Levothyroxine Sodium – An Overview of Challenges Related to Interchangeability and Stability of Different Formulations
Obinna Esomchukwu1, Martin Matuvi1, Syed Ali Imran2 and Remigius U Agu1*
1 Biopharmaceutics and Drug Delivery Lab, College of Pharmacy Dalhousie University, Canada
2Division of Endocrinology and Metabolism, Dalhousie University, Canada
Submission: April 09, 2021; Published: May 17, 2021
*Corresponding author:Remigius Agu, Biopharmaceutics and Drug Delivery Lab, College of Pharmacy Dalhousie University, Halifax, Canada
How to cite this article:Obinna E, Martin M, Syed Ali I, Remigius U A. Levothyroxine Sodium – An Overview of Challenges Related to Interchangeability and Stability of Different Formulations. J Endocrinol Thyroid Res. 2021; 6(1): 555679. DOI: 10.19080/JETR.2021.06.555679
Abstract
Since the early 1950s, oral levothyroxine sodium (L-T4) has been the primary form of treatment for hypothyroidism. There are currently several brands and generic options available to patients, some of which are considered bioequivalent. In the last two decades, however, several reports have shown loss of therapeutic control in patients after switching to a different, albeit bioequivalent, L-T4 product. Such observations can partly be explained by L-T4’s narrow therapeutic index, which means small changes in the dose can lead to therapeutic failure or adverse events. Thyroxine (T4) is also a labile compound and several products have been recalled due to stability or potency issues. These recalls have led regulatory agencies, such as the FDA, to revise the methods used to determine bioequivalence. Notwithstanding, several professional organizations have suggested that the current methods of determining bioequivalence are inadequate for establishing the therapeutic equivalence of levothyroxine sodium products. In addition to bioequivalence concerns, L-T4 tablets have been subject to several recalls due to stability and potency issues. This article examines how formulation properties, excipients, and tablet handling practices can influence the stability of L-T4 tablets. In addition, it provides a summary of L-T4 interchangeability in North America and provides an overview of L-T4 tablets recalls due to sub potency. Finally, it explores some of the challenges with the current method of establishing bioequivalence and discusses some of the risks associated with unintended dose changes of levothyroxine sodium.
Keywords: Thyroid; Bioequivalence; Hormone; Formulation
Introduction
Hypothyroidism is a common endocrine disorder affecting 2-5 % of the general population [1,2]. Oral levothyroxine sodium (L-T4), the mainstay of replacement therapy, is currently available under various brand names and generic versions [3]. Regulators, such as the United States Food and Drug Administration (FDA) and Health Canada, are responsible for determining the bioequivalence of the various L-T4 preparations marketed in their jurisdiction. In the US, several L-T4 preparations are considered bioequivalent, thus allowing patients to hypothetically switch between products without experiencing adverse drug reactions or therapeutic failure. However, there are well documented cases of either therapeutic failure or adverse events occurring when switching from one bioequivalent product to another [4,5]. This led the American Thyroid Association (ATA) and American Association of Clinical Endocrinologists (AACE) to classify L-T4 as a narrow therapeutic index or critical dose drug capable of causing significant adverse outcomes including dyslipidemia, poor pregnancy and fetal outcomes, atrial fibrillation, bone loss, and exacerbation of heart disease from small dose changes [6,7].
Over the last two decades, the FDA has made several changes in the regulatory requirements for marketing L-T4 tablets. In 1997, after several cases of adverse events being reported as a result of switching between brands and differences related to stability and potency of different preparations, the FDA declared L-T4 as a new drug; therefore, all L-T4 manufacturers in the US had to submit a new drug application (NDA) and in the process, establish bioequivalence with an approved reference product [8]. L-T4 products which did not satisfy the stability requirements of the FDA were discontinued. Subsequently, after numerous drugs recalls due to sub-potency, the FDA further tightened the stability requirements of L-T4 tablets in 2007 and the potency requirement during normal shelf-life was increased from 90 % - 110 % to 95 % -105 % [9]. Despite these measures, reports of loss of therapeutic efficacy and adverse events continue to occur in patients switching between brands or generics.
