Statistical Review: Abuse Potential of Pregabalin
Mahasen Al Qallaf
General Department of Criminal Evidence, Toxicology Laboratory, Kuwait
Submission:January 20, 2021;Published:February 01, 2021
*Corresponding author:Mahasen Al Qallaf, Ministry of Interior, General Department of Criminal Evidence, Toxicology Laboratory, Kuwait
How to cite this article:El Hadji O N. Pregnant Women Victims of Physical Domestic Violence in Dakar. J Forensic Sci & Criminal Inves. 2020; 15(1):555901 DOI:10.19080/JFSCI.2021.15.555903.
Abstract
Pregabalin (PGB) marketed under brand name Lyrica, it is a medication used to treat epilepsy, neuropathic pain, and generalized anxiety disorder. Ministry of Health in Kuwait subjected pregabalin for only medical prescription users, and classified it as dangerous drugs, since the highly exposure to pregabalin is associated with adverse effects such as sleepiness and fatigue, dizziness, anxiety, euphoria, and depression, which is reported as physical dependence. According to widely spread among young people in last three years, especially when users know that there is no criminal legislation about this drug. Ministry of interior in Kuwait attempt to category pregabalin as psychotropic substances punishable by law to reduce the spread and consumption. The aim of this study is to present statistical review of the data concerning the abuse potential of pregabalin. Several cases report abuse of illicit pregabalin either alone or with combined with other pharmaceutical or narcotics drugs, that is further confirmed by LC/MS/MS.
Keywords: Pregabalin; Lyrica; Statistical; Misuse; Kuwait; LC/MS/MS
Abbreviations: GABA: Gamma-Aminobutyric Acid; HRAM: High-Resolution, Accurate-Mass; CDS: Chromatography Data System; HESI: Heated Electrospray Ionization Source; THC: Tetrahydro Cannabinoids
Introduction
Pregabalin, (S)-3-(amino methyl)-5-methylhexanoic acid is a novel analogue of the neurotransmitter gamma-aminobutyric acid (GABA) with analgesic, anticonvulsant, and anxiolytic activity. Pegabalin is minimally metabolized and primarily excreted through urine in an unchanged form. The amount of pregabalin metabolized in humans is negligible, 98% of the drug is eliminated unchanged in urine, 0.9% is eliminated renally as N-methylpregabalin, and 4% is eliminated as an unidentified derivative. Less than 0.1% of pregablin is eliminated in feces. The elimination half-life is 6 h (range 4.6-6.8h) for all doses of pregabalin [1]. The potential for pregabalin abuse or addiction is possible, even the addiction rank in the category of low risk. However, tolerance and withdrawal symptoms have been reported in pregabalin dependence case reports and were found to be associated with symptoms of behavioral dependence. Evidence from preclinical and therapeutic clinical trials suggests the development of tolerance to the euphoric effects. Evaluation the abuse potential of a drug is a complex task that cannot be based on a single test but rather should be based on the overall pharmacokinetic and pharmacodynamics properties of the drug, as well as data from both preclinical and clinical studies. In addition, empirical evidence from clinical use may also indicate abuse potential. Indication of abuse include non-prescribed use or use for non-medical purposes in patients with substance abuse [2-4].
Materials and Methods
Chemicals and standards
All chemicals and standards were purchased from Lipomed (Arlesheim, Switzerland) in 10mg sealed vials, and methanol, water, and acetonitrile were LC/MS grade from Fisher Chemical, while phosphate, buffer and ethyl acetate were purchased from Sigma Aldrich. Other chemicals are available in toxicology laboratory.
Immunoassay screening
Randox screening (urine): Randox Immunoassay testing offers rapid separation of presumptive positive and negative specimens, prior to more costly and time-consuming chromatographic confirmation. Evidence Investigator Biochip Array Technology is used to perform simultaneous detection of multiple analytes from a single sample. The core of the technology is the Randox Biochip; a solid-state device with array of discrete testing regions containing immobilized antibodies specific to different drugs of abuse compound classes. The Randox DoA V Urine kit (Randox laboratories Limited, 55 Diamond Road, Crumlin, County Anntrim, UK) used in this paper employs a competitive chemiluminescent immunoassay, where the drug in the specimen and drug labelled with horse radish peroxidase (HRP) are in direct competition for the antibody binding sites. Increased levels of drug in a specimen will lead to reduced binding of drug labelled with HRP and thus a reduction in the chemiluminescent signal emitted. The light signal generated from each of the test regions on the biochip is detected using digital imaging technology and compared to that from a stored calibration curve. The cutoff concentration of a drug or its metabolites in the matrix establish for assigning negative and positive specimens. In an immunoassay, cutoff concentrations can be selected at the assay’s optimal sensitivity, selectivity, and efficiency reducing the number of false positive and false negative specimens [5,6].
