A Rare Case of Subacute Thyroiditis Simultaneously Complicated by Graves’ Disease: A Case Report and Review of the Literature
Ayako Fukushima, Makito Tanabe, Yuichi Terawaki, Takashi Fukuda, Takashi Nomiyama, and Toshihiko Yanase*
Department of Endocrinology and Diabetes Mellitus, Fukuoka University, Japan
Submission: July 27, 2017; Published: August 28, 2017
*Corresponding author: Toshihiko Yanase, MD, Department of Endocrinology and Diabetes Mellitus, School of Medicine, Fukuoka University, 7-45-1 Nanakuma, Jonan-ku, Fukuoka 814-0180, Japan, Tel:+81-92-801-1011; Fax: +81-92-865-5163; Email: tyanase@fukuoka-u.ac.jp
How to cite this article: Ayako F, Makito T, Yuichi T, Takashi F, Takashi N, et al. A Rare Case of Subacute Thyroiditis Simultaneously Complicated by Graves’ Disease: A Case Report and Review of the Literature.J Endocrinol Thyroid Res. 2017; 2(4):555595. DOI: 10.19580/JETR.2017.02.555595.
Abstract
A 46-year-old female presented with neck pain and thyrotoxicosis. Further examination revealed that she had both subacute thyroiditis (SAT) and Graves’ disease (GD). These diagnoses were made based on increased thyroid hormone levels, high TSH-R antibody titers, echographic findings of the thyroid and concomitant inflammatory findings. Her thyrotoxicosis, inflammatory findings and TSH-R antibodies were gradually normalized by oral administration of prednisolone. Interestingly, she possessed characteristic HLA typing associated with susceptibility to both SAT and GD. A review of four other cases showing simultaneous onset of SAT and GD in the literature revealed a similar clinical course and comparable changes in TRAb in all cases. The occurrence of SAT may trigger thyroid antigen release and cause the simultaneous onset of transient GD in some susceptible cases with latent GD.
Keywords: Graves’ disease; Subacute thyroiditis; Hyperthyroidism; TRAb; Thyroid receptor antibody; TSAb; Thyroid-stimulating antibody
Abbreviations: GD: Graves’ Disease; HLA: Human Leukocyte Antigen; SAT: Subacute Thyroiditis; Trab; TSH: Receptor Antibody; TSH: Thyroid Stimulating Hormone
Introduction
Graves’ disease (GD) and destructive thyroiditis are representative thyroid diseases characterized by thyrotoxic conditions. Destructive thyroiditis usually includes painless thyroiditis and subacute thyroiditis (SAT). SAT is typically a viral infectious disease and usually accompanies anterior neck pain and tenderness of the thyroid gland. The thyrotoxic phase by inflammation is self-limited or improved by steroid treatment. Thyroid function gradually returns to normal but occasionally develops into hypothyroidism as a result of thyroid damage. In contrast, GD is an autoimmune disease as evidenced by the presence of TSH receptor antibodies (TRAb) or thyroid stimulating antibodies (TSAb). The thyrotoxicosis of GD is usually not self-limited and requires treatment, such as antithyroid drugs, radioisotopes or thyroidectomy. Thus, the differential diagnosis of SAT and GD is very important in clinical practice [1].
As an essential tool for differential diagnosis, 131I or Technetium-99m (99mTc) scintigraphy is useful. Radioactive iodide uptake into the thyroid is usually decreased in SAT because of suppressed TSH and inflammation. The inflammation leads to destruction of thyroid follicles [2,3]. In contrast, uptake is increased in GD because of the autonomous increase in the synthesis of thyroid hormones. Thus, there appears to be no etiological relationship between the two diseases. However, 34 patients with GD following SAT with various time intervals have been noted [4-14]. In a report by Nakano et al. [14] describing 7 patients with GD following SAT, the interval between the onset of SAT and GD ranged from 1 to 8 months (mean, 4.7 months). However, cases with concurrent onset of SAT and GD are very rare, and only four cases have previously been reported [15-18].
We observed a rare case in which GD and SAT were thought to develop simultaneously. In these patients, the clinical course of GD was unusual, because the apparent increase in TRAb at the onset of disease was gradually decreased by prednisolone treatment alone, and antithyroid medication was not required. We report this case with discussion and a review of four other similar cases in the literature.
