X-Ray Fluorescence Analysis: Useful For Forensic Examination
Shailendra Jha and Mukesh Sharma*
Phys. Division, State Forensic Science Laboratory, India
Submission: February 23, 2016; Published: March 15, 2016
*Corresponding author: Mukesh Sharma, Phys. Division, State Forensic Science Laboratory, 15 Vashundhara Colony, Tonk Road, Jaipur - 302018 Rajasthan, India, Tel: +919460986307. Email: mksphy@gmail.com
How to cite this article: Madhu B, Anika S. X-Ray Fluorescence Analysis: Useful For Forensic Examination J Forensic Sci & Criminal Inves. 2016; 1(1): 555553. DOI: 10.19080/JFSCI.2016.01.555553
Abstract
In the field of forensic science, it is very important to investigate of evidential samples obtained at various crime scenes. X-ray fluorescence (XRF) is used widely in forensic science. Its main strength is its non-destructive nature, thus preserving evidence. This paper, deals with the application of XRF to examine the evidences like purity gold and silver jewelry (Indian Ornaments), remnants of glass pieces and paint chips recovered from crime scenes. The experimental measurements on these samples have been made using X-ray fluorescence spectrometer (LAB Center XRF-1800) procured from Shimazdu Scientific Inst., USA. The results are explained in terms of quantitative/ qualitative analysis of trace elements how useful for forensic point of view.
Keywords: X-ray Fluorescence; Forensic Science
Abbreviations: XRF: X-ray Fluorescence; State FSL: State Forensic Science Laboratory; GRIM: Glass Refractive Index Measurement; RI: Refractive Index; EPMA: Electron Probe Microanalysis/p>
Introduction
Various kinds of forensic evidential samples have been controlled by criminal laws and could not be destroyed carelessly, even if any analytical examinations should be needed on the samples, while those samples should be examined rapidly on the basis of the human rights of the concerned suspects or victims. Those evidential samples have been observed and have been examined by many forensic experts [1]. Such samples have thus needed to be analyzed for elemental composition, in turn leads to the investigating agency to know the type and origin of the clue material to reach the criminal or victim. X-ray Fluorescence (XRF) spectroscopy is a non-destructive technique of analysis widely used in forensic science for the identification of elements in pigments, metal alloys, and other materials of evidential clues recovered from the scenes of crime [2,3].
In this paper, we have reported the application of XRF to examine the evidences like purity of gold and silver jewelry (Indian Ornaments) and remnants of glass pieces recovered from crime scenes. In this study, we have also intended to explore the advantages of the XRF method [4], the capabilities of simultaneous multi-element non-destructive, analysis and automatic handling of a large number of samples. XRF spectrometry is widely used technique for the analysis of metals, alloys, composite (glass) and polymers (paint-chip). This paper reports the quantitative and qualitative elemental analysis of silver brick and glass fragments using the WD XRF spectrometer and the FP method.
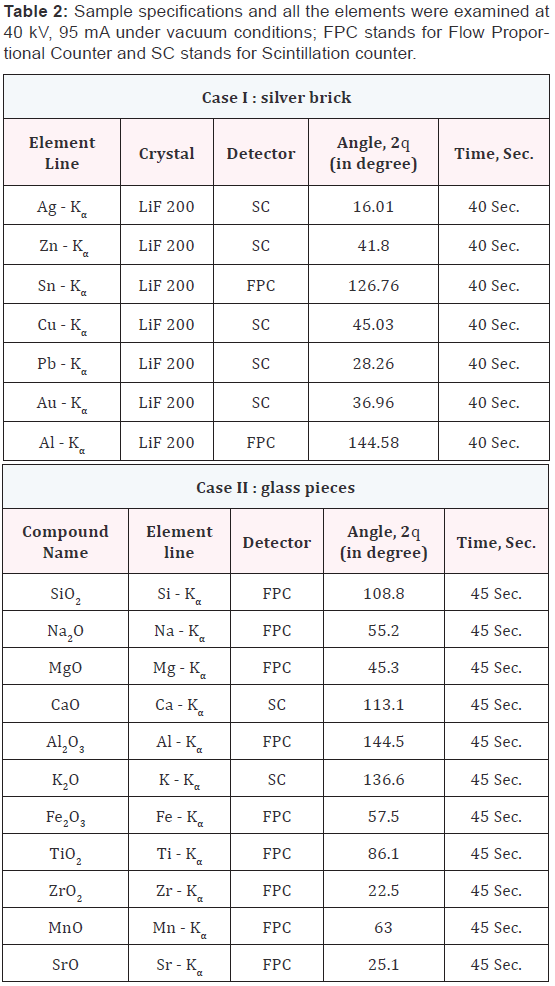
Case I: In this case, a jeweller was cheated by two people. Those had supplied him a fake silver brick having 15.4 cm x 13.2 cm x 3.9 cm dimensions, quoting that the brick is made of 80 to 85 % of silver. After purchasing this by an amount of 1.5 million Indian rupees, he doubted that the brick was fake and the persons, who had sold him the brick, were fraud. So, he lodged a complaint against them in police station. The brick was forwarded to the State Forensic Science Laboratory, Rajasthan (State FSL) for examination of its purity.
