Recombinant Human Zona Pellucida 2 Protein was not Detected to Bind to the Non-Viable Spermatozoa in Vitro
Xinrui Yang1,2, Zhixin Huo1,3 and Hao Pang1*
1Department of Forensic Genetics and Biology, China Medical University, China
2School of Forensic Medicine, Wannan Medical College, China
3Department of Forensic Medicine, Inner Mongolia Medical University, China
Submission:January 21, 2021;Published:February 08, 2021
*Corresponding author:Hao PANG, School of Forensic Medicine, Department of Forensic Genetics and Biology, China Medical University, Shenyang 110122, PR China
How to cite this article:Xinrui Y, Zhixin H, Hao P. Recombinant Human Zona Pellucida 2 Protein was not Detected to Bind to the Non-Viable Spermatozoa in Vitro. J Forensic Sci & Criminal Inves. 2021; 15(1): 555905 DOI:10.19080/JFSCI.2021.15.555905.
Abstract
Zona pellucida (ZP) binds to spermatozoa is the first step for sperm-egg interaction. ZP2, one of four human ZPs, is considered as a secondary zona ligand during fertilization to be related to acrosome exocytosis and block to polyspermy. Various studies have been performed employing either purified native or recombinant proteins to gain insight into the role of ZP2 with the capacitated spermatozoa. In the current study recombinant human ZP2 (rhZP2) was first produced by overexpression in HEK293 cell, and the overexpressed rhZP2 was next purified. Presence of the rhZP2 was identified by Western blotting analysis. The effect of the rhZP2 binding to non-viable spermatozoa was assessed by indirect immunofluorescence and ELISA. After the non-viable spermatozoa were incubated with the rhZP2 in vitro, the interactions reflected by their dependent fluorescent labeling were not observed under microscope. The rhZP2 present in resultant supernatant was not significantly reduced by ELISA analysis after the rhZP2 was reacted with spermatozoa. Further, there was no relation found between spermatozoon number or incubation time and the amount of rhZP2 bound to the non-viable spermatozoa. The present results provide experimental evidence that the rhZP2 has not shown a character binding to non-viable spermatozoa in vitro, suggesting that the capacitated spermatozoa have still been preferentially employed in fertilization. An insufficiency of direct hit interaction, as a ligand vs an acceptor, between the rhZP2 and non-viable spermatozoa implies that the rhZP2 has not applicability in separation of spermatozoa from the mixtures with vaginal fluid in forensic cases.
Keywords: Recombinant, ZP2, Purified rhZP2, Capacitation, Non-viable spermatozoa, Binding
Introduction
Zona pellucida (ZP) is a kind of glycoprotein matrix secreted by oocytes and granulosa cells that is essential for mammalian fertilization. In human, the extracellularly glycoproteinaceous coat is composed of four ZPs, ZP1-4. Reproductive studies prove that ZP glycoproteins have a major part to play in specific recognition of the spermatozoa to the oocyte and subsequent acrosome reaction of spermatozoa. In addition, ZPs can also prevent the formation of non-viable polypoid embryos against polyspermy and protect the embryo development [1]. Human ZP2 glycoprotein is one member of the ZP glycoprotein complex. Its 745 amino acid encoded by the Zp2 gene on chromosome 16 contain all structural domains of ZP protein family [2]. The 34 amino acids of N-terminal are first cleaved and secreted as a signal peptide, the remaining polypeptide as a functional protein is effused into the extracellular matrix. A transmembrane region of ZP2 protein distributes on the carboxyl terminal side and its neighbored amino acid sequence can be recognized by furin. ZP2 has been implicated as a secondary spermatozoon ligand that binds spermatozoon only after the induction of the spermatozoa acrosome reaction and is modified by the acrosome reaction to undergo a proteolytic cleavage. Spermatozoon binding to ZP2 is necessary for successful mammalian fertilization. Further studies on the molecular basis of gamete recognition have indicated that the N terminus of ZP2 is a key target region for spermatozoon binding. Recombinant amino acid residues 39-154 of human ZP2 produced in baculovirus can in vitro bind to their cognate spermatozoon [3]. These results not only cumulate experimental data for the role of ZP2 in mediating spermatozoon binding to the ZP and but also support human sperm-egg recognition with dependent on ZP2 involvement [4].