L-T4 requires a strict and consistent dosing regimen to achieve therapeutic effects. In addition to drug potency, drug and food interactions, drug-drug interactions, comorbidities, and poor compliance affect bioavailability and consequently lead to variability in the efficacy of L-T4. For instance, Hashimoto’s thyroiditis, which is the commonest cause of hypothyroidism in North America, is associated with a higher incidence of immunemediated inflammatory gastrointestinal conditions, which can affect L-T4 absorption. While a detailed discussion of the underlying mechanism is outside the scope of this article, many commonly used foods, drugs and supplements can interfere with LT4 absorption. Certain drugs and supplements like bile acid sequestrants, calcium carbonate, and iron supplements tend to form stable and insoluble complexes with L-T4 interfering with its absorption [10-12]. On the other hand, drugs such as proton pump inhibitors and cholestyramine also inhibit L-T4 absorption by altering the gastrointestinal (GI) motility and pH [13-15].
Therefore, patients are advised against taking supplements or medications at least one hour after taking their L-T4 dose [16]. It is also recommended that L-T4 be taken on an empty stomach, because many food products including coffee can affect bioavailability [16]. Given these considerations and the narrow therapeutic index of L-T4, it is recommended that patients take L-T4 at the same time either each morning or at bedtime to ensure the consistency of each dose. Other regimens such as taking a slightly larger dose of L-T4 2-3 times a week instead of a daily dose have also been recommended to improve absorption [17]. While other reviews have addressed some of the issues regarding L-T4 stability, methods of establishing bioavailability and challenges associated with switching between L-T4 products this paper highlights the often-neglected effects of physicochemical properties of L-T4, excipients and tablet handling practices that affect stability and efficacy causing loss of therapeutic control [18- 22].
Methods
A comprehensive literature search was conducted in Embase and Medline (via Pubmed) using a combination of the following keywords: levothyroxine AND interchangeability OR bioequivalence AND stability OR potency. The literature search was conducted between December 2019 and September 2020 and it included articles published from 1997, the year the FDA updated its regulation on levothyroxine, to 2020. No conference proceeding or presentation was included. Discussion The Interchangeability of L-T4 Products in Canada and the US Prior to 1962, the FDA did not require drug manufacturers to prove the efficacy of their products. As long as the product was safe, it could be sold to the public. However, following the 1962 Kefauver-Harris Drug Amendments, manufacturers were required to prove both safety and efficacy of their products [23]. Consequently, the FDA conducted a review of all the drugs that had been approved between 1938 and 1962. This evaluation was conducted under the Drug Efficacy Study Implementation (DESI) program. Since L-T4 had been available in the US prior to 1938, it was exempted from the FDA evaluation. In 1997, however, the FDA declared L-T4 as a new drug mandating the submission of a New Drug Application (NDA). This process was integral to the regulation of the number of L-T4 products currently available in Canada and the US. Currently, two brands of L-T4 are being sold in Canada: Synthroid (BGP Pharma ULC) and Eltroxin (Aspen Pharmacare Canada Inc.). These brands have been in the market since the 1950s. They are not interchangeable and do not have any generic equivalents available for humans use [24].
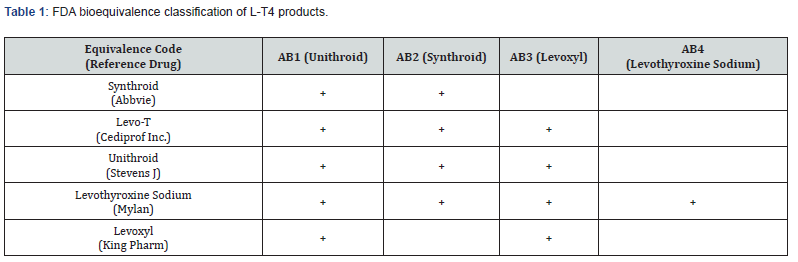
In the US, there are nine L-T4 manufactures: seven brands and two generics. The section of the FDA Orange Book (which lists the therapeutic equivalence of drugs in the US) concerned with L-T4 bioequivalence is summarized in Table 1. The FDA currently has four reference standards for establishing L-T4 bioequivalence. Each reference (i.e., Unithroid, Synthroid, Levoxyl, and Levothyroxine sodium) has a corresponding bioequivalence or AB code (Table 1). A product that shows bioequivalence with a reference shares the same AB code. L-T4 manufactures can choose to establish bioequivalence with any or all the reference standards. For example, AB3 is the code for the third reference standard drug, Levoxyl. Manufacturers wishing to obtain AB3 status will have to compare their product with Levoxyl. Currently, only Levo-T, Unithroid, and Levothyroxine have AB3 status. Therefore, patients on Levoxyl should, in theory, experience a similar therapeutic effect with Unithroid, Levo-T, or Levothyroxine.
