The Role of Hepcidin in the Occurrence of Anemia in Type 2 Diabetes Mellitus with NAFLD
Yermek Rakhat*, Romualdas Tomas Preikša, Zhangenthan Abilayuly and Samal Saginova
Kazakh National Medical University named after S.D. Asfendiyarov, Kazakhstan
Submission: January 30, 2019; Published: May 23, 2019
*Corresponding author:Yermek Rakhat, Kazakh National Medical University named after S.D. Asfendiyarov, Tolebi street №94, Kazakhstan
How to cite this article:Yermek R, Romualdas T P, Zhangenthan A, Samal S. The Role of Hepcidin in the Occurrence of Anemia in Type 2 Diabetes Mellitus with NAFLD. J Endocrinol Thyroid Res. 2019; 4(4): 555641. DOI: 10.19080/JETR.2019.04.555641
Introduction
Diabetes mellitus is a group of metabolic diseases characterized by chronic hyperglycemia resulting from defects in insulin secretion, insulin action, or both. Metabolic abnormalities in carbohydrates, lipids, and proteins result from the importance of insulin as an anabolic hormone [1-3]. 425 million people in the world have diabetes, 212 million undiagnosed diabetic patients, over 1 million children and adolescence have DM1, 279 million people with diabetes live in urban area, 327 million people with diabetes are working-age (aged 20-64), 98 million people are 65-79 years [4]. Type 2 diabetes is the most common type of diabetes, accounting for around 90% of all cases of diabetes. In type 2 diabetes, hyperglycemia is the result of an inadequate production of insulin and inability of the body to respond fully to insulin, defined as insulin resistance. During a state of insulin resistance, insulin is ineffective and therefore initially prompts an increase in insulin production to reduce rising glucose levels but over time a state of relative inadequate production of insulin can develop. Type 2 diabetes is the most commonly seen in older adults, but it is increasingly seen in children, adolescents and younger adults due to rising levels of obesity, physical inactivity and poor diet.
Insulin resistance in DM2 has many manifestations that include obesity, nephropathy, essential hypertension, dyslipidemia, ovarian hyperandrogenism and premature adrenarche, non-alcoholic fatty liver disease and systemic inflammation [5,6]. The presence of abdominal obesity and accompanying insulin resistance provide fertile conditions for the development of NAFLD. Indeed, NAFLD is often considered as the hepatic manifestation of insulin resistance and the metabolic syndrome [7]. It is well known that the prevalence of NAFLD associated with several risk factors such as obesity, metabolic syndrome, insulin resistance and type 2 diabetes [8,9]. Compared to healthy populations, patients with type 2 diabetes mellitus show increased risk for catching of advanced liver disease including fibrosis, cirrhosis and hepatocellular carcinoma [10]. A significant increase in the prevalence of NAFLD in type 2 diabetes mellitus patients was observed. The pooled prevalence was 54% (95% CI 45% - 64%). Whereas, in eligible studies involved in this meta-analysis, the prevalence of NAFLD in type 2 diabetes mellitus patients ranged from 5% (24) to 87% [11].
Here, the high prevalence of NAFLD in type 2 diabetes mellitus patients indicated the importance of management and early evaluation of NAFLD in type 2 diabetes patients [12]. Obesity and iron deficiency (ID) are considered the two most common nutritional disorders worldwide [13]. The association between obesity and iron status was first described by Wenzel in 1962, who noted that obese adolescents had lower serum iron compared to non-obese adolescents [14]. More recently, research has focused on the role of systemic, obesity-related, low-grade inflammation leading to ID via increased hepcidin expression [15-17].
When the body is iron replete, hepcidin concentrations are high, and the iron supply to the plasma is reduced; however, when iron demands are high, hepcidin concentrations are reduced, and more iron enters the circulation. Major iron-exporting cells include macrophages, which recycle heme-derived iron from senescent RBCs; hepatocytes, which are the major site of iron storage; and intestinal enterocytes, which facilitate dietary intake of iron.