In mammalian, spermatozoon capacitation leads to its movement hyperactivated and prepares the spermatozoon to go through acrosome reaction. At molecular level, cholesterol depletion from the spermatozoon plasma membrane associated with spermatozoon capacitation, which quickens membrane fluidity, modulates intracellular ion concentrations [5], and hyperpolarizes the spermatozoon plasma membrane [6]. During mammalian fertilization, spermatozoa must pass through the female reproductive tract to capacitate or in vitro may be capacitated by incubation with chemical analog, and then the capacitated spermatozoon is able to bind to the ZP. In contrast to the sperm-egg binding investigations, which the capacitated spermatozoa were overwhelmingly employed to explore its interactions with ZP glycoproteins during fertilization in humans, the incapacitated and non-viable ones were rarely exploited. With hamster gametes in vitro, it was founded that an incapacitated spermatozoon would bind specifically to ZP of the same species. The examination from the binding of ZP3 to incapacitated mouse spermatozoon indicates that maturation and capacitation of spermatozoon are prerequisites for spermatozoon to expose its ZP3 binding sites [7]. The results from human spermatozoa-ZP recognition in vitro revealed that uncapacitated spermatozoa had an obviously lower ZP-binding capacity when compared with the capacitated ones [8,9]. In human, primary spermatozoon receptors interacting with ZP3 and ZP4 located in the surface of the intact spermatozoon acrosome, whereas secondary spermatozoon receptors binding to ZP2 were only exposed after the acrosome reaction [9,10]. Most models of gamete recognition suppose that a single ligand in the extracellular ZPs interacts with a spermatozoon surface receptor [11,12]. Although the ligand can bind a cell surface receptor and trigger relative downstream signaling cascades resulting in diverse cellular responses, it may be a showing rationality that the interaction between the ligand and receptor is experimentally evaluated at the protein level in vitro. In addition, except for the potential application in genesiology, it is a very meaningful exploration for ZPs-spermatozoa binding in vitro to separate spermatozoa from the mixtures with vaginal fluid in forensic cases.
A biologically active synthetic ZP, including recombinant human ZP proteins (rhZPs), would be distinguishingly useful as ligands for investigating spermatozoon-ZP recognitions. Thus, the purpose of this study was to evaluate the non-viable human spermatozoon binding capacity to rhZP2 in vitro.
Materials and Methods
Construction of rhZP2 vector and its expression in eukaryocyte
The cDNA encoding full-length human ZP2 (NM_003460.2) was firstly synthesized, and then cloned into the pcDNA 3.1(+)/6His expression vector, which contains an additional C-terminal 6-histidine (6×His) tag (Produced by Taihe Biotechnology, Beijing, China). The recombinant vector and a negative control were transfected into the human embryonic kidney cell line 293 (HEK293) cells. After the HEK293 cells were harvested, and finely crushed, the resultant lysates were stored at -80℃ until used either for identification or protein purification of the rhZP2.
Detection of recombinant proteins by western blotting
The cell lysates were tested for the presence of the rhZP2 proteins. The samples were mixed with a reducing sample buffer, boiled for five minutes. Proteins were separated by SDSpolyacrylamide gel electrophoresis (SDS-PAGE) and transferred to 0.45mm nitrocellulose (Merck Millipore Bioscience). Membranes were blocked with 5% skim milk in Tris buffered saline containing 0.05% Tween-20 (TBST) for 1h at room temperature. The anti-His monoclonal antibody (1:5000, TransGen Biotech, Beijing, China) and anti-ZP2 polyclonal antibody (1:5000, Invitrogen, Carlsbad, California) were diluted in TBST as the primary antibody, respectively. Second antibody, goat anti-mouse IgG-HRP or goat anti-rabbit IgG-HRP, was used to incubate the corresponding membrane. After washing, the immunoreactive proteins were visualized with enhanced chemiluminescence (TransGen Biotech, Beijing, China).
Purification of rhZP2 protein
The expressed rhZP2 protein was with affinity purified using the 6×His tag binding capacity of the Ni-NTA agarose resin (Merck Millipore Bioscience). The cell lysate was mixed with preequilibrated Ni-NTA resin and incubated overnight. After extensive washing with the buffer containing 10mmol/L Tris-HCl (pH 8.0), 0.3mol/L NaCl, and 5mmol/L imidazole, the resins were resuspended in the washing buffer. The rhZP2 was eluted off by a series of imidazole solutions with different concentrations. The eluted solutions, which the rhZP2 showed higher concentration characterized by Western blot, were together dialyzed against 10mmol/L Tris-HCl (pH 8.0) and 0.3mol/L NaCl buffer. The collected solution through dialysis was condensed in Ultrafree-15 Centrifugal Filter Units (MW 10kDa cut off, Millipore, MA, USA). The above finalized rhZP2 was in quality and quantity assessed by Western blots.