Stability of Oral L-T4
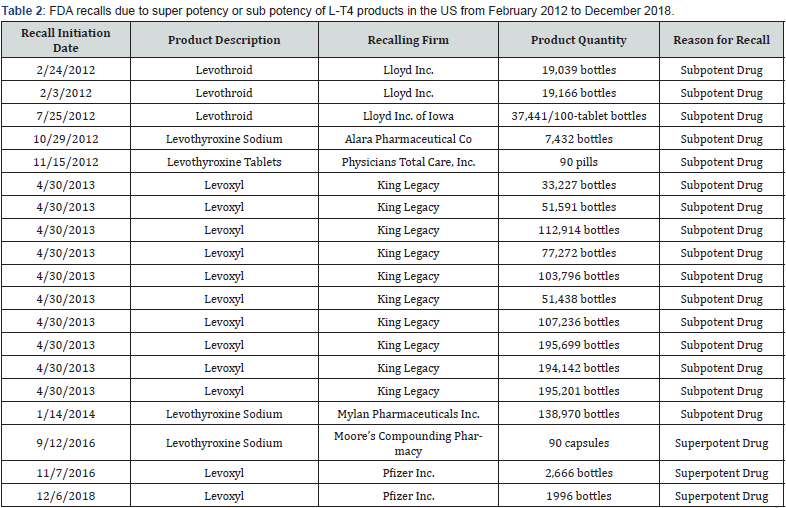
L-T4 is one of the most recalled drugs in the US. From February 2012 to December 2018, nineteen recalls due to sub potency, and super potency of L-T4 tablets occurred in the US as summarized in Table 2. The most common reason for these recalls was subpotency during the shelf life of the drug. The expiry date of a drug is usually determined from the results obtained from stability testing in which the drug potency will remain between 95-105% [25]. A 10% reduction for most drugs may not pose any health risk, but because L-T4 is a narrow therapeutic index drug, such a decrease in dose could result in lowering of the therapeutic effect. For example, the next lowest dose for a patient on Synthroid 150 μg is the 137 μg tablet and if 10% of the 150 μg tablet degrades, the effective dose would be 135ug, which would be less than the next available lower dose [26]. Before the 2007 regulatory changes in potency requirements, the FDA obtained results of stability studies of all oral L-T4 products available in the US from 2003-2005 [13].
The stability data showed that some L-T4 products degraded significantly before the expiration date which led FDA to impose a more stringent potency requirement of 95-105% over the shelf life. The cause of this loss of stability, despite controlling for the known destabilizing factors like heat, light, humidity, oxidation and pH, remains unclear [27]. Consequently, instead of improving the stability of L-T4, the new potency requirement may have led to a reduction in the shelf-life of L-T4. Several explanations for the loss of stability have been suggested. Patel et al. suggested that it might have been due to formulation excipients and formulation pH and recommend the use of non-hygroscopic excipients and basic pH modifiers [28]. They also showed that high moisture content and low pH are closely associated with L-T4 degradation. Another study proposed that L-T4 stability is dependent on the impact of oxygen and moisture and suggested that the loss of the bound hydrates in L-T4 is responsible for the observed instability [29].
They surmised that the stability of L-T4 is compromised after dehydration by the presence of oxygen and that L-T4 is more stable in moist conditions with high relative humidity than in dry conditions and recommended against the use of hygroscopic excipients which disrupt the bound water molecules in L-T4. Similarly, a study using the solid-state characterization found that L-T4 loses its hydrates at relative humidity of less than 45% and showed that L-T4 is susceptible to degradation by high temperatures, low humidity, and oxygen [30]. Together, these findings suggest that L-T4 is unstable in low humidity but stable in high humidity as long as its water molecules are maintained.
