Iodine Deficiency Amongst Vulnerable Population of Endemic Districts of India
Neha Sareen and Umesh Kapil*
Department of Gastroenterology and Human Nutrition, All India Institute of Medical Sciences, India
Submission: April 04, 2017; Published: May 30, 2017
*Corresponding author: Umesh Kapil, Professor and Head, Department of Gastroenterology and Human Nutrition, All India Institute of Medical Sciences, New Delhi, Email: umeshkapil@gmail.com
How to cite this article: Neha Sareen and Umesh Kapil.Iodine Deficiency Amongst Vulnerable Population of Endemic Districts of India. J Endocrinol Thyroid Res. 2017; 2(2): 555581. DOI:10.19080/JETR.2017.02.555581
Abstract
Iodine deficiency (ID) is the preventable cause of mental retardation worldwide. Himachal Pradesh (HP) and Uttarakhand (UK) states are known endemic regions to ID. Hence, the present study was conducted to assess the current iodine nutritional status amongst vulnerable population of endemic districts of India. Three districts from each state namely; Kangra, Kullu and Solan from Himachal Pradesh and Udham Singh Nagar, Nainital amd Pauri Garhwal from Uttarakhand state were selected for the present study. In each district, 30 clusters were identified by utilizing population-proportional-to-size cluster sampling methodology. Total of 5748 (HP) and 6143 (UK) School Age Children (SAC); 1711 (HP) and 1727 (UK) Pregnant Mothers (PMs) and 1934 (HP) and 2013 (UK) Neonates were included in study. Clinical examination of thyroid of each child and PM was conducted. Casual urine samples were collected from children and PMs. Cord blood samples were collected for estimation of Thyroid Stimulating Hormone (TSH) amongst neonates.
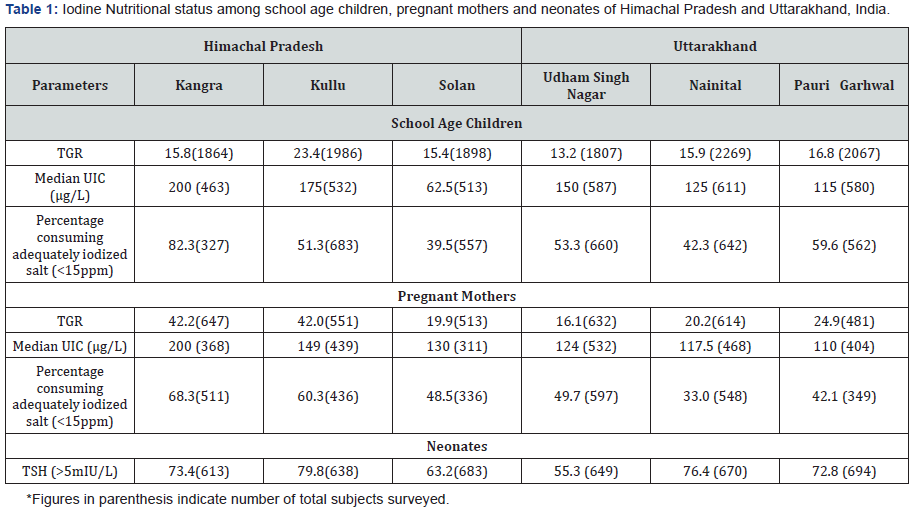
School age children: Total Goitre Rate (TGR) was 15.8% (Kangra), 23.4% (Kullu) and 15.4% (Solan) in HP and 13.2% (Udham Singh Nagar), 15.9% (Nainital) and 16.8% (Pauri Garhwal) in UK. Median Urinary Iodine Concentration (UIC) level was 200μg/l (Kangra), 175μg/l (Kullu) and 62.5μg/l (Solan) in HP and 150μg/l (Udham Singh Nagar), 125μg/l (Nainital) and 115μg/l (Pauri Garhwal) in UK.
Pregnant mothers: TGR was 42.2% (Kangra), 42.0 % (Kullu) and 19.9% (Solan) in HP, and 16.1% (Udham Singh Nagar), 20.2 % (Nainital) and 24.9% (Pauri Garhwal) in UK. Median UIC level was 200μg/l (Kangra), 149μg/l (Kullu) and 130μg/l (Solan) in HP and 200μg/l (Udham Singh Nagar), 149μg/l (Nainital) and 130μg/l (Pauri Garhwal) in UK.