Spermatozoon samples preparation
Freshly ejaculated semen was obtained by masturbation from three healthy normozoospermic donors after 3 to 4 days of abstinence, following the clearance from Human Biosafety and Ethical Committee in China Medical University (2014/062). Ejaculates could liquefy at 37℃ for 30 minutes and were assessed using standard methods (World Health Organization, 2001). Samples were centrifuged, and resulting pellets were washed with PBS. After washing, spermatozoon concentration was adjusted, either immediately or later using as a non-viable spermatozoon sample, for forthcoming experiments [13].
Indirect immunofluorescence on non-viable spermatozoon
A reaction system containing 5ng of purified rhZP2 and approximately 105 non-viable spermatozoa in PBS buffer was incubated at 37℃ for 1 h, 3 h, and 6 h, respectively. After centrifugation at 800 × g for 5min, the supernatant was stored at -70℃ for the following competitive inhibition assay. The precipitated spermatozoa were firstly washed twice times with PBS, and then fixed on slide with immunostaining fix solution (Beyotime Institute of Biotechnology, China) for 10min at room temperature. After washing, the slide was incubated in immunostaining blocking buffer (Beyotime Institute of Biotechnology, China) for 60min at room temperature, and then reacted with anti-ZP2 polyclonal antibody (1:100; Invitrogen, Carlsbad, California) for 1h at room temperature. Washing in PBS again, the spermatozoa on the slide were incubated in Alexa Fluor 488-conjugated goat anti-rabbit IgG (1:100, sino biological) for 30min at room temperature. The images were acquired using an invert fluorescence microscope (Leica, Germany).
Enzyme-linked immunosorbent assay (ELISA)
Purified rhZP2 was coated onto a 96-well microtiter plate in a final concentration of 0.039ng, 0.078ng, 0.156ng, 0.313ng, 0.625ng, 1.25ng, 2.5ng, and 5ng per well, respectively, and then incubated overnight at 4℃. After blocking with PBS containing 3% BSA for 2h at 37℃, whole wells were washed three times with 200μl of PBST. Next, the wells were incubated with anti-His monoclonal antibody (1:1000 in PBST) or anti-ZP2 polyclonal antibody (1:2000 in PBST) for 1h at 37℃, followed incubation by goat anti-mouse IgG-HRP or goat anti-rabbit IgG-HRP secondary antibody (1:500 in PBST) for 1h at 37℃. After the wells were washed, and subsequently exposed to single component TMB colorant for 30min at room temperature and terminated by 1M sulfuric acid solution. Absorbance at 405nm was determined with a microplate reader (M2, Molecular Devices, USA). The wells without rhZP2 loaded and subjected to the same procedures were used as background wells [14].
Statistical analysis
Data in this study were collected from at least three repeat experiments and presented as means ± standard deviation (SD). The comparisons between experimental and control groups were analyzed with Student’s t-test. P value less than 0.05 was used to determine significant difference.
Results
Characterization of rhZP2 expressed in HEK293 cells and its purification
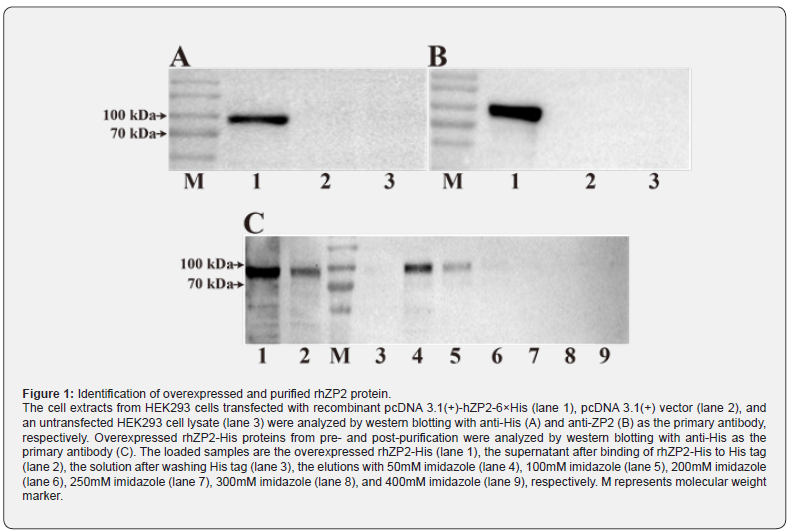
A synthesized 2253 bp DNA product, including whole human ZP2’s coding region and 6 histidine sequences, was cloned into pcDNA3.1(+) expression vector. This pcDNA3.1(+)-rhZP2-6×His plasmid was overexpressed in HEK293 cells to obtain recombinant fusion protein. Western blotting analysis of the cell lysate from each transfectant was shown in (Figure 1A & 1B) confirming the identity of rhZP2-His fusion protein by their cross-reactivity using an anti-ZP2 or anti-His antibody. The results showed that a band with molecular weight of approximate 96kDa was equally recognized by two specific antibodies. Molecular weight of the rhZP2-His protein in the current study is higher than the one expressed in Sf9 cell and lower than the one expressed also in HEK293 cell [14,15], suggesting that the slight differences might have generated from cell line difference or posttranslational modification. Purification of rhZP2-His protein using Ni-NTA agarose resins resulted in a major protein band at 96kDa in a few lanes (Figure 1C). The quantity of the protein was estimated by gray value of band with imageJ software. The purification efficiency for the rhZP2-His protein was about 15% in the current study. After collecting all elution with rhZP2-His protein, dialysis and concentration, the working solution of the purified rhZP2-His was successfully obtained.