Tablet Properties
To determine bioequivalence, the FDA adapted The Biopharmaceutics Classification System (BCS), which is used to predict drug bioavailability based on the solubility and permeation of the drug. Thus, rather than testing bioequivalence of certain batches, disintegration, dissolution, and friability test are done, and bioequivalence and potency can be estimated based on the BCS designation of the drug. Since L-T4 has a highly variable individual absorption it is often classified as a Class III drug (high solubility and low permeability) [31]. Rate and extent of dissolution are factors considered to be critical for the absorption of a solid oral dosage form. To maintain consistency among oral preparations, it is important that they share similar dissolution profiles to ensure rapid and complete absorption. Minor differences in dissolution rates have been observed between L-T4 preparations, which may contribute to some of the observed variations in absorption.
Although, it is suggested that small discrepancies in dissolution rates are clinically irrelevant to most patients, the dependence on this property in determining bioequivalence and the potential variability in the gut, especially hypothyroid patients with absorption disorders exploration remains unclear. This was borne out in a study by Pabla et al. showing significant difference in the rate of dissolution with changes in pH between L-T4 products otherwise considered bioequivalent [32]. They reported that the dissolution of three different products decreased with an increase in pH, and the variation in the rate of dissolution was significantly higher between pH=2 and pH=5. Patients with altered gastric acid production, such as those on proton pump inhibitors or with diseases such as chronic gastritis, could experience less drug dissolution, especially considering the low pH of the stomach [33- 35]. L-T4 absorption primarily occurs in the distal jejunum and ileum and drug dissolution occurs primarily because of the pH in those regions. Similarly, patients with autoimmune inflammatory bowel disorders, which are associated with Hashimoto’s thyroiditis are susceptible to variability in dissolution rates because of the alteration in the pH of the GI system [36].
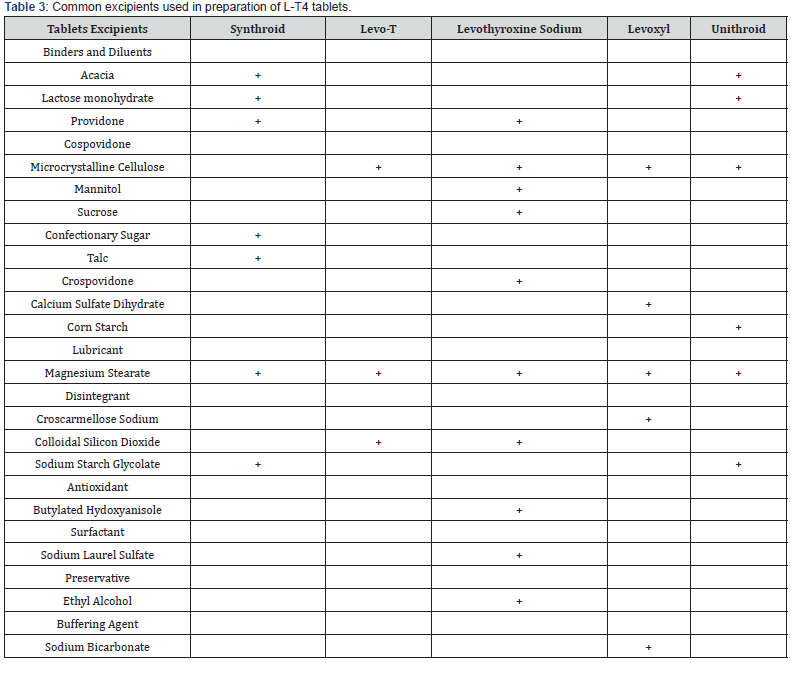
L-T4 is an amphoteric molecule with three pKa values: 2.2 (COOH), 6.7 (OH), and 10.1 (NH2). Drug solubility is high below pH 2.2 and above 10.1, with its lowest solubility between pH 4 and 5 [37,38]. As a result, L-T4 dissolution is highest in the stomach, where the pH is very low [33]. The various effects of pH on the dissolution rates of different L-T4 formulations are further complicated due to differences in the drug particle sizes, excipients, and manufacturing processes of each product which can also affect dissolution. In most L-T4 formulations, excipients constitute the bulk of the drug; the amount of L-T4 in a tablet can be as small as 25 μg, so the excipient to drug ratio is usually high. Table 3 shows five current L-T4 brands and the different excipients which are usually present in the different products.