It is important to note, in Indian context, that a number of different techniques are used to fabricate jewellery which results in samples that vary considerably in homogeneity, and hence their suitability for accurate XRF analysis. Lost wax investment casting, extrusion - rolling - stamping, pressed powder, and electroforming processes are all used to design jewellery. Each of these methods results in jewellery samples that possess unique XRF measurement characteristics due in part to the macroscopic metallurgical properties of the alloy being used. One can study the phase diagram for the Ag – Cu system to understand that the Silver-rich fraction of higher melting point will solidify first that in some cases, creates a silver-rich ‘skin’ on the surface of the sample. In addition, a piece of jewellery may have areas of solder, electro-plating, or multiple coloured alloys juxtaposed with the parent alloy. Since XRF is essentially a surface analysis technique with an effective depth of approximately 10um for typical jewellery alloys, it is possible for ‘sample - prep’ errors to be introduced into the measurement due to heterogeneity [5].
Case II: In this case, an accident was happened between a car and truck. The police had sent many glass fragments collected from the scene of occurrence to the State FSL, Jaipur with control sample of the suspect vehicles. Preliminary identification of glass fragment was made using GRIM-2 (Glass Refractive Index Measurement) of Foster-Freeman, UK. The best match RI (Refractive Index) fragment were taken for XRF analysis, two of them were marked as Q-1 and Q-2 and one control sample marked as exhibit C-1. The investigating agency was interested to know whether the suspect’s vehicle had hit the victim’s vehicle causing death. Applications by XRF [6-8], total – reflection XRF [9,10], XRF and electron probe microanalysis (EPMA) [11,12], neutron activation analysis [13] and XRF with pyro-electric X-ray generator [14] on the analysis of glass and ceramics have been reported already available in the literature.
Experimental
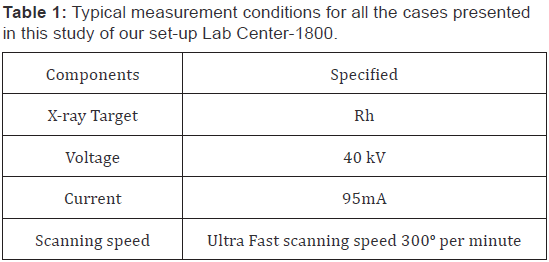
The XRF analysis of the samples were performed with a Shimazdu sequential X-ray fluorescence spectrometer Lab Center XRF-1800 Wave-length dispersive XRF spectrometer [15] using the 10 mm to 20 mm sample aperture, the instrument condition are given in Table 1 and the case examined listed in Table 2 with dimensions and detection parameter. Samples were rotated at 300o per minute. An end window X-ray tube with a Rhodium target was used to generate X-rays.
Discussion
ADH = Time (hrs.) X (Average temp. – Minimum development threshold temperature).
Whole formulae for PMI calculation by using ADH method is depicted in Table 4. The PMI estimated was 9.6 days and her death may occur on 17th October 2014 (Table 4). The autopsy was performed 2 days after recovery of corpse. According to autopsy surgeon the PMI of corpse is 10 to 12 days without intimating any known reason of death. This case study illustrates the importance of using insect evidence to estimate minimum Postmortem Interval and to reconstruct a possible scenario of the events.