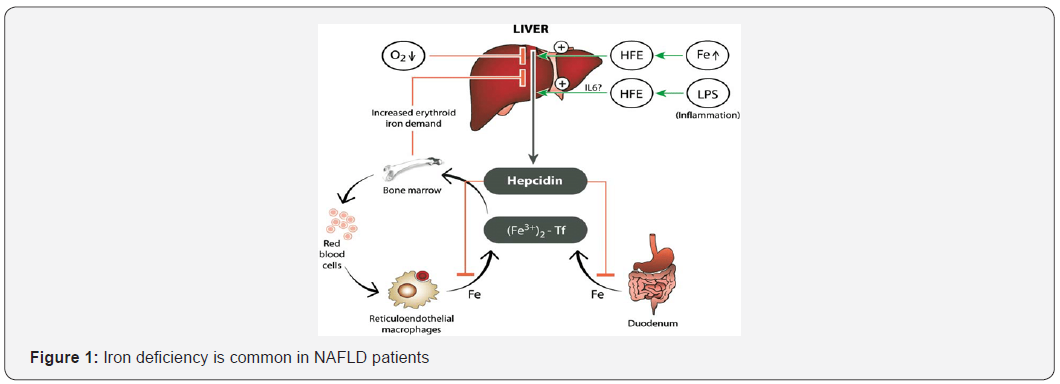
However, most cells can export iron through FPN1 and, thus, are potential hepcidin targets. The bone morphogenetic protein-SMAD regulatory pathway is central to hepcidin regulation, and it is likely the pathway through which the iron dependent regulation of hepcidin occurs but inflammatory cytokines hypoxia and several other factors [18-22] also can activate signaling pathways that lead to alterations in hepcidin transcription. Increased iron stores and inflammation enhance hepcidin expression, whereas reduced iron stores and hypoxia lower expression. Hepcidin expression increased in chronic inflammation, by inflammatory cytokines interleukin-6 (IL-6) and interleukin-1β (IL-1β) via STAT3 [23]. Hepcidin is predominantly expressed in the liver, but also in subcutaneous and visceral adipose tissue, albeit at such a low level it may not contribute to systemic hepcidin levels [24,25] (Figure 1). Iron deficiency is common in NAFLD patients. Circulating serum hepcidin levels in NAFLD patients with iron deficiency are low reflecting an appropriate physiologic response of hepcidin signaling to iron deficiency. Obesity and female sex are likely the most important risk factors of ID in NAFLD subjects; however, alcohol consumption, presence of diabetes and potentially racial background may also be contributing factors. Increased hepcidin as a result of obesity-induced systemic inflammation may initially contribute to iron deficiency but once ID is established hepcidin is appropriately down regulated [26].
Conclusion
Thus, the impact of obesity-induced hepcidin upregulation and the relationship between liver vs adipose-derived hepcidin, and iron regulation in the setting of obesity is not well understood. Moreover, as we can see from the conducted numerous studies, the occurrence of anemia was studied in fatty hepatosis (NAFLD), especially the emphasis was on the role in appearance of anemia of the insufficiently explored protein hepcidin secreted by the liver. According to the latest data, hepcidin is a cellular bioregulator of iron metabolism in the body. Thus, since fatty hepatosis (NAFLD) causes inflammatory and destructive changes in the liver, it can cause an increase of hepcidin levels which in turn will inhibit the absorption of iron through the intestines and the release of iron from macrophages, which in turn leads to iron deficiency anemia. As we all know, in 60-70 percent of all cases, fatty hepatosis (NAFLD) appears in patients with type 2 diabetes, and therefore we decided to conduct a study in this direction. We want to prove the role of hepcidin in the appearance of anemia in diabetes mellitus with fatty hepatosis (NAFLD).
References
- American Diabetes Association (2014) Diagnosis and classification of diabetes mellitus. Diabetes Care 37 (Suppl 1): S62-S69.
- Craig ME, Hattersley A, Donaghue KC (2009) Definition, epidemiology and classification of diabetes in children and adolescents. Pediatr Diabetes 10 Suppl 12: 3-12.
- Galtier F (2010) Definition, epidemiology, risk factors. Diabetes Metab 36: 628-651.
- IDF DIABETES ATLAS (2017) In: 8th (Edn)
- Rosenbloom AL, Silverstein JH, Amemiya S, Zeitler P, Klingensmith GJ (2009) Type 2 diabetes in children and adolescents. Pediatr Diabetes 10 Suppl 12: 17–32.