Case Report
A 46-year-old female first noted a cough and nasal discharge. On day 4, she experienced a sore throat, swallowing pain and frontal neck pain. From day 5, an occipital headache was also noted. During this time, a fever was not evident. On day 9, she consulted a doctor. At this time, a goiter was observed, and the patient was diagnosed with primary hyperthyroidism based on suppressed serum TSH level (less than 0.01ng/ml) and increased FT4 level (4.82ng/ml; reference range, 0.9 to 1.7). On day 19,for further examinations, she visited our department with a consultation letter from the first doctor. Weight loss of 5kg was observed during the approximate 2 weeks before the visit to our hospital. Although she felt no palpitations or hand tremors, she was afflicted with general fatigue and hyperhidrosis. She had no remarkable personal or family history. She was medicated with loxoprofen sodium (60mg/day) and rebamipide (100mg/day).
The patient’s physical examination revealed the following characteristics: height, 154cm; body weight, 68kg; body mass index, 28.67kg/m2; supine blood pressure, 113/68mmHg; pulse, 88 beats/min; body temperature, 36.7 °C. While occipital pain was still observed, symptoms, including sore throat, swallowing difficulty and spontaneous pain of the front neck, were not remarkable. The thyroid gland was enlarged (right < left), elastic and hard. The patient felt mild tenderness on the left lobe of the thyroid. No other physical abnormalities were observed.
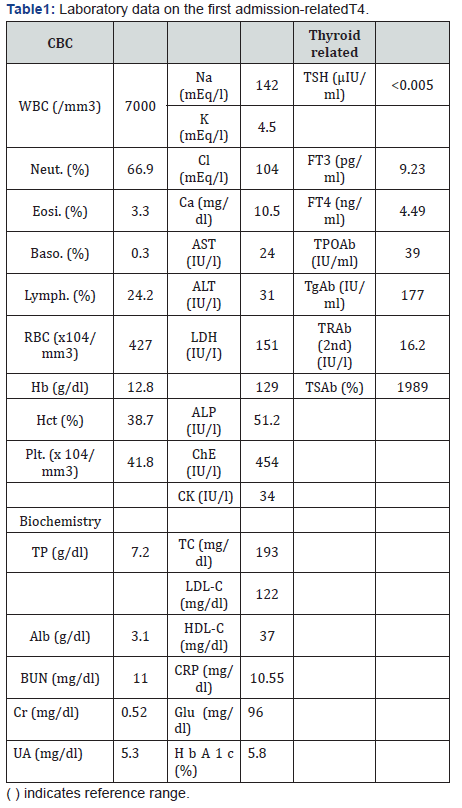
Laboratory data on admission is shown in Table 1. Biochemical findings revealed elevated AST, g-GTP, ALP and ChE levels, suggesting possible thyrotoxicosis-induced liver dysfunction or fatty liver when we considered her body mass index of 28.67 kg/ m2. In addition, CRP was increased to 10.55mg/dl, indicating an inflammatory response. Endocrinological examinations revealed that TSH levels were below the reference range (0.1μU/ml or less), and serum FT4 and FT3 levels were elevated, with values of 4.49ng/dl and 9.23ng/dl, respectively. Anti-TPO antibody, antithyroglobulin antibody, TRAb and TSAb titers were all elevated.
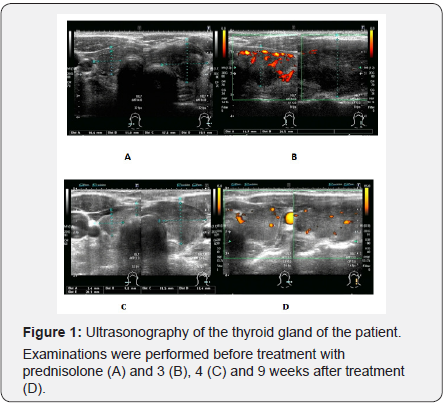
Thyroid ultrasonography before treatment was performed 1 week after the first visit to our hospital on day 26 (Figure 1). The examination revealed increased internal blood flow in the right lobe on color Doppler ultrasound, which was consistent with a diagnosis of GD. In contrast to the findings in the right lobe, the left lobe was enlarged with a heterogeneous hypoechoic region and accompanied by relatively decreased internal blood flow, suggesting SAT. Although we could not completely rule out the possibility that GD and SAT occurred throughout both lobes of the thyroid gland, GD appeared to be predominant in the right lobe, whereas SAT appeared to be in the left lobe.