Effects of Excipients on Stability
Excipients are integral components of oral therapies. They can affect drug dissolution, stability, and other factors that may affect bioequivalence. The effects of excipients are significant in L-T4 because of its small dose concentration in the tablet. One of the most apparent differences between L-T4 brands is the excipients present in their formulations. Although excipients are often physiologically inactive, their physicochemical properties can affect drug stability. The FDA data on L-T4 recalls showed that some brands had more recalls than others due to sub potency [25].
One study looked at the effects of humidity and heat on the degradation and analyzed how different excipients can affect L-T4 degradation [39] and showed that significant degradation of L-T4 occurred at high temperature and humidity, especially in the presence of crospovidone, povidone, and sodium lauryl sulfate. Povidone and crospovidone showed significant hygroscopicity (>10% water gain), making moisture the likely degrading agent. Sodium lauryl sulfate, another common excipient in L-T4 tablet formulations showed deliquescent activity in the presence of moisture, which is significant, as this will also affect tablet hardness as well as promote drug degradation. These specific excipients are present in several, but not all, of the available L-T4 formulations. Brands containing such excipients are susceptible degradation, especially if the drug products are not correctly stored. Another study showed similar effects of crospovidone and povidone on the stability of L-T4 and also showed that L-T4 degrades much faster at lower pH than it does at higher pH and reported that pH modifiers, including sodium bicarbonate, reduced L-T4 degradation [28].
Tablet Splitting
Given the wide range of L-T4 dosages, tablet splitting is not generally required. However, patients may decide to split their L-T4 tablets to save costs or reduce the drug into ingestible pieces. The latter mostly occurs when administering tablets to children or individuals swallowing difficulties. Tablets can either be split manually or mechanically; however, studies have shown an uneven distribution of active compounds between the two halves, as well as significant loss of the tablet, which is fractured off during the splitting process. Some studies have questioned the validity and safety of this process, especially with narrow therapeutic index drugs such as L-T4 showing heterogeneous distribution of the active compound, depending on the manufacturing process and tablet binder used [40]. Tablet splitting also leads to the increased surface area to volume ratio and disruption of the external coating. As a result, splitting a tablet might accelerate the effects of hygroscopic water adsorption, fragmentation, friability, and degradation of L-T4. Thus, if degradation is increased by tablet splitting, the differences in the stability of the divided tablet may also be affected.
Implications of Unintended Dose Changes in a Narrow Therapeutic Index Drug
A narrow therapeutic index drug is defined as any drug that has a less than 2-fold difference between the minimum toxic concentration and minimum effective concentration [41]. Drugs like warfarin, tacrolimus, phenytoin, carbamazepine, and L-T4 are examples of narrow therapeutic index drugs. Small unintended changes in L-T4 doses can lead to significant clinical effects [42]. Although overt symptoms related to grossly abnormal thyroid hormone levels may occur due to unintended dose changes, more subtle subclinical changes are not uncommon. which may account for approximately 90% of the observed adverse events in patients [4]. The study also reported that switching from brand products to generic formulations was associated with a higher incidence of adverse events which included development of new symptom or loss of therapeutic control of TSH levels and found that in over 90% cases switching to a different L-T4 product was done either by the pharmacy or patient without the clinician’s knowledge [4]. These findings emphasize importance of consistent dosing in the treatment of hypothyroidism. The prevalence of subclinical hypothyroidism is estimated between 14-21% in patients treated with L-T4 [4]. A meta-analysis involving 55,287 hypothyroid patients (3450 with subclinical hypothyroidism) showed a significant increase in the risk of coronary heart disease (CHD) and cardiovascular mortality in patients with subclinical hypothyroidism compared to euthyroid patients [43]. These risks were independent of age, gender, or preexisting cardiovascular conditions. These findings were supported by another metaanalysis that involve 14,449 patients [44]. Other studies have also shown an association between subclinical hypothyroidism and increased risks in left ventricular systolic and diastolic dysfunction, increased systolic time interval, elevated total cholesterol, low density lipoprotein cholesterol (LDL-C) and triglyceride levels, and homocysteinemia [45-47].