Discussion
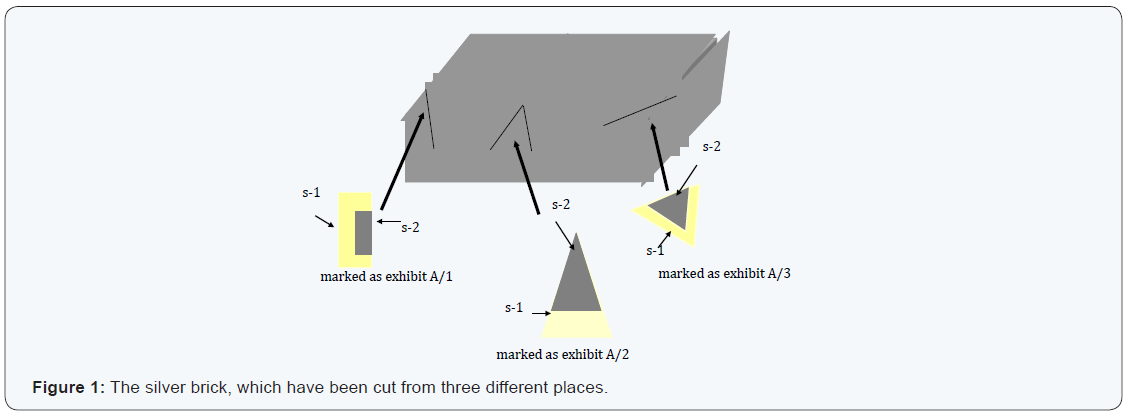
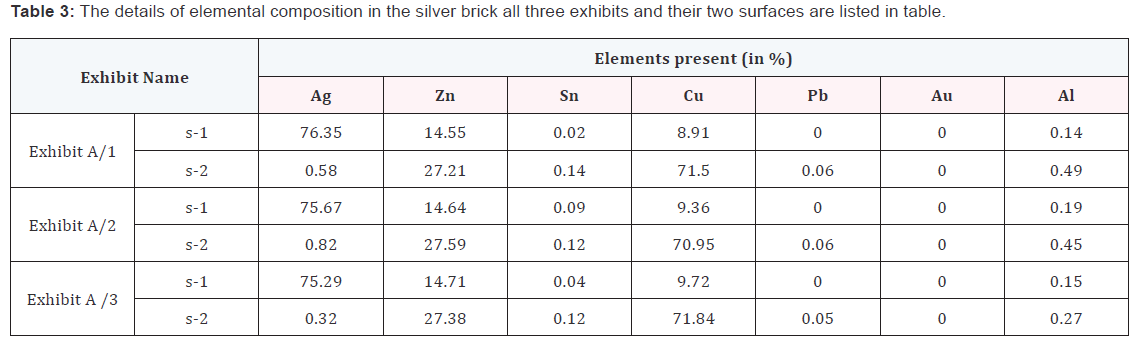
Case study I: In this case we have applied the chemical test of silver, observed that all surfaces having silver layer. So, it gave us misguiding results. Then the brick was cut from three different corners and all the three cut pieces have been marked as exhibits A/1, A/2 and A/3. In these three exhibits having two surfaces, Figure 1 demonstrated the cut exhibits. The inner and outer surfaces have further been marked as s-1 & s-2 surfaces, respectively. The results of examination of the three exhibits and their surfaces in the silver brick are summarised in the Table 3. From Table 3, it is clear that the brick has two different layers. The outer layer having purity about 70-75 % silver on the other hand in the inner layer the brick has about 70 -71 % cooper and the other elements like Zn, Pb, Al and Sn were in traces.