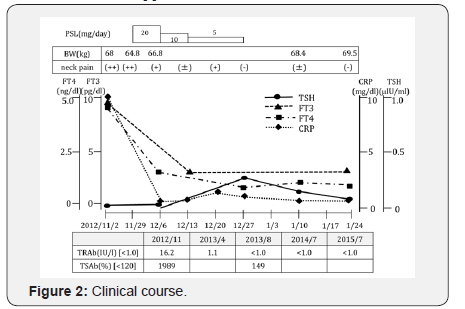
It was relatively easy to diagnose SAT in this patient because of the inflammatory findings in both clinical and laboratory assessments (Figure 2). A diagnosis of GD was also suspected primarily because of high TRAb and TSAb titers. The early performance of thyroid scintigraphy was unfortunately not possible because of the patient’s circumstances. Ultimately, 99mTc scintigraphy was performed 19 days after initiation of prednisolone treatment on day 45. Contrary to our prediction, the actual results revealed relatively increased uptake in the left lobe compared with that of the right lobe, and the overall uptake rate was within the normal range of 2.4% at 20 minutes (data not shown). Because treatment with prednisolone can suppress inflammation and thyroid hormone synthesis, the results may not reflect the actual condition.
Oral administration of PSL 20mg/day was initiated for treatment of SAT on day 26. Prednisolone dosage was optimized by confirming the improvement of anterior neck pain and inflammatory markers in blood. Since thyroid function was improved rapidly after the start of 20mg PSL, the dose was gradually decreased and terminated after 1 month (Figure 2).
Thyroid ultrasonography performed at 3, 4 and 9 weeks after treatment with prednisolone revealed gradual but clear improvements in the left lobe each week. Specifically, the enlarged left lobe was gradually reduced, the observed hypoechoic region was mostly returned to isochoric, and the decreased internal blood flow on color Doppler ultrasound was gradually recovered (Figure 1A-D) In contrast, the increased internal blood flow in the right lobe was gradually reduced. At 9 weeks, echogenic levels of both lobes were comparable (Figure 1D).
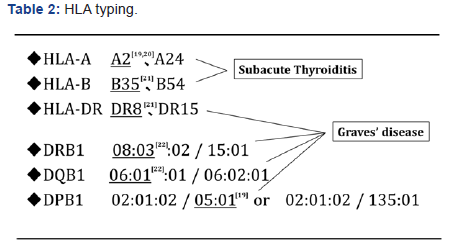
Because genetic susceptibility to both SAT and GD has been shown to be strongly linked to HLA subtype [19-22], we investigated this point. The patient’s HLA was found to have A2 [19,20], B35 [21] and DR8 [21], which are associated with SAT susceptibility, and DRB1 *08:03 [22], DQB1*06:01 [22] and DQB1*05:01 [19], which are associated with GD susceptibility. The patient seemed to be sensitive to both SAT and GD in the constitutional character (Table 2).
Subsequently, thyroid function was maintained in a normal range, and oral administration of antithyroid medication was unnecessary. TRAb was improved to 1.1 IU/ml after 3 months of prednisolone treatment and became negative after 7 months of the treatment. Over the next 2 years, thyroid function and TRAb were maintained in a normal range.
Discussion
We observed a patient afflicted by both SAT and GD. Consistent with SAT, she presented with neck pain, laboratory evidence of systemic inflammation and thyrotoxicosis. In addition, initial laboratory data revealed high titers of TRAb and TSAb, suggesting underlying autoimmune GD. Her thyrotoxicosis possibly resulted from the release of thyroid hormone from destroyed thyroid tissue by SAT and from increased thyroid hormone synthesis by GD. The concurrent occurrence of GD and SAT is relatively rare. To our knowledge, there have been only 34 cases reported of patients affected with GD after SAT with variable time differences in the onset [4-14]. Only four cases with simultaneous onset of both diseases have been reported [15-18]. Our case appears to be the fifth rare case.