Neonates: TSH levels of >5mIU/L were found in 73.4% (Kangra), 79.8% (Kullu) and 63.2% (Solan) of neonates in HP and 55.3% (Udham Singh Nagar), 76.4% (Nainital) and 72.8% (Pauri Garhwal) of neonates in UK. According to the TGR and median UIC levels; SAC are in a transition phase from iodine deficient to iodine sufficient. PMs and neonates of all the districts surveyed were iodine deficient according to the median UIC level of <150μg/l and TSH levels of more than 5mIU/l.
Keywords:Iodine deficiency; Goiter; Thyroid stimulating hormone; India
Introduction
Iodine is an essential micronutrient required for normal thyroid function, growth and development. Iodine deficiency (ID) is the single most important and preventable cause of mental retardation worldwide. During pregnancy, recommended dietary allowance of iodine is increased by 50% due to
- Physiological increase in maternal and fetal thyroid hormone production and
- Increase in renal iodine losses [1].
Hence, the recommended dietary allowance of iodine required by PM is higher (250μg/day) as compared to normal adult (150μg/day) [2]. If the Pregnant Mother (PM) is iodine deficient, there is a decreased synthesis of thyroxin by fetal thyroid whichleads to compromised mental and physical development of fetus [3]. Iodine deficiency in pregnant women poses additional reproductive risks, including overt hypothyroidism, infertility and increased abortions in pregnant women [4]. ID in fetus results in miscarriages, stillbirths, brain disorders, retarded psychomotor development, speech and hearing impairments [2].
People living in an areas affected by severe ID may have an intelligence quotient (IQ) of up to 13.5 points below that of those from areas where there is no ID [2]. Himachal Pradesh and Uttarakhand states, India are known endemic regions for iodine deficiency. School age children (6-12 years) (SAC), pregnant mothers (PMs) and neonates are the most vulnerable groups as they are especially sensitive to even marginal iodine deficiency.There are some reports covering small populations from different districts of Himachal Pradesh and Uttarakhand which have reported high prevalence of iodine deficiency amongst school age children [5-7]. The National Family Health Survey (NFHS- 4) also reported only the household iodized salt consumption in India [8]. There was a lack of data available on the effects of iodine deficiency on these 3 vulnerable age groups (SAC, PMs and Neonates) from Himachal Pradesh and Uttarakhand states, India. Hence, the present study was conducted to assess the current iodine nutritional status amongst vulnerable population (SAC, PMs and Neonates) of endemic districts of India.
Methodology
Study Participants
A cross sectional survey was conducted in Himachal Pradesh and Uttarakhand states, India. Himachal Pradesh state has three geographical regions namely:
- Kangra
- Mandi and
- Shimla.
One district was selected from each region i.e. Kangra (Kangra region), Kullu (Mandi region) and Solan (Shimla region). The state of Uttarakhand has 13 districts (Dehradun, Uttarkashi, Chamoli, Dehradun, Pauri Garhwal, Tehri Garhwal, Rudraprayag, Haridwar, Almora, Pithoragarh, Nainital, Bageshwar, Champawat and Udham Singh Nagar) which are distributed in three geographical regions namely
- Terai (Plain),
- Kumaon and
- Garhwal
One district was selected from each region i.e. Udham Singh Nagar (Terai region), Nainital (Kumaon region) and Pauri Garhwal (Garhwal region). From each district iodine nutritional status was assessed amongst; SAC (6-12 years), PMs and Neonates.
School age children
The 30 clusters (Schools) in each district were selected by using Population Proportionate to Size Sampling (PPS) methodology recommended by WHO/UNICEF/ICCIDD [2]. In each school, the children were briefed about the objectives of the study and the informed consent was undertaken.
4.2.1. Sample size: Keeping in view the anticipated prevalence of 15%, a confidence level of 95%, absolute precision of 2.0, and a design effect of 1.5, a total sample size of 1800 was calculated for each district. However, we included 1864 (Kangra), 1986 (Kullu) and 1898 (Solan), Himachal Pradesh and 1807 (Udham Singh Nagar), 2269 (Nainital), and 2067 (Pauri Garhwal), Uttarakhand of SAC.
Pregnant mothers
The 30 clusters (antenatal) in each district were selected by using Population Proportionate to Size Sampling (PPS) methodology recommended by WHO/UNICEF/ICCIDD [2]. From each antenatal clinics seventeen PMs who were attending the antenatal clinics were included.
Sample size: Keeping in view the anticipated prevalence of 5%, a confidence level of 95%, relative precision of 3.0, and a design effect of 2, a total sample size of 450 from each district as calculated. However, we included 647 (Kangra), 551 (Kullu) and 513 (Solan), Himachal Pradesh and 632 (Udham Singh Nagar), 614 (Nainital), and 481 (Pauri Garhwal), Uttarakhand of pregnant mothers in present study. Pregnant mothers who were consuming drugs that could influence their thyroid status were excluded from the study.