Samples preparation and extraction
Urine samples were obtained from different healthy volunteers and used as negative blank urine, whereas positive urine samples were collected either from hospitals or police station as suspicious samples of misuse of drugs. The urine samples were collected in plastic containers and stored in freezer at -5 °C until analysis.
Liquid-Liquid extraction of pregabalin in human urine:
Aliquot volumes of human urine samples were transferred into small separation funnel. Then 5mL of carbonate buffer pH-9.4 (prepared by dissolving 26.5g sodium carbonate and 21.0g sodium bicarbonate in 500mL distilled water) was added and solution was mixed well. The mixture was then extracted with 3 x 5mL of diethyl ether. The ether extract was collected and evaporated. The residue was dissolved in 2mL of mobile phase and to be injected to LC/MS/MS [7].
Solid phase extraction of pregabalin in human urine
ISOLUTE C18 cartridge is used for extraction of a range of analytes from aqueous samples using a non-polar retention mechanism. Columns were set on a glass block vacuum manifold and the pretreated samples were loaded and the vacuum (10mm Hg) was applied for a slow drop-wise sample flow. 0.5mL of methanol used for cartridge conditioning and then 0.5mL + 1.0 % formic acid for equilibrate. The urine sample then load in 5mL cartridge and later to be wash with water/methanol with 1.0 5 formic acid. The eluents were collected with 0.5mL methanol and 2.0% ammonium hydroxide solution and were evaporated to dryness under a stream of nitrogen at 40°C and reconstituted with mobile phase [8].
Q-Exactive Focus -LC/MS/MS Orbitrap
A Q-ExactiveTM Hybrid Quadrupole-OrbitrapTM Mass Spectrometer (Thermo Fisher Scientific, Bremen, Germany) was used to confirm the results generated from the screening test of the biological urine and blood samples. The Q Exactive system provides very good analytical performance in terms of reproducibility, linearity, and signal-to-noise, and addresses an extremely wide range of masses. The mass spectrometer is a benchtop LC/MS/MS system that combines quadruple precursor ion selection with high-resolution, accurate-mass (HRAM) Orbitrap detection. Samples (5μL) were injected in a 2.6 mm Accucore TM Phenyl-Hexyl column (100 x 2.1mm) and the LC column was heated to 40C. Analytes were resolved at 0.5mL/min using a mobile phase consisting of two solvents. The Mobile phases for LC-Screening are (Phase A: H2O, [NH4]+ [HCOO]- 2mM, 0.1 % HCOOH ), for 1L mobile phase A use 1L of water and add 126mg of ammonium formate and 1mL of formic acid. (Phase B: [NH4] + [HCOO]- 2mM, MeOH/ACN 50:50, 0.1% HCOOH, 1% H2O), for 1L of mobile phase B use 495mL of methanol, 495mL of acetonitrile, 10mL of water, and add 126mg of ammonium formate and 1mL of formic acid. The Q-Exactive mass spectrometer was equipped with a heated electrospray ionization source (HESI-II) and was operated in the positive ionization mode. For data acquisition, the Trace Finder 4.1 software from Thermo Scientific was used, and the Thermo Scientific TM Chromeleon TM Chromatography Data System (CDS) was used to ensure data quality and manage the analytical processes.