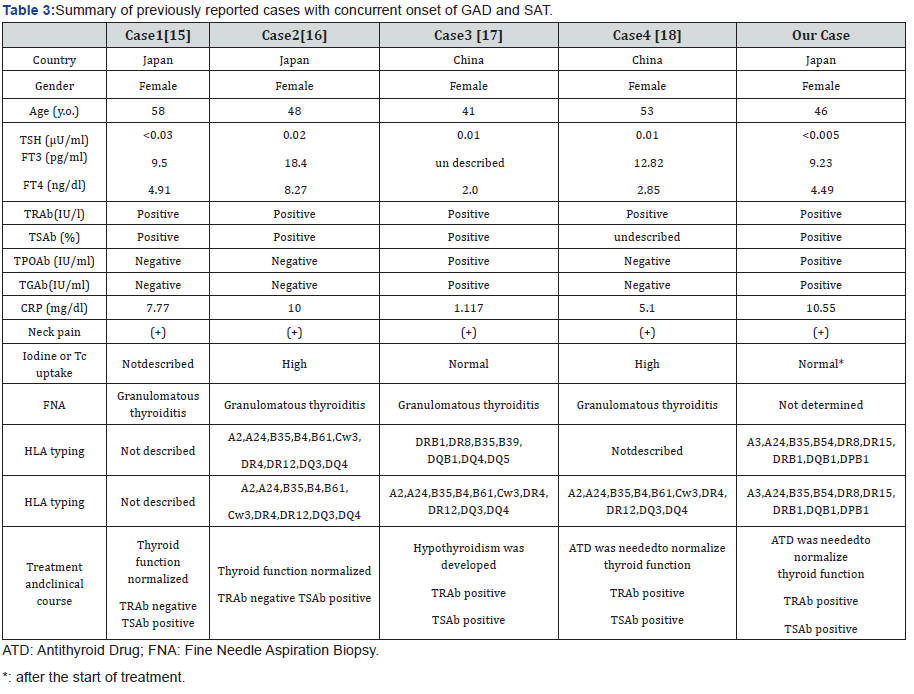
We reviewed the four other cases in the literature [15-18] and compared them with our case (Table 3). Interestingly, all cases were middle-aged female patients from Asia (Japan and China). Our patient displayed a similar clinical phenotype and clinical course, including the presence in TRAb or TSAb, to all other cases (Table 3). TRAb-associated thyroid dysfunction following SAT has also been noted in another study [23]. The difference among 5 cases is whether TRAb or TSAb became negative (case 2 and our case) or kept present (cases 1, 3 and 4) after the treatment of SAT. Case 2 and our case seem to be transient GD manifested from latent GD, while cases 1, 3 and 4 seem to have developed into overt GD triggered by SAT.
The simultaneous occurrence of both destructive thyroid disease and GD is likely not coincidental, as the two diseases are strongly associated with each other. Fukata et al. [24] reported that one of four patients had both GD and destructive Hashimoto’s disease at the same time by immunological events. Thus, SAT and GD could also occur simultaneously by viral infection or an autoimmune mechanism. Thyroid antigen release in SAT may be similar to that in patients with GD after radiotherapy. Importantly, this patient had HLA typing associated with susceptibility to both SAT and GD [19-21]. Similar susceptibility to both diseases was also found in cases 2 [16] and 3 [17] (Table 3). Taken together, the occurrence of SAT may trigger thyroid antigen release and cause the simultaneous onset of transient GD in some cases with latent GD.
The simultaneous presence of both SAT and GD at initial presentation may explain why iodine and Tc uptake are not necessarily high or low as typically observed in GD or SAT, respectively (Table 3). Not only the circulating TSAb by GD but also the increased tissue vascularity in the affected thyroid in GD and markedly decreased vascularity in SAT [25] may affect the results of echographic or scintigraphic examinations in a complicated manner. Based on echographic examinations, theleft lobe of the thyroid appeared to be more affected by SAT in our case. It is unclear whether the inflammatory process was localized to a single lobe. This possibility appears to be rare but has been reported [26].
In our case, thyrotoxicosis, inflammatory findings, TRAb and TSAb were gradually normalized by oral administration of prednisolone alone. Antithyroid medications were not needed. Thus, continuous overproduction of TRAb or TSAb was not observed. This clinical time course also suggests that the occurrence of SAT may trigger thyroid antigen release and cause the simultaneous onset of transient GD in some susceptible cases with latent GD.
Conclusion
In conclusion, we observed a rare case in which GD and SAT coexisted. When examining hyperthyroidism, it is necessary to consider the rare but possible coexistence of both diseases and carefully select treatment by distinguishing the characteristics of each disease.
Acknowledgement
We thank Lynley Pound, Ph.D., from Edanz Group (www. edanzediting.com/ac) for editing a draft of this manuscript.
Conflict of Interest
The authors have no conflict of interest to declare.
References
- Mandel SJ, Larsen PR, Davis TF (2011) Willianms Textbook of Endocrinology (12th edn), Saunders, Philadelphia, USA, pp. 362-405.
- Hamburger JI, Kadian G, Rossin HW (1965) Subacute thyroiditis-- evolution depicted by serial 131-I scintigram. J Nucl Med 6(8): 560- 565.
- Fragu P, Rouqier P, Schlumberger M, Tubiana M (1982) Evolution of thyroid 127I stores measured by X-ray fluorescence in subacute thyroiditis. J Clin Endocrinol Metab 54(1): 162-166.
- Sheets RF (1955) The sequential occurrence of acute thyroiditis and thyrotoxicosis. JAMA 157(2): 139-140.
- Perloff WH (1956) Thyrotoxicosis following acute thyroiditis: a report of 5 cases. J Clin Endocrinol Metab 16(4): 542-546.