Non-viable spermatozoon binding ability to the rhZP2
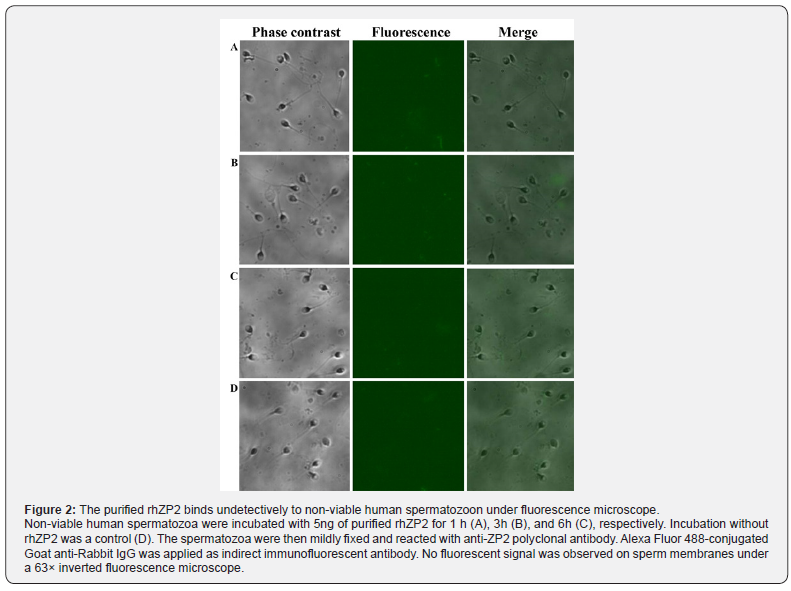
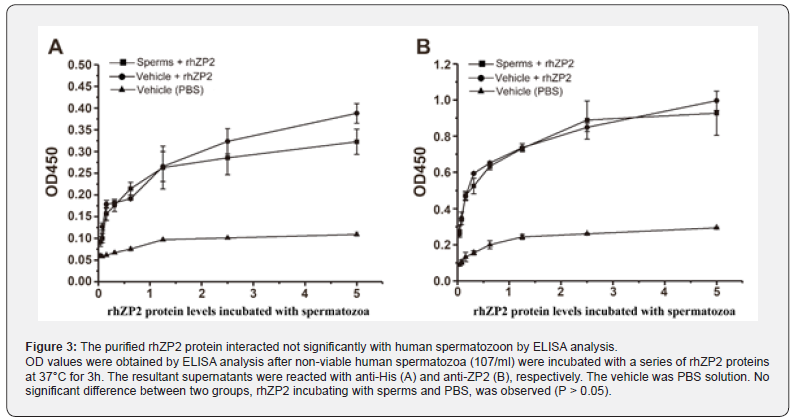
To explore a direct hit interaction as ligand vs receptor in vitro, a binding ability of the rhZP2 to non-viable human spermatozoon membrane was determined by immunofluorescence. As shown in Figure 2, after the non-viable spermatozoa and rhZP2-His protein was incubated at 37℃ for 1h, 3h, and 6h, respectively, there was not any visible fluorescence, an indicator produced from fluorescent-label secondary antibody reacting specifically with anti-ZP2, observed on the non-viable spermatozoon membrane compared with control without rhZP2-His protein incubation (Figure 2A-2D). In a competitive inhibition experiment, the absorption values that the rhZP2-His protein was incubated with vehicle (PBS) increase gradually with increasing concentration by ELISA analysis (Figure 3A & 3B). However, under the same condition, the absorption values that the rhZP2-His protein was incubated with non-viable spermatozoa did not show significant differences compared with ones from the rhZP2-His protein incubating with vehicle (P > 0.05). The results suggested that the rhZP2 didn’t exhibit a competence binding to non-viable human spermatozoon in vitro.