Neonates
In selected districts, all the hospitals/institutions providing obstetric services were identified and enlisted. Out of which 5-6 hospitals/ institutions that provided maximum deliveries were selected. A total of 510 births occurred in these institutions during the study period were included from each district. The informed consents were undertaken from mother of the neonates. Caesarean deliveries, deliveries in which iodine preparations were used and PMs who were on anti-thyroid therapies were excluded from the study.
Sample Size: Keeping in view the anticipated prevalence of 2.9%, a confidence level of 95%, absolute precision of 2.0, and a design effect of 2, a total sample size of 541 from each district was calculated. However, we included 613 (Kangra), 638 (Kullu) and 683 (Solan), Himachal Pradesh and 649 (Udham Singh Nagar), 670 (Nainital), and 694 (Pauri Garhwal) of neonates in the present study.
Clinical thyroid examination
Clinical examination of the thyroid gland of each child and PM was conducted by trained field investigator. The grading of the goitre was done according to the criteria recommended jointly by WHO/UNICEF/ICCIDD
- Grade 0- not palpable and not visible
- Grade I- palpable but not visible
- Grade II- palpable and visible) [2].
The sum of grades I and II provided the Total Goiter Rate (TGR) of the study population. When in doubt, the investigators recorded the immediate lower grade. The intra and inter observer variation was minimized by repeated training of the investigator and by random examinations of goitre grades by second author.
Laboratory measurements
Urine sample: In each cluster, a minimum of fifteen SAC and ten PMs (from the subjects who were enrolled for clinical thyroid examination) were requested to provide casual urine samples inthe plastic bottles with the screw caps provided to them. The urine samples were stored in the refrigerator until analysis. The UIC analysis was done within 2 months using the wet digestion method [9].
Salt sample: A minimum of ten SAC and eleven PMs were selected and were requested to bring four tea spoons of salt (about 20g) in the auto-seal polythene pouches from their kitchen. The iodine content of the salt was analyzed by using standard Iodometric Titration (IT) method [10].
Quality control measures
We adopted Internal Quality Control (IQC) methodology, in which a pooled urine sample was prepared. This pooled sample was analyzed 25 times with standards and blank in duplicate. The mean (X) UIC and standard deviation (SD) of this pooled sample was calculated. This was considered as IQC sample. The IQC sample was stored in refrigerator and analyzed with every batch of UIC estimation. The 95% confidence interval for mean of UIC of IQC sample was then calculated. This was used as the operating control range. The methodology adopted was as follows:
Sample mean (X)±2 (SD)
The X – 2(SD) = the lower confidence limit or Lower Concentration Value (LCV)
The operating control range for IQC sample was between LCV and UCV. A regular linear graph paper was utilized to prepare Levey Jennings plots. The mean urinary iodine concentration of the IQC sample was plotted as a continuous horizontal line on the Y-axis. The lower concentration value (LCV) was plotted below the mean line on the Y-axis scale and the upper concentration value (UCV) was plotted above the mean line on the Y-axis scale. The X-axis was used to plot the date on which the IQC sample was analyzed. This chart was used to plot the date specific analysis. The urinary iodine concentration obtained for the IQC sample for each batch. If the value of the IQC sample was between the two limit lines of LCV and UCV, then the UIC test was deemed in control, and all results were accepted. If any value of the IQC sample was plotted outside the two limit lines of LCV and UCV then, the test was considered as out-of-control, and the entire batch was repeated [11].
The X – 2(SD) = the lower confidence limit or Lower Concentration Value (LCV)
The X – 2(SD) = the lower confidence limit or Lower Concentration Value (LCV)
Umbilical cord blood collection
Cord blood was collected before placental delivery within five minutes after birth to avoid clotting. One drop of blood was applied to filter paper. The spots were dried at room temperature and the filter papers were sealed and kept in a freezer until assayed in the laboratory. The samples were stored at 4 °C before analysis.