Result
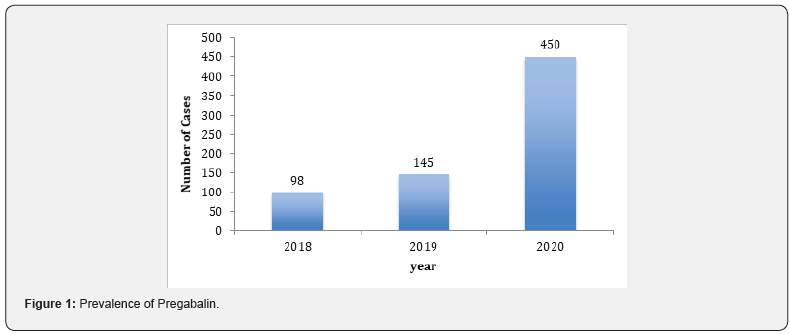
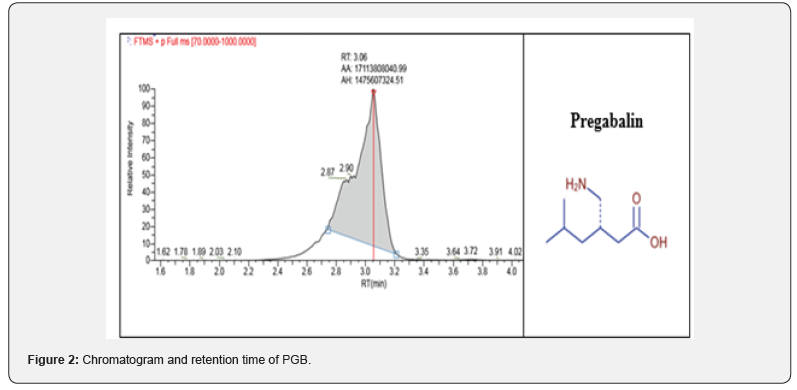
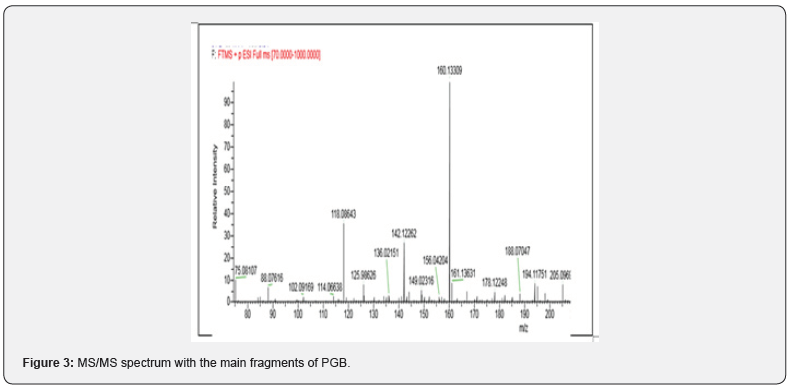
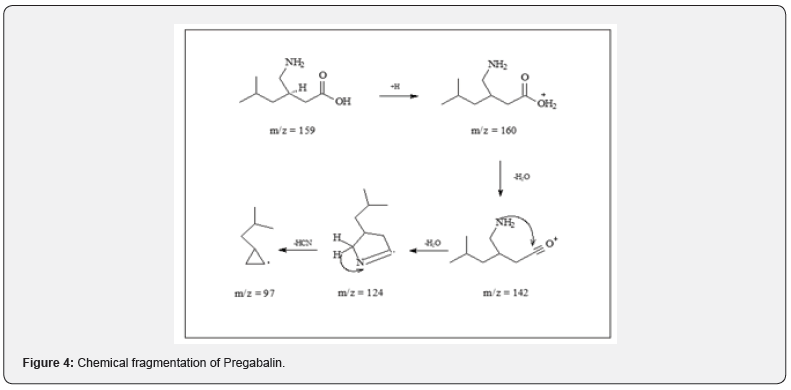
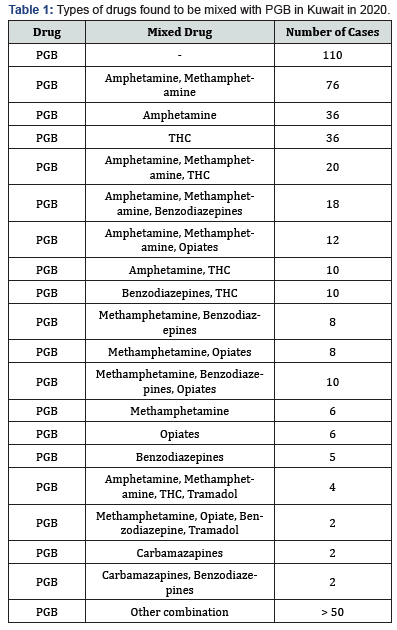
Reports of abuse and misuse of pregabalin (Lyrica) have been risen in recent years and become more common among young adults. In the last three years, the misuse of lyrica increased by fourfold or higher in Kuwait since 2018, especially when abuser know that there is no criminal legislation about this drug. In current study, statistical survey of pregabalin in 2018 to 2020 was shown the widely increases of misuse of the drug. Most of the cases received either from hospitals or from different police stations. In Figure 1, 2018 there was not much abuse of pregabalin between young users, but the curve started to expand to reach the highest rate in 2020. The data collected out of 1000 positive urine samples for each year that have other drugs than pregabalin. The growing pregabalin misuse not restricted only on pregabalin itself but also extended to be mixed with other pharmaceutical or narcotics drugs can cause a real addiction. In current study, more details about addiction in 2020 were fully analyzed and recovered as in particular analysis of misuse of pregabalin alone or mixed. The samples were first analyzed in screening test by using Randox immunoassay testing that offers rapid separation of presumptive positive and negative specimens. The types of cases include pregabalin with different types of drugs mixed, including central nervous system stimulants, depressants, and hallucinogens. The majority was pregabalin alone, followed by PGB mixed with Methamphetamine and Amphetamine, then Amphetamine and Tetrahydro cannabinoids (THC), and many other combinations as shown in Table 1. The risk of pregabalin increases when combined with other stimulants, depressants, and hallucinogens, or other drugs depress the central nervous system, which affects a person’s breathing, like benzodiazepines, heroin and opioids. Less than 10% of data used pregabalin as medication since few cases were received from hospitals in Kuwait and they have medical prescription to treat several types of diseases. Whereas the most cases comes from police stations where there is a misuse of pregabalin with the purpose of pleasure, euphoria, and relaxation. Thus, government should reclassify it as a controlled drug and to be punishable by law to reduce the spread and consumption. Further analysis was done to confirm the screening results by using LC/MS/MS orbitrap confirmation analysis. Where clearly three base peaks was shown in mass spectrometry in all libraries that is proceed all reference standards. The retention time of both standard and positive urine sample was in 3.1 to 3.2min, (Figure 2). In order to confirm that the peak m/z=160 corresponded to pregabalin and no other compound, MS/MS experiments were carried out. For this, first, pregabalin was tuned individually by direct infusion of standard. Three adequate MS/MS transitions were found (m/z = 142, m/z = 124, m/z = 97) (Figure 3). Thus, the identity