Effects of spermatozoon number and incubation time on the rhZP2 binding spermatozoa
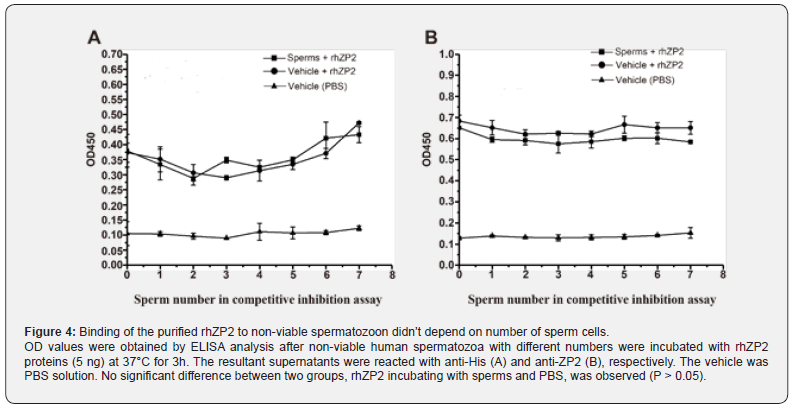
To examine whether binding capability of the rhZP2 on non-viable spermatozoon could be improved, loading different spermatozoon numbers and selecting incubation time course were designed in the current competitive inhibition assay. The results showed that the absorption values in different numbers of the non-viable spermatozoon (101 - 107/each well) had not significant changes by ELISA analysis with either anti-His (Figure 4A) or anti-ZP2 (Figure 4B) (P > 0.05). In a time, course of the quantified rhZP2-binding spermatozoon, the detectable levels based on absorption values by ELISA analysis with either anti- His (Figure 5A) or anti-ZP2 (Figure 5B) didn’t show meaningfully increasing over time either (P > 0.05). Thus, neither existence of a spermatozoon number-dependent nor incubation timedependent manner indicated further that the rhZP2 didn’t possess an ability to binding non-viable human spermatozoon in vitro.
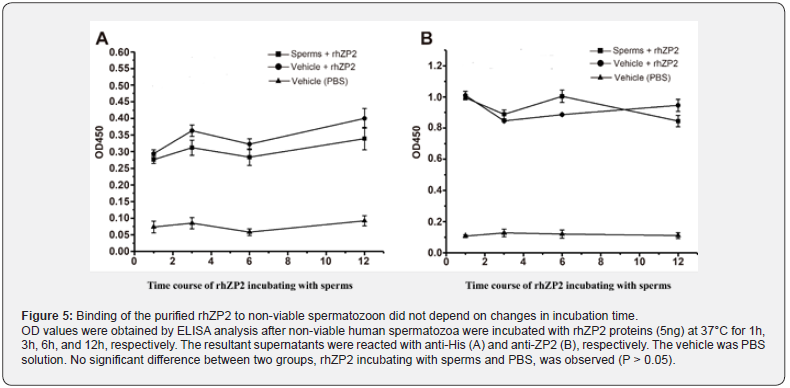
Discussion
With the accumulation of experimental data from both in vivo and in vitro studies, further insights on the ZPs’ role during fertilization have been deeply harvested by either purified native or recombinant ZP proteins as a gamete because it may be a prerequisite that the proper ZPs molecules are recognized by the spermatozoa in fertilization. Two main functions analyzed by recombinant ZPs, which are attributed to their binding characteristics to spermatozoa, are active in promoting acrosomal exocytosis (AE) and in inducing alterations in motility patterns. The results proved amply that the rhZPs could be valuable to study the interaction between spermatozoon and ZPs [16,17]. ZP1 mainly provides stability and structural integrity to the ZP matrix by cross-linking to ZP2-ZP3 heterodimers [18]. A research showed also that the ZP1 could bind to the acrosomal cap of the capacitated human spermatozoa and a dose dependent increase in AE was observed when capacitated spermatozoa were incubated with rhZP1 [19]. Although the overexpressed rhZP2 did not bind to the capacitated human spermatozoon, they were able to bind to the equatorial region of acrosome-reacted spermatozoon [20]. Employing recombinant peptides, the 51-149 amino acid residues in N-terminus of ZP2 were identified as a real binding site of ZP2 to human spermatozoon [3]. The ZP2 region is also related to human gamete recognition and penetration through the ZP matrix [3,4]. Overexpressed rhZP3 may bind to anterior head and equatorial region of the capacitated spermatozoa, while acrosome-reacted spermatozoon shows its binding with the rhZP3 primarily in the equatorial region [20]. In addition, rhZP3 binding spermatozoon has dose-dependent increase in the AE ([16,20,21]. Interestingly, rhZP4 also binds to the acrosomal cap and equatorial region of the capacitated human spermatozoon, showing similar binding profile as observed in rhZP3. In contrast to ZP3, the major binding profile of ZP4 was observed on the acrosomal cap. However, once spermatozoon goes to stage of acrosome reaction, its binding with ZP4 was restricted to only equatorial region [20]. Whatever the expressed recombinant ZPs were produced in either prokaryocyte or eukaryocyte, all showed to some extent binding characteristics to spermatozoon in vitro. Although real binding mechanisms between two haploid gametes based on all ZPs interacting with spermatozoon have still unknown, the recombinant individual and complex ZPs proteins as an in vitro experiment tool have played a significant role in whole fertilization progression. The role played by individual ZP2 protein during sperm-egg binding has been largely studied but still remains as unknown and controversial issues. Different binding characteristics and sites to spermatozoa were observed under the condition of applying overexpressed and native hZP2 respectively [8,20]. The binding of capacitated human spermatozoon to a protein band, which is specific rhZP2 separated by electrophoresis and immobilized on a membrane, suggested that the rhZP2 may be loosely recognized by spermatozoon in vitro [13]. Furthermore, the binding of rhZP2 proteins to capacitated human spermatozoa in a dosedependent manner was also reported. Thus, the previous studies showed that the ability of rhZP2 to bind to spermatozoon might depend on spermatozoon’s capacitation or