The samples were estimated for Thyroid Stimulating Hormone (TSH) by using sandwich Enzyme Linked Immuno- Sorbent Assay (ELISA) method. Dry Blood Spots were eluted in anti- TSH antibodies coated with micro wells and were incubated with peroxidase labeled anti-TSH monoclonal antibodies. After washing, the unbound antibodies were washed off and the bound conjugate remained in the micro well. These bound conjugates further react with substrate 3,3’,5,5’ Tetramethylbenzidine (TMB) and produce a colour product. The concentration of TSH is directly proportional to the colour produced. Absorbance was read at 450nm and a value of TSH was expressed in the units’ mlU/l of blood. In order to measure the concentration of TSH in the test sample, the calibration standards and controls were used. The calibration standards and controls were assayed for producing a standard curve of TSH by O.D versus TSH concentration (mlU/l). Therefore, by comparing the O.D of the test samples to this standard curve, the concentration of the TSH was determined [12-14].
Ethical clearance
The project was approved by ethical committee of All India Institute of Medical Sciences, New Delhi. The informed consent was taken from the mother/guardian of the subjects studied.
Results
Total goiter rate
School age children: TGR was found to be 15.8% (Kangra), 23.4% (Kullu) and 15.4% (Solan), Himachal Pradesh and 13.2% (Udham Singh Nagar), 15.9% (Nainital) and 16.8% (Pauri Garhwal), Uttarakhand (Table 1).
Pregnant mothers: TGR was found to be 42.2% (Kangra), 42.0% (Kullu) and 19.9% (Solan) in Himachal Pradesh and 16.1% (Udham Singh Nagar), 20.2% (Nainital) and 24.9% (Pauri Garhwal) in Uttarakhand state (Table 1).
Urinary iodine concentration
The UIC levels and percentage of non iodized salt (salt with iodine content of <15ppm) consumed by SAC and PMs is depicted in Table 1.
School age children: The median UIC levels were 200μg/l (Kangra), 175μg/l (Kullu) and 62.5μg/l (Solan), in Himachal Pradesh and 150μg/l (Udham Singh Nagar), 125μg/l (Nainital) and 115μg/l (Pauri Garhwal) in Uttarakhand (Table 1).
Pregnant mothers: The median UIC Levels were 200μg/l (Kangra), 149μg/l (Kullu) and 130μg/l (Solan), in HimachalPradesh and 124μg/l (Udham Singh Nagar), 117.5μg/l (Nainital) and 110μg/l (Pauri Garhwal) in Uttarakhand (Table 1).
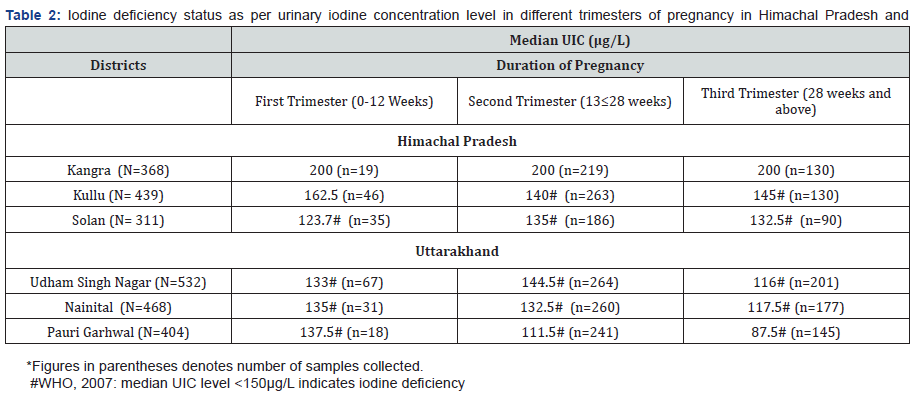
Median UIC level according to different trimesters of pregnancy
The median UIC level according to trimester wise is depicted in Table 2. It was found that in district Solan there was a rise in median UIC level from 1st trimester to 2nd trimester and further there was a decline in median UIC level from 2nd trimester to 3rd trimester of pregnancy. Whereas, in districts Udham Singh Nagar, Nainital and Pauri Garhwal, there was a decline in median UIC levels from 1st trimester to 3rd trimester of pregnancy (Table 2).
Thyroid stimulating hormone (TSH) levels
The TSH levels of more than 5mIU/l were found in 73.4% (Kangra), 79.8% (Kullu) and 63.2% (Solan) of neonates of Himachal Pradesh and 55.3% (Udham Singh Nagar), 76.4% (Nainital) and 72.8% (Pauri Garhwal), neonates of Uttarakhand state, indicating high prevalence of ID in the population studied (Table 1).
Discussion
Urinary Iodine Concentration is an indicator of recent dietary intake of iodine in last the 24h, as most of the iodine absorbed in the body appears in the urine. Total Goiter Rate indicates past chronic status of iodine in the population. According to WHO/ UNICEF/ICCIDD, the median UIC level of <100μg/l amongst SAC and <150μg/l amongst PMs indicates ID in the community [2].