of compound was determined by retention time and at least three MS/MS transitions. Pregabalin undergoes little or no metabolism. In experiments using nuclear medicine techniques, it was revealed that approximately 98% of the radioactivity recovered in the urine was unchanged pregabalin. The main metabolites is N- methyl pregabalin [9,10]. In current study, the presence of the metabolite N- methylated pregabalin (m/z = 174) was not detected in any urine sample. This is in accordance with a low renal elimination (Figure 4) show the fragmentation of PGB.
Discussion
Pregabalin is well tolerated and associated with dose dependent adverse effects that are mild to moderate and are usually transient. The greater risk increases when combined with other stimulants, depressants, and hallucinogens. Also, it is dangerous to take pregabalin with alcohol or other drugs depress the central nervous system, which affects a person’s breathing, like benzodiazepines, heroin and opioids. This means that the using any combination of these types of drugs with or without alcohol increases the risk of overdose and death. Pregabalin also lowers opioid tolerance meaning that the risk of overdose and death increases when they are used together with opioids.
Conclusion
The present study suggests an important abuse potential of pregabalin. Prescribers should pay attention to signs of abuse, especially in patients with a history of substance abuse. Further studies should address the extent of abuse and individual factors that may increase liability towards abuse of pregabalin.
References
- Rodriguez J, Castaneda G, Munoz L (2013) Direct determination of pregabalin in human urine by nonaqueous CE-TOF-MS. Electrophoresis 34: 1429-1436.
- N Manjushree, Chakrabory A, Shashidhar K, Naraynaswamy S (2015) A review of the drug pregabalin. Int J Basic Clin Pharmacol 4(4): 601-605.
- Schjerning O, Rosenzweig M, Pottegard A, Damkier P (2016) Abuse Potential of Pregabalin. CNS drugs 30(1): 9-25.
- Foroutan N, Nikvarz N (2016) Role of pregabalin in management pruritus: A literature review. J Pharm Pharm Sci 19 (4): 465-474.
- Chirachariyavej T, Srisont S, Peonim V (2010) Application of the multiple drugs immunoassay test for rapid detection of drug abuse in postmortem urine. J Med Assoc Thai 3 (11): 1301-1309.
- Biochip Immunoassays, Randox Laboratories.
- Gujral RS, Haque SM, Kumar S (2009) A novel method for the determination of pregabalin in bulk pharmaceutical formulations and human urine samples. African Journal of Pharmacy and Pharmacology 3(6): 327-334.
- Bansal A, Tewari A, Garg S, Gupta A (2009) Pregabalin: pharmacology and use in pain management. J Anaesth Clin Pharmacol 25(3): 321-326.
- Spigset O, Westin AA (2013) Detection Times of pregabalin in urine after illicit use: when should a positive specimen be considered a new intake. The Drug Monit 35: 137-140.
- Gujral RS, Haque SM, Shanker P (2009) Development and validation of pregabalin in bulk, Pharmaceutical formulations and in human urine samples by UV spectrophotometry. International Journal of Biomedical Science (2): 175-180.






