acrosome-reacted status. Another informative point was the rhZP2 could bind to human spermatozoon in vitro although the binding sites were detected on different regions of spermatozoon, implying that application of recombinant ZP2 on fertilization has its potential value for validation of sperm-zona interaction. In viewpoint of genesiology, two main episodes of in vitro fertilization are the spermatozoa’s capacitation and acrosome reaction, which lead to spermatozoon’s structural and functional alteration. Therefore, almost research associated with ZP-sperm interaction have been performed under the condition with the capacitated spermatozoa, although the uncapacitated ones were uncommonly employed [22]. The nature of capacitation and its relationship to important events of fertilization have not been elucidated unambiguously so far. Spontaneous AE of spermatozoon can be considered as a true physiological event (Gupta, 2015). The stimulation of AE by ZPs could regulate the appropriate timing of events during fertilization [23]. However, considering ZP glycoproteins have been proposed as ligands for spermatozoon (also call ZPs as receptor) [19,24], a direct interaction between rhZPs and non-viable spermatozoon has not been reported yet. Thus, there is considerable interest in developing new strategies to decode receptor (spermatozoon)- ligand (egg) affinity which will advance our understanding of in vitro fertilization and improve assisted human reproduction and separation of spermatozoa from mixed vaginal fluid in forensic caseworks.
A ligand-receptor recognition is customarily described as its interaction at the molecular level on membranes. The basic principles of mechanistic interaction have been well understood and a variety of models have currently been developed [25]. A direct hit between a free ligand and receptor target on membrane is probably an event [26]. ZP2, as a spermatozoon ligand as described above, moves to recognize its receptor on the spermatozoa membrane [27]. But the identity of possible counterpart molecules (receptor) of ZP2 on spermatozoon remains elusive [28,29]. The emergence of fluorescence-based methods represents a potentially higher throughput approach to assess unlabeled ligand kinetics [30]. In addition to a direct molecular interaction of a ligand with the binding site of a receptor, competition association binding experiments were used to determine an affinity [31]. In the current study, the indirect immunofluorescence and competitive inhibition assays were used to test binding status of rhZP2 (ligand)-non-viable spermatozoon (receptor) in vitro. However, neither specific immunofluorescence on the membranes of non-viable spermatozoa nor the reduction of absorption values by absorption-inhibition was observed. Even no, any significant improvements were followed in spermatozoon number- and binding time-dependent manners. The present results demonstrated that the binding of rhZP2 to non-viable human spermatozoa was undetectable, differing from those to either capacitated or incapacitated human spermatozoa as receptors reported previously [13]. In contrast to the competitive experiment based on rhZP2-non-viable spermatozoon absorption-inhibition, about 20% inhibition rate from the rhZP3 as ligand was observed previously (our unpublished data). Thus, in combination with the previous and present data, it is speculated that there are three concerns on the rhZP2-non-viable spermatozoon binding in in vitro experiment. One of them is that the capacitation and AE of spermatozoon might have not been neglected yet and might have been a prerequisite at least under rhZP2 binding to spermatozoon. Second one is that an interaction between rhZP2 and spermatozoon may depend on a matching on conformation and functional alteration. Consequently, a complete explanation may be more rationale when the corresponding identity on spermatozoon membrane to interact with ZP2 is disclosed and 3D protein structure of ZP2 is obtained. Third one is that both the rhZP2 and the antibody generated from the rhZP2 may be valuable tools for investigating fundamental aspects of fertilization and development of ZP2-based contraceptive vaccine. But the rhZP2 has been considered insufficient for separating spermatozoon from mixture with vaginal fluid, because forensic DNA analysis of sexual assault evidence requires separation of DNA from epithelial (victim) and spermatozoon (perpetrator) cells [32-38].