Reliance on Pharmacokinetic Parameters for Bioequivalence
Bioequivalence of LT4 is typically assessed by administering the reference drug and potential equivalent to healthy, euthyroid individuals. Serum or plasma samples are then taken at regular time intervals to determine pharmacokinetic (PK) parameters such as time taken to reach maximum concentration (Tmax), areaunder- the-curve (AUC), and peak concentration (Cmax). Currently, plasma Cmax and AUC of thyroid hormone (T4) are required to be within 80-125% of each other when the two L-T4 products are given to healthy euthyroid individuals [25]. L-T4, however, is a synthetic variant of the endogenous T4 which is metabolized to its active metabolite triiodothyronine (T3) within the cells thus making it difficult to measure intracellular T3 levels. To reduce the effect of endogenous T4, the FDA Guidance for Industry stipulates that bioequivalence studies are to be done using pharmacological L-T4 dose of 600 μg. The FDA also recommends pre-dose baseline correction to account for endogenous T4 levels [19].
Despite these measures it is suggested that the current equivalence methods are inadequate for determining L-T4 bioequivalence [7]. Consequently, some experts suggest the addition of a pharmacodynamics approach to bioequivalence studies. The rationale being that since L-T4 is administered in euthyroid patients, TSH levels are suppressed as T4 levels increase; as a result, bioequivalent drugs will show not only comparable PK results but also show similar TSH levels [48]. While some have supported the advantages of using TSH as a pharmacodynamics marker in bioequivalence analysis [26], it is noteworthy that there may a test-to-test variability in TSH making it hard to compare different results. Another contentious issue is the use of healthy euthyroid individuals to determine bioequivalence. Since endogenous T4 cannot yet be effectively distinguished from exogenous L-T4, healthy individuals with functioning pituitary-thyroid feedback system will down-regulate TSH production, resulting in less plasma T4 which has led some to suggest that thyroidectomized patients are the ideal group for establishing bioequivalence [26]. Since thyroidectomized patients cannot endogenously produce thyroid hormones, baseline T4 will strictly be dependent on the administered L-T4 dose. Furthermore, lower, and safer doses of L-T4 will be required for bioequivalence studies if treated thyroidectomized patients are used instead of healthy individuals [26]. A study showed that dosages varying as much as 12.5% with baseline correction and 35% without correction for baseline could pass equivalence studies [26]. Such findings emphasize the need for a revision of the current method, especially when the differences in marketed tablet strengths are usually between 25-33%.
Conclusion
Patients should, ideally, be able to substitute drug products with bioequivalent products without concern of adverse events or therapeutic failure. However, the labile nature of L-T4 requires strict monitoring when switching between brands or from brands to generic formulation. Since excipients constitute a significant portion of L-T4 tablets, their effect on the stability either through pH or moisture regulation should be carefully considered by manufactures of L-T4. The recent studies on L-T4 stability shows the importance of maintaining its waters of hydration and proper understanding of its solid-state characteristics. L-T4 is not only a narrow therapeutic index drug, but a peculiar drug which is more stable in the high relative humidity, than in low relative humidity. Therefore, storage conditions of L-T4 should appropriately reflect these unique characteristics and also guide manufacturers in improving formulation practices. Finally, regulatory agencies such as the FDA should consider the benefits of including the assessment of pharmacodynamics parameters in establishing bioequivalence.
References
- Clemens K, Payne W, Van Uum SHM (2011) Central hypothyroidism. Canadian family physician Medecin de famille canadien 57(6): 677-680.
- Golden SH, Robinson KA, Saldanha I, Anton B, Ladenson PW (2009) Clinical review: Prevalence and incidence of endocrine and metabolic disorders in the United States: a comprehensive review. J Clin Endocrinol Metab 94(6): 1853-1878.
- Vaidya B, Pearce SHS (2008) Management of hypothyroidism in adults. BMJ 337: 284-289.
- Hennessey J, Malabanan A, Haugen B, Levy E (2010) Adverse Event Reporting in Patients Treated with Levothyroxine: Results of the Pharmacovigilance Task Force Survey of the American Thyroid Association, American Association of Clinical Endocrinologists, and The Endocrine Society. Endocr Pract 16(3): 357-370.
- Dong BJ, Brown CH (1991) Hypothyroidism resulting from generic levothyroxine failure. J Am Board Fam Pract 4(3):167-170.
- Baskin HJ, Cobin RH, Duick DS, Gharib H, Guttler RB, et al. (2002) American Association of Clinical Endocrinologists medical guidelines for clinical practice for the evaluation and treatment of hyperthyroidism and hypothyroidism. Endocr pract: 8(6): 457-469.