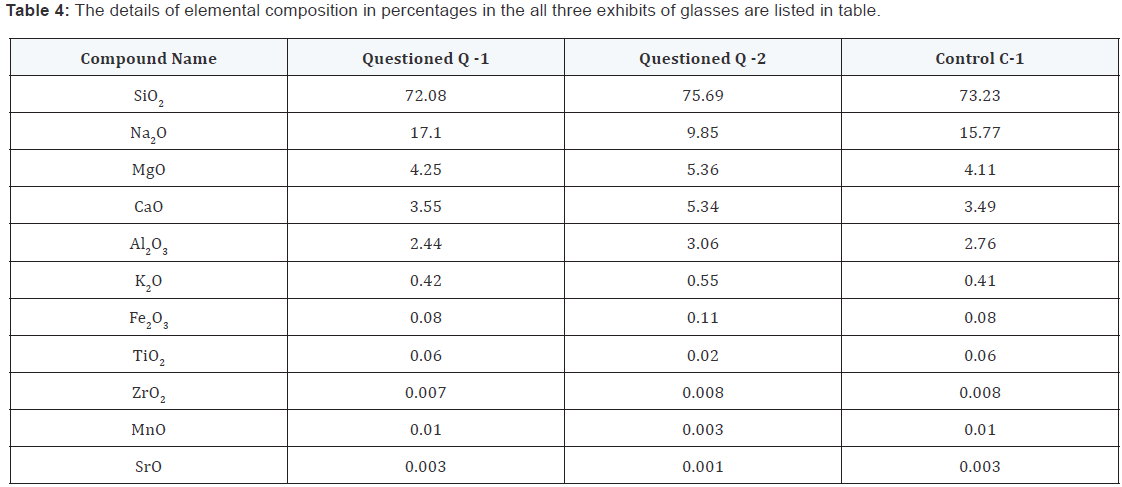
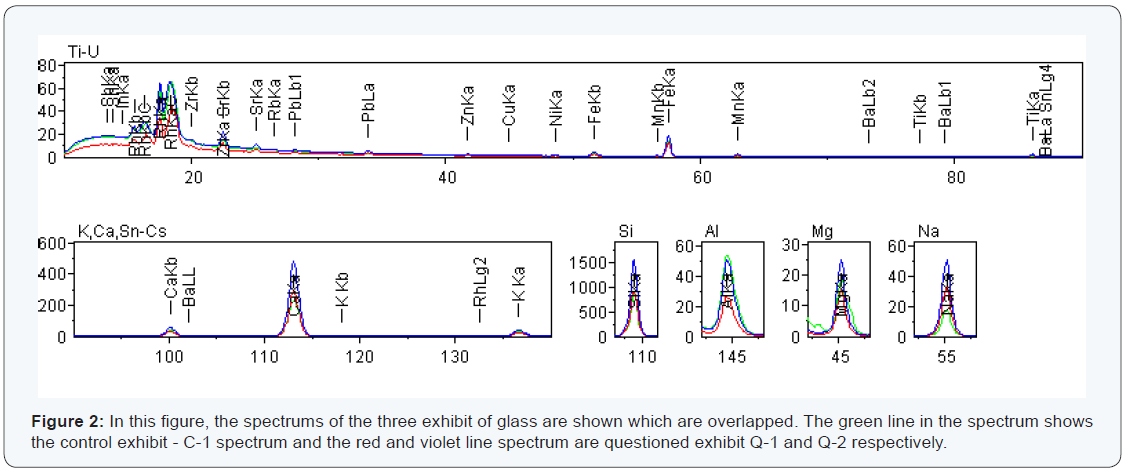
Case II: Table 4, shows that the elements detected in each sample are same in both exhibits but their relative intensity and percentages are different. When the elements detected are same, two exhibits are similar or not with the control sample can easily be identified. The same elements (Si, Ca, Mg, Fe, Al and K) were detected in the questioned marked as Q-1, Q-2 and control sample as C-1, where the relative peak intensity is differ in all. But question exhibit Q-1 and control exhibit C-1 are almost having same intensity as shown in Figure 2. The results of the three exhibits are summarised in the Table 4.
Results
In the present paper the quantitative analysis of sample, made it possible to estimate the approximate concentrations of elements by a simple quantification. This kind of highly sensitive spectrometer [15] is useful for rapid identification elements possible in the field of forensic science.
Case I: Table 3 shows that the element detected in inner and outer surfaces of the exhibits are different from each other. Even the detected elements are different, the sample can be compared for identified, i.e. whether the two surfaces are same or not can be concluded. The same element (Ag, Zn, Sn, Pb, Al and Cu) were detected in the outer surface and inner surface, where the relative peak intensity of inner and outer surfaces were different from each other. From the Table 3, it is clearly seen that the after 3 mm depth from the outer surface the brick having no silver. The elements in the inner surfaces are mainly Cu, Zn, Pb and Sn in traces has been observed.
Case II: In this case one can easily demonstrate that the XRF can be very useful to investigate the elemental composition in glass fragments. The accuracy of the elemental analysis of these glass pieces by XRF has been assessed by comparison with the control samples. The result with SiO2 and Na2O are in agreement within 1.1 % in control sample C-1 and questioned sample Q-1. Furthermore, CaO, Al2O3, K2O, Fe2O3, TiO2, ZrO2, MnO and SrO all agree within 0.10 percent. It is clear that from these results that the elemental analysis of these glass fragments determined by this XRF technique is in excellent agreement between the questioned Q-1 and control C-1.