Acknowledgement
We are thankful to all the volunteers, who donated spermatozoa sample in this research. This work was supported by National Natural Science Foundation (NSF) of China Grant Number 81471826.
References
- Fahrenkamp E, Algarra B, Jovine L (2020) Mammalian egg coat modifications and the block to polyspermy. Mol Reprod Dev 87:326-340.
- LF Liang, J Dean (1993) Conservation of mammalian secondary sperm receptor genes enables the promoter of the human gene to function in mouse oocytes. Dev Biol 156: 399-408.
- Avella MA, Baibakov B, Dean J (2014) A single domain of the ZP2 zona pellucida protein mediates gamete recognition in mice and humans. J Cell Biol 205: 801-809.
- Baibakov B, Boggs NA, Yauger B, Baibakov G, Dean J (2012) Human sperm bind to the N-terminal domain of ZP2 in humanized zonae pellucidae in transgenic mice. J Cell Biol 197: 897-905.
- Visconti PE, Krapf D, de la Vega Beltrán JL, Acevedo JJ, Darszon A (2011) Ion channels, phosphorylation and mammalian sperm capacitation. Asian J Androl 13: 395-405.
- González EOH, Sosnik J, Edwards J, Acevedo JJ, Lujambio IM (2006) Sodium and epithelial sodium channels participate in the regulation of the capacitation-associated hyperpolarization in mouse sperm. J Biol Chem 281: 5623-5633.
- Kerr CL, Hanna WF, Shaper JH, Wright WW (2002) Characterization of zona pellucida glycoprotein 3 (ZP3) and ZP2 binding sites on acrosome-intact mouse sperm. Biol Reprod 66: 1585-1595.
- Wong CW, Lam KKW, Lee CL, Yeung WSB, Zhao WE, et al. (2017) The roles of protein disulphide isomerase family A, member 3 (ERp57) and surface thiol/disulphide exchange in human spermatozoa-zona pellucida binding. Hum Reprod 32: 733-742.
- Philip CN Chiu, Ben ST Wong, CL Lee, Ronald TK Pang, Kai Fai Lee (2008) Native human zona pellucida glycoproteins: purification and binding properties. Hum Reprod 23: 1385-1393.
- H Tsubamoto, A Hasegawa, Y Nakata, S Naito, N Yamasaki, et al. (1999) Expression of recombinant human zona pellucida protein 2 and its binding capacity to spermatozoa. Biol Reprod 61: 1649-1654.
- Gahlay G, Gauthier L, Baibako B, Epifano O, Dea J (2010) Gamete recognition in mice depends on the cleavage status of an egg's zona pellucida protein. Science 329(5988): 216-219.
- Rankin TL, Coleman JS, Epifano O, Hoodbhoy T, Turner SG (2003) Fertility and taxon-specific sperm binding persist after replacement of mouse sperm receptors with human homologs. Dev Cell 5: 33-43.
- Chirinos M, Cariño C, González MEG, Arreola E (2011) Characterization of human sperm binding to homologous recombinant zona pellucida proteins. Reprod Sci 18: 876-885.
- JA Maldera, M Weigel Muñoz, M Chirinos, D Busso, F GE Raffo (2014) Human fertilization: epididymal hCRISP1 mediates sperm-zona pellucida binding through its interaction with ZP3. Mol Hum Reprod 20: 341-349.
- Martic M, Moses EK, Adams TE, Liu DY, Gook DA (2004) Recombinant human zona pellucida proteins ZP1, ZP2 and ZP3 co-expressed in a human cell line. Asian J Androl 6: 3-13.
- Campo PC, Chirinos M, Fan XJ, González MEG, Chavarría MG (2006) Biological effects of recombinant human zona pellucida proteins on sperm function. Biol Reprod 74: 760-768.
- Yang X, Zha Y, Yang X, Kan FWK (2015) Recombinant hamster oviductin is biologically active and exerts positive effects on sperm functions and sperm-oocyte binding. PLoS One 10: e0123003.