- Werner SC (1979) Graves’ disease following acute (subacute) thyroiditis. Arch Intern Med 139(11): 1313-1315.
- Wartofsky L, Shaaf M (1987) Graves’ disease with thyrotoxicosis following subacute thyroiditis. Am J Med 83(4): 761-764.
- Fukata S, Matsuuka F, Kobayashi A, Hirai K, Kuma K, Sugawara M (1992) Development of Graves’ disease after subacute thyroiditis: two unusual cases. Acta Endocrinol (Copenh) 126(6): 495-496.
- Bartalena L, Bogazzi F, Pecori F, Martino E (1996) Graves disease occurring after subacute thyroiditis: report of a case and review of the literature. Thyroid 6(4): 345-348.
- Bennedbeak FN, Gram J, Hegedüs L (1996) The transition of subacute thyroiditis toGraves’ disease as evidenced by diagnostic imaging. Thyroid 6(5): 457-459.
- Grunenberger F, Chenard MP, Weber JC, Jaeck D, Schlienger JL (1998) Relapse of Graves’ disease after subacute thyroiditis. Thyroid 8(8): 683-685.
- Iitaka M, Kakinuma S, Yamanaka K, Fujimaki S, Oosuga I, et al. (2001) Induction of autoimmune hypothyroidism and subsequent hyperthyroidism by TSH receptor antibodies following subacute thyroiditis: a casereport. Endocr J 48(2): 139-142.
- Wang X, Renedo MF (2004) Graves’ disease occurring afterhyperparathyroidismand subacute thyroiditis. Endocr Pract 10(6): 509-511.
- Nakano Y, Kurihara H, Sasaki J (2011) Graves Disease Following Subacute Thyroiditis. Tohoku J Exp Med 225(4): 301-309.
- Mitani Y, Shigemasa C, Kouchi T, Taniguchi S, Ueta Y, et al. (1992) Detection of thyroid-stimulating antibody in patients with inflammatory thyrotoxicosis. Horm Res 37(4-5): 196-201.
- Fujii S, Miwa U, Seta T, Ohoka T, Mizukami Y (2003) Subacute thyroiditis with highly positive thyrotropin receptor antibodies and high thyroidal radioactive iodine uptake. Intern Med 42(8): 704-709.
- Hoang TD, Mai VQ, Clyde PW, Shakir MK (2011) Simultaneous occurrence of subacute thyroiditis and Graves’ disease. Thyroid 21(12): 1397-1400.
- Fang F, Yan S, Zhao L, Jin Y, Wang Y (2016) Concurrent Onset of Subacute Thyroiditis and Graves’ Disease. Am J Med SCi 352(2): 224-226.
- Dong RP, Kimura A, Okubo R, Shinagawa H, Tamai H, et al. (1992) HLA-A and DPB1 loci confer susceptibility to Graves disease. Hum Immunol 35(3): 165-172.
- Wan XL, Kimura A, Dong RP, Honda K, Tamai H, et al. (1995) HLA-A and -DRB4 genes in controlling the susceptibility to Hashimoto’s thyroiditis. Hum Immunol 42(2): 131-136.
- Kobayashi N, Tamai H, Nagai K, Matsubayashi N, Matsuzuka F, et al. (1985) Studies on the pathogenesis of Subacute Thyroiditis. Nihon Naibunpi Gakkai Zasshi 61(7): 737-743.
- Katsuren E, Awata T, Matsumoto C, Yamamoto K (1994) HLA class II alleles in Japanese patients with Graves’ disease: weak associations of HLA-DR and -DQ. Endocr J 41(6): 599-603.
- Iitaka M, Momotani N, Hisaoka T, Yoshimura H, Ishikawa N, et al. (1998) TSH receptor antibody-associated thyroid dysfunctionfollowing subacute thyroiditis. Clin Endocrinol 48(4): 445-453.
- Fukata S, Matsuzuka F, Hara T, Mukuta T, Kuma K, et al. (1993) Rapidly progressive thyroid failure in Graves’ disease after painful attack in the thyroid gland. Arch Intern Med 153(18): 2157-2161.
- Hiromatsu Y, Ishibashi M, Miyake I, Soyejima E, Yamashita K, et al. (1999) Color Doppler ultrasonography in patients with subacute thyroiditis. Thyroid9(12): 1189-1193.
- Hamburger JI (1986) The various presentations of thyroiditis: diagnostic considerations. Ann Intern Med 104(2): 219-224.






























