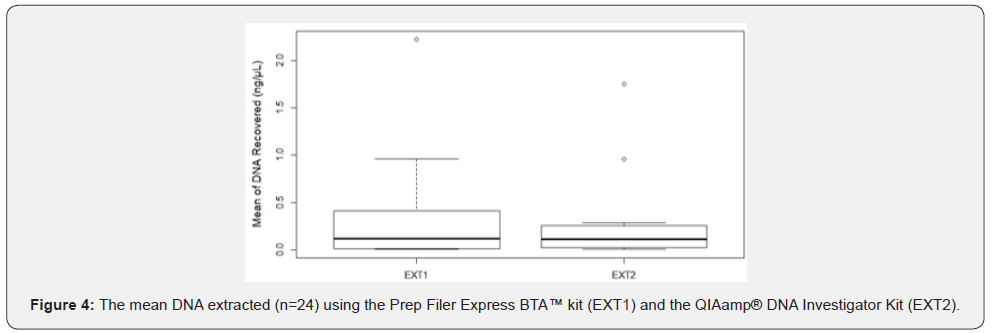
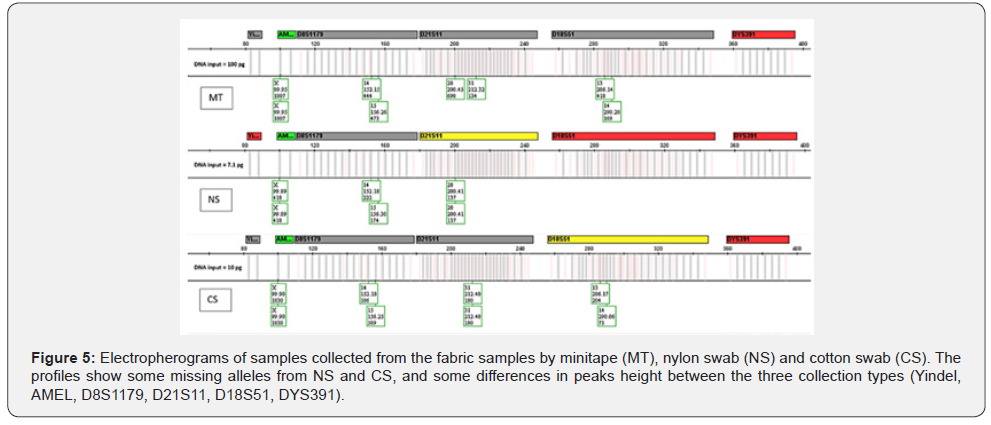
The amount of collected Touch DNA from the fabric was significantly affected by collection type (p < 0.05), with minitape (MT) being more efficient than a cotton swab (CS) and nylon swab (NS) to recover Touch DNA from fabric samples (5 x 7 cm, 65% polyester and 35% cotton) (Figure 2 & 3). Similarly, previous studies reported that the use of minitapes can be more efficient than other collection methods from other types of porous surfaces such as paper [4]. Samples collected by the three collection methods were not affected by extraction type (p > 0.05) when 460μL of lysis buffer was used with EXT1 instead of 230μL (Figure 4). A previous study by Alketbi [4] investigated the interaction between the collection method and extraction of the three collection methods used in this study and suggested that the Prep Filer Express BTA™ kit is more effective on samples collected by minitapes than the QIAamp® DNA Investigator Kit. Some Touch samples collected from the samples were amplified to validate their quality. All the samples collected by MT produced full STR Profiles, whereas half of the samples collected by NS and CS produced full STR Profiles and some produced partial STR Profiles (Figure 5). Blanks were taken from surfaces after sterilization and negative controls for the collection and extraction methods, all of which proved negative for DNA when quantified and amplified.
Conclusion
Collection of Trace DNA from clothes can be impacted by the type of collection method used, with the use of tape, such as minitape, being more effective for recovering DNA from porous surfaces like fabric.
Conflict of interest
None.
Acknowledgement
This study was approved by the General Department of Forensic Science and Criminology in Dubai Police and ethical approval was granted by the School of Forensic and Applied Sciences, and the University of Central Lancashire’s Research Ethics Committee (ref. no. STEMH 912). Many thanks to COPAN DIAGNOSTICS INC. for supporting this experiment with free swabs and to ThermoFisher Scientific™ for product discounts.
References
- Alketbi SK (2018) The affecting factors of Touch DNA. Journal of Forensic Research 9(3): 424.
- Verdon TJ, Mitchell RJ, Oorschot RA (2014) Swabs as DNA collection devices for sampling different biological materials from different substrates. Journal of Forensic Science 59(4): 1080-1089.
- Alketbi SK (2020) Collection of Touch DNA from Rotten Banana Skin. International Journal of Forensic Sciences 5(4): 204.
- Alketbi SK, Goodwin W (2019) The effect of surface type, collection, and extraction methods on touch DNA. Forensic Science International Genetics Supplement Series 7(1): 704-706.
- Alketbi SK, Goodwin W (2019) The effect of time and environmental conditions on Touch DNA. Forensic Science International. Genetics Supplement Series 7(1): 701-703
- Alketbi SK, Goodwin W (2019) The effect of sandy surfaces on Touch DNA. Journal of Forensic Legal & Investigative Sciences 5: 34.
- Alketbi SK, Goodwin W (2019) Validating Touch DNA collection techniques using cotton swabs. Journal of Forensic Research 10(3): 445.
- Alketbi SK, Goodwin W (2019) Touch DNA Collection Techniques for Non-Porous Surfaces Using Cotton and Nylon Swabs. Journal of Scientific & Technical Research 36(3): 28608-28612.
- Textile Exchange (2017) Preferred Fiber Material Market Report.






