- Kraemer FB, Ginsberg HN (2014) Gerald M. Reaven, MD: Demonstration of the central role of insulin resistance in type 2 diabetes and cardiovascular disease. Diabetes Care 37: 1178–1181.
- Anstee QM, Targher G, Day CP (2013) Progression of NAFLD to diabetes mellitus, cardiovascular disease or cirrhosis. Nat Rev Gastroenterol Hepatol 10(6): 330-344.
- Marchesini G, Brizi M, Bianchi G, Tomassetti S, Bugianesi E, et al. (2001) Nonalcoholic fatty liver disease. Diabetes 50: 1844-50.
- Forlani G, Giorda C, Manti R, Mazzella N, De Cosmo S, et al. (2016) The burden of NAFLD and its characteristics in a nationwide population with type 2 diabetes. J Diabetes Res 2016: 2931985.
- Bondini S, Kleiner DE, Goodman ZD, Gramlich T, Younossi ZM (2007) Pathologic assessment of non-alcoholic fatty liver disease. Clin Liver Dis 11: 17-23.
- Prashanth M, Ganesh H, Vima M, John M, Bandgar T, et al. (2009) Prevalence of nonalcoholic fatty liver disease in patients with type 2 diabetes mellitus. J Assoc Physicians India 57: 205-10.
- Yi M, Chen RP, Yang R, Chen H (2017) Increased prevalence and risk of non‐alcoholic fatty liver disease in overweight and obese patients with Type 2 diabetes in South China. Diabet Med 34(4): 505-513.
- Harris RJ (2004) Nutrition in the 21st century: what is going wrong. Arch Dis Child 89(2): 154-158.
- Wenzel BJ, Stults HB, Mayer J (1962) Hypoferraemia in obese adolescents. Lancet 2: 327-328.
- Yanoff LB, Menzie CM, Denkinger B, et al. (2007) Inflammation and iron deficiency in the hypoferremia of obesity. Int J Obes (Lond) 31: 1412-1419.
- Bekri S, Gual P, Anty R, et al. (2006) Increased adipose tissue expression of hepcidin in severe obesity is independent from diabetes and NASH. Gastroenterology 131: 788-796.
- Tussing-Humphreys LM, Nemeth E, Fantuzzi G, et al. (2010) Elevated Systemic Hepcidin and Iron Depletion in Obese Premenopausal Females. Obesity 18(7): 1449-1456.
- Ganz T (2013) Systemic iron homeostasis. Physiol Rev 93(4): 1721-1741.
- Ganz T, Nemeth E (2015) Iron homeostasis in host defence and inflammation. Nat Rev Immunol 15(8): 500-510
- Peyssonnaux C, Nizet V, Johnson RS (2008) Role of the hypoxia inducible factors HIF in iron metabolism. Cell Cycle 7(1): 28-32.
- Guo W, Bachman E, Li M, Roy CN, Blusztajn J, et al. (2013) Testosterone administration inhibits hepcidin transcription and is associated with increased iron incorporation into red blood cells. Aging Cell 12: 280-291.
- Hou Y, Zhang S, Wang L, Li J, Qu G, et al. (2012) Estrogen regulates iron homeostasis through governing hepatic hepcidin expression via an estrogen response element. Gene 511(2): 398-403.
- Lee P, Peng H, Gelbart T, et al. (2005) Regulation of hepcidin transcription by interleukin-1 and interleukin-6. Proc Natl Acad Sci U S A 102(6): 1906-1910.
- Bekri S, Gual P, Anty R, et al. (2006) Increased adipose tissue expression of hepcidin in severe obesity is independent from diabetes and NASH. Gastroenterology 131: 788-796.
- Tussing-Humphreys L, Frayn KN, Smith SR, et al. (2011) Subcutaneous adipose tissue from obese and lean adults does not release hepcidin in vivo. Scientific World Journal 11: 2197-2206.
- Asma Siddique, James E. Nelson, Bradley Aouizerat, Matthew M Yeh, and Kris V Kowdley (2014) Iron Deficiency in Patients with Nonalcoholic Fatty Liver Disease is Associated with Obesity, Female Sex, and Low Serum Hepcidin. Clin Gastroenterol Hepatol 12(7): 1170-1178.






