- Blakesley V, Awni W, Locke C, Ludden T, Granneman GR, et al. (2004) Are bioequivalence studies of levothyroxine sodium formulations in euthyroid volunteers reliable? Thyroid 14(3):191-200.
- Federal Register: Prescription Drug Products; Levothyroxine Sodium.
- FDA: FDA Acts to Ensure Thyroid Drugs Don’t Lose Potency Before Expiration Date.
- Singh N, Weisler SL, Hershman JM (2001) The acute effect of calcium carbonate on the intestinal absorption of levothyroxine. Thyroid 11(10): 967-971.
- Dharmarajan TS, Gunturu SG, Pitchumoni CS (2012) Iron, Copper, and Zinc. In: Geriatric Gastroenterology. Springer, New York, pp. 177-183.
- Abbott Laboratories. Synthroid - Levothyroxine Sodium Tablet.
- Benvenga S, Bartolone L, Squadrito S, Lo Giudice F, Trimarchi F (1995) Delayed intestinal absorption of levothyroxine. Thyroid. 5(4): 249-253.
- Stedman CA, Barclay ML (2000) Review article: comparison of the pharmacokinetics, acid suppression and efficacy of proton pump inhibitors. Aliment Pharmacol Ther 4(8): 963-978.
- Annese V, Bassotti G, Napolitano G, Usai P, Andriulli A, et al. (1997) Gastrointestinal motility disorders in patients with inactive Crohn’s disease. Scand J Gastroenterol 32(11): 1107-1117.
- Benvenga S, Bartolone L, Pappalardo MA, Russo A, Lapa D, et al. (2008) Altered intestinal absorption of L-thyroxine caused by coffee. Thyroid 18(3): 293-301.
- Grebe SKG, Cooke RR, Ford HC, Fagerström JN, Cordwell DP, et al. (1997) Treatment of Hypothyroidism with Once Weekly Thyroxine 1. J Clin Endocrinol Metab 82(3): 870-875.
- Klein I, Danzi S (2003) Evaluation of the therapeutic efficacy of different levothyroxine preparations in the treatment of human thyroid disease. Thyroid 13(12): 1127-1132.
- Blakesley VA (2005) Current methodology to assess bioequivalence of levothyroxine sodium products is inadequate. AAPS J 7(1): E42-46.
- Bolton S (2005) Bioequivalence studies for levothyroxine. AAPS J 7(1): E47-53.
- Hennessey JV (2006) Levothyroxine dosage and the limitations of current bioequivalence standards. Nat Clin Pract Endocrinol Metab 2(9): 474-475.
- Benvenga S, Carlé A (2019) Levothyroxine Formulations: Pharmacological and Clinical Implications of Generic Substitution. Adv Ther 36(Suppl 2): 59-71.
- FDA Part I: The 1906 Food and Drugs Act and Its Enforcement.
- Health Canada. Status of Levothyroxine Products in Canada Issue What is the Status of Levothyroxine Products in the USA?
- US FDA. Guidance for Industry Levothyroxine Sodium Tablets-In Vivo Pharmacokinetic and Bioavailability Studies and In Vitro Dissolution Testing Clinical Medical.
- Eisenberg M, Distefano JJ (2009) TSH-based protocol, tablet instability, and absorption effects on L-T4 bioequivalence. Thyroid. 19(2):103-110.
- Kannamkumarath SS, Wuilloud RG, Stalcup A, Caruso JA, Patel H, et al. (2004) Determination of levothyroxine and its degradation products in pharmaceutical tablets by HPLC-UV-ICP-MS. Journal of Analytical Atomic Spectrometry 19(1): 107.
- Patel H, Stalcup A, Dansereau R, Sakr A (2003) The effect of excipients on the stability of levothyroxine sodium pentahydrate tablets. Int J Pharm 264(1-2): 35-43.
- Hamad ML, Engen W, Morris KR (2015) Impact of hydration state and molecular oxygen on the chemical stability of levothyroxine sodium Impact of hydration state and molecular oxygen on the chemical stability of levothyroxine sodium. Pharm Dev Technol 20(3): 314-319.