- Paul MG, M Wassarman (1985) Mouse egg extracellular coat is a matrix of interconnected filaments possessing a structural repeat. J Mol Biol 181: 253-264.
- Ganguly A, Bansal P, Gupta T, Gupta SK (2010) ZP domain' of human zona pellucida glycoprotein-1 binds to human spermatozoa and induces acrosomal exocytosis. Reprod Biol Endocrinol 8: 110.
- S Chakravarty, S Kadunganattil, P Bansal, RK Sharma, SK Gupta (2008) Relevance of glycosylation of human zona pellucida glycoproteins for their binding to capacitated human spermatozoa and subsequent induction of acrosomal exocytosis. Mol Reprod Dev 75: 75-88.
- José O, Hernández OH, Chirinos M, González MEG (2010) Recombinant human ZP3-induced sperm acrosome reaction: evidence for the involvement of T- and L-type voltage-gated calcium channels. Biochem Biophys Res Commun 395: 530-534.
- RN Peterson, P Campbell, WP Hunt, JJ Bozzola (1991) Two-dimensional polyacrylamide gel electrophoresis characterization of APz, a sperm protein involved in zona binding in the pig and evidence for its binding to specific zona glycoproteins. Mol Reprod Dev 28: 260-271.
- Kim KS, Gerton GL (2003) Differential release of soluble and matrix components: evidence for intermediate states of secretion during spontaneous acrosomal exocytosis in mouse sperm. Dev Biol 264: 141-152.
- JD Bleil, PM Wassarman (1980) Mammalian sperm-egg interaction: identification of a glycoprotein in mouse egg zonae pellucidae possessing receptor activity for sperm. Cell 20: 873-882.
- Kim H, Lim HS (2018) Mechanistic ligand-receptor interaction model: operational model of agonism. Transl Clin Pharmacol 26(3): 115-117.
- G Vauquelin, A Packeu (2009) Ligands, their receptors and ... plasma membranes. Mol Cell Endocrinol 311: 1-10.
- Gupta Sk (2018) The Human Egg's Zona Pellucida. Curr Top Dev Biol 130: 379-411.
- Aydin H, Sultana A, Li S, Thavalingam A, Lee JE (2016) Molecular architecture of the human sperm IZUMO1 and egg JUNO fertilization complex. Nature 534: 562-565.
- Ohto U, Ishida H, Krayukhina E, Uchiyama S, Inoue N (2016) Structure of IZUMO1-JUNO reveals sperm-oocyte recognition during mammalian fertilization. Nature 534: 566-569.
- Sykes DA, Stoddart LA, Kilpatrick LE, Hill SJ (2019) Binding kinetics of ligands acting at GPCRs. Mol Cell Endocrinol 485: 9-19.
- Thompson AJ, Verheij MHP, Verbeek J, Windhorst AD, de Esch IJP, et al. (2014) The binding characteristics and orientation of a novel radioligand with distinct properties at 5-HT3A and 5-HT3AB receptors. Neuropharmacology 86: 378-388.
- Horsman KM, Barker SLR, Ferrance JP, Forrest KA (2005) Separation of sperm and epithelial cells in a microfabricated device: potential application to forensic analysis of sexual assault evidence. Anal Chem 77: 742-749.
- Bray C, Son JH, Meizel S (2002) A nicotinic acetylcholine receptor is involved in the arosome reaction of human sperm initiated by recombinant human ZP3. Biol Reprod 67: 782-788.
- Ganguly A, Bukovsky A, Sharma RK, Bansal P, Bhandari B (2010) In humans, zona pellucida glycoprotein-1 binds to spermatozoa and induces acrosomal exocytosis. Hum Reprod 25: 1643-1656.
- Gupta SK, Bansal P, Ganguly A, Bhandari B, Chakrabarti K (2015) Role of zona pellucida glycoproteins during fertilization in humans. J Reprod Immunol 108: 90-97.
- JF Hartmann, RB Gwatkin, CF Hutchison (1972) Early contact interactions between mammalian gametes in vitro: evidence that the vitellus influences adherence between sperm and zona pellucida. Proc Natl Acad Sci 69: 2767-2769.
- Espinosa PS, Retamal NH, Gómez LR, Avilés M, Aizpurua J (2020) Influence of in vitro capacitation time on structural and functional human sperm parameters. Asian J Androl 22: 447-453.
- World Health Organization (2001) Laboratory manual of the WHO for the examination of human semen and sperm-cervical mucus interaction. Ann Ist Super Sanita 37: I-XII: 1-123.






