Conclusion
In case I it was found that the brick cleverly designed by a person who is expert in moulding and the skill was used in cheating. XRF revealed that the silver brick was a fake one. Case II was a case of hit and run on a highway by the analysis of the glass fragments it was established the suspect’s vehicle hit the victim’s vehicle.
Summary
X-ray fluorescence is a useful and versatile analytical technique in any laboratory, let alone a forensic science lab. The purpose of this research paper is to raise the awareness of XRF, in a forensic context, be almost as important as the analysis itself. The prevention of evidence, contact trace evidence, may be vital, so non-destructive method analysis is essential. One can conclude that the XRF analysis is a useful and non-destructive tool for measuring the elemental composition of metals, glass and ceramics.
Acknowledgement
The authors are thankful to the Director, FSL, Jaipur, Rajasthan (India) for his support and help at every step. The work is partly presented in International Conference INTERNATIONAL CONFERENCE & EXHIBTION ON NEUTRON & X-RAY SCATTERING, Malaysia in 2009.p>
References
- Ida H, Kawai J (2004) Identification of steel by X-ray fluorescence analysis with a pyroelectric X-ray generator. Anal Bioanal Chem 379(4): 735-738.
- Kugler W (2003) X-Ray Diffraction Analysis In The Forensic Science: The Last Resort In Many Criminal Cases. Advances in X-ray Analysis 46(1): 1-16.
- Ida H, Kawai J (2004) X-ray fluorescence analysis with portable instruments. Adv X-Ray Chem Anal Japan 35: 81-92.
- Jaklevic JM, French JR, Clarkson TW, Greenwood MR (1978) Adv X-Ray Anal 21: 1971.
- Kloos D (2000) Analysis of Gold Karat Alloys Using Proportional Counter Based Micro-EDXRF; Proceedings of the 24th International Precious Metal Conference, June 2000, p. 1-22.
- Jokubonics C, Wobrauschek P, Zamini S, Karwowski M, Trnka G, et al. (2003) Results of quantitative analysis of Celtic glass artefacts by energy dispersive X-ray fluorescence spectrometry. Spectrochimica Acta Part B 58: 627-633.
- Mialazzo M (2004) Nucl Inst and Methds Phys Res Part B 213: 683.
- Bronk H, Röhrs S, Bjeoumikhov A, Langhoff N, Schmalz J, et al. (2001) ArtTAX--a new mobile spectrometer for energy-dispersive micro X-ray fluorescence spectrometry on art and archaeological objects. Fresenius J Anal Chem 371(3): 307-316.
- Wegstein M, Urban H, Rostam-Khani P, Wittershagen A, Kolbesen BO (1997) Total-reflection X-ray flourescence spectrometry, a powerful tool for semi-quantitative analysis of archaeological glass samples. Spectrochim. Acta Part B 52(7): 1057-1061.
- Cariati F, Fermo P, Gilarodoni S, Galli A, Mialazzo M (2003) Spectrochimica Acta Part B 58: 177.
- http://serials.unibo.it/cgi-ser/start/en/spogli/df-s.tcl?prog_art=7302634&language=ENGLISH&view=articoli
- Jembrih D, Schrenier M, Peev M, Kresjsa P, Clausen C (2000) Identification and classification of iridescent glass artifacts with XRF and SEM/EDX. Mikrochimica Acta 133(1-4): 151-157.
- Heck M, Hoffman P (2002) Analysis of early medieval glass beads - The raw materials to produce green,orange and brown colours. Mikrochimica Acta 139: 71-76.
- Ramos SS, Rieg FB, Adelantado JVG, Marco DJY, Carbo AD (2002) Application of XRF, XRD, Thermal Analysis, and Voltammetric Techinues to the study of Ancient Ceremics. Anal Bioanal Chem 373(8): 893-900.
- Ida H, Kawai J (2004) Identification of glass and ceramics by X-ray fluorescence analysis with a pyroelectric X-ray generator. Anal Sci 20(8): 1211-1215.
- Lab Manual (2005), Shimadzu, USA.