- Shah HS, Chaturvedi K, Hamad M, Bates S, Hussain A, et al. (2019) New Insights on Solid-State Changes in the Levothyroxine Sodium Pentahydrate during Dehydration and its Relationship to Chemical Instability. AAPS PharmSciTech 20(1): 39.
- Lindenberg M, Kopp S, Dressman JB (2004) Classification of orally administered drugs on the World Health Organization Model list of Essential Medicines according to the biopharmaceutics classification system. Eur J Pharm Biopharm 58(2): 265-278.
- Pabla D, Akhlaghi F, Zia H (2009) A comparative pH-dissolution profile study of selected commercial levothyroxine products using inductively coupled plasma mass spectrometry. Eur J Pharm Biopharm 72(1): 105-110.
- Sachmechi I, Reich DM, Aninyei M, Wibowo F, Gupta G, et al. (2007) Effect of proton pump inhibitors on serum thyroid-stimulating hormone level in euthyroid patients treated with levothyroxine for hypothyroidism. Endocr Pract 13(4): 345-349.
- Gubbins PO, Bertch KE (1991) Drug absorption in gastrointestinal disease and surgery. Clinical pharmacokinetic and therapeutic implications. Clin Pharmacokinet 21(6): 431-447.
- Parsons RL (1977) Drug Absorption in Gastrointestinal Disease with Particular Reference to Malabsorption Syndromes. Clin Pharmacokinet 2(1): 45-60.
- Carvalho GA de, Fighera TM (2014) Effect of gastrointestinal disorders in autoimmune thyroid diseases. Translational Gastrointestinal Cancer 4(1): 76-82.
- Marathe PH, Wen Y, Norton J, Greene DS, Barbhaiya RH, et al. (2000) Effect of altered gastric emptying and gastrointestinal motility on metformin absorption. Br J Clin Pharmacol 50(4): 325-332.
- Evans DF, Pye G, Bramley R, Clark AG, Dyson TJ, et al. (1988) Measurement of gastrointestinal pH profiles in normal ambulant human subjects. Gut 29(8):1035-1041.
- Collier JW, Shah RB, Gupta A, Sayeed V, Habib MJ, et al. (2010) Influence of formulation and processing factors on stability of levothyroxine sodium pentahydrate. AAPS PharmSciTech 11(2): 818-825.
- Shah RB, Collier JS, Sayeed VA, Bryant A, Habib MJ, et al. (2010) Tablet Splitting of a Narrow Therapeutic Index Drug: A Case with Levothyroxine Sodium. AAPS PharmSciTech 11(3): 1359-1367.
- Midha KK, McKay G (2009) Bioequivalence; Its History, Practice, and Future. AAPS J 11(4): 664-670.
- Surks MI, Ortiz E, Daniels GH, Sawin CT, Col NF, et al. (2004) Subclinical thyroid disease: scientific review and guidelines for diagnosis and management. JAMA 291(2): 228-238.
- Rodondi N, Den Elzen WPJ, Bauer DC, Cappola AR, Razvi S, et al. (2010) Subclinical hypothyroidism and the risk of coronary heart disease and mortality. JAMA 304(12): 1365-1374.
- Ochs N, Auer R, Bauer DC, Nanchen D, Gussekloo J, et al. (2008) Meta-analysis: Subclinical thyroid dysfunction and the risk for coronary heart disease and mortality. Ann Intern Med 148(11): 832-845.
- Ye Y, Xie H, Zeng Y, Zhao X, Tian Z, et al. (2014) Association between subclinical hypothyroidism and blood pressure - A meta-analysis of observational studies. Endocr Prac 20(2): 150-158.
- Pearce EN (2012) Update in lipid alterations in subclinical hypothyroidism. J Clin Endocrinol Metab 97(2): 326-333.
- Adrees M, Gibney J, El-Saeity N, Boran G (2009) Effects of 18 months of l-T4 replacement in women with subclinical hypothyroidism. Clin Endocrinol 71(2): 298-303.
- Carr D, Mcleod DT, Parry G, Thornes HM (1988) Fine adjustment of thyroxine replacement dosage: comparison of the thyrotrophin releasing hormone test using a sensitive thyrotrophin assay with measurement of free thyroid hormones and clinical assessment. Clin Endocrinol 28(3): 325-333.






