In the present study amongst school age children, the TGR was high and median UIC level were adequate indicating that the SAC are in a transition phase from iodine deficient (as indicated by TGR) to iodine sufficient (as indicated by median UIC level). Pregnant Mothers were iodine deficient in all the three districts surveyed as reported by median UIC level of <150μg/l. This could be possibly due to consumption of salt with less than 15ppm of iodine. Similar studies conducted amongst PMs of different districts of the country reported the median UIC level of <150μg/l indicating iodine deficiency amongst the PMs studied [5,15-18]. A study conducted in United Kingdom has documented that children of mothers who had ID during pregnancy are more likely to have low verbal intelligent quotient and poor reading accuracy and comprehension [19].
Trimester-specific MUI has been reported only in a few studies from India and also from worldwide [20-22]. In pregnant women with iodine restriction, the UIC may transiently show an early increase, due to an increase in the glomerular filtration rate, and thereafter, a steady decrease in UIC from the first to the third trimesters of gestation, thus revealing the underlying tendency toward iodine deficiency associated with the pregnant state, whereas, in iodine-sufficient areas there may be no decline [23].
In the present study, in district Solan, Himachal Pradesh there was a rise in median UIC level from 1st trimester to 2nd trimester and further there was a decline in median UIC level from 2nd trimester to 3rd trimester of pregnancy. Whereas in all the three districts studied from Uttarakhand state, there was a decline in median UIC levels from 1st trimester to 3rd trimester of pregnancy. An earlier study reported that UIC level in the first, second and third trimester was 285μg/L, 318μg/L and 304μg/L, respectively. Thus indicating a rise from the first trimester to the second trimester, followed by a small fall again in the third trimester, but it was not statistically significant [23]. Another similar study conducted reported that insufficient iodine nutrition was most prevalent in the 3rd trimester (40.0%). The figures for insufficient iodine nutrition increased from 30% in first trimester to 37.0% in second trimester and to 40% in third trimester by [15].
Iodine deficiency in neonates leads to cretinism including mental deficiency with a mixture of Mutism, spastic diplegia, squint, hypothyroidism and short stature. Neonates are the most vulnerable group for ID. According to WHO, raised TSH in neonates at birth is an indicator for ID. WHO (2007) reported that a <3% frequency of TSH concentrations above 5mIU/L in samples collected 3-4 days after birth indicates iodine sufficiency in a population [2].
The prevalence of neonates with elevated TSH levels is a valuable indicator of the severity of ID in a given population. In the present study, high percentage of neonates from both the states (Himachal Pradesh and Uttarakhand) of India reported TSH levelof 5mlU/l and more, indicating presence of ID in both the states surveyed. This could be possibly due to higher percentage of families consuming salt with iodine content of less than 15ppm. A similar study conducted in West Bengal has reported the TSH level of more than 5mlU/l in 2.95 % of the neonates [17]. Studies conducted in different countries have reported the TSH level of more than 5mlU/l; Western Uganda (20-32%), Estonia (18.1%), Italy (14.4%), Spain (9.0%), Thailand (8.9%), Australia (6.8%), Poland (3-5%) and Iran (3.6%), respectively [24-31].
Conclusion
The findings of the present study indicate that there is an urgent need to strengthen the IDD control programme for prevention of ID in Himachal Pradesh and Uttarakhand states, India. There is a need for revitalizing the National IDD control program to ensure supply of salt with adequate iodine content of 15ppm and more to achieve elimination of IDD in both the states. Since, most vulnerable group for ID for health consequences is the fetus and hence the assessment of ID status of the pregnant mothers should be included in the monitoring of IDD control program. Moreover, there is an urgent need of initiating a neonatal screening program for assessment of neonatal hypothyroidism and also for early detection of children with iodine deficiency.
Limitations of the Study
- The intra- and inter-observer variation in goitre examination was controlled by repeated training and random examination of goitre grades by an expert. However, despite all of the training for quality control, there is still the possibility for misclassification of a normal thyroid gland as goitre grades I and vice versa.
- We could not assess the size of the thyroid gland using ultrasound due to a lack of resources.
- Iodine deficiency can be detected using maternal free thyroxine during the first trimester of pregnancy. We could not assess the same due to a lack of resources for this investigation.
- Cord blood sample taken immediately after delivery could have false positive high values of TSH due to physiological neonatal TSH surge that elevates TSH level.
References
- Li M, Eastman CJ (2012) The changing epidemiology of iodine deficiency. Nat Rev Endocrinol 8(7): 434-440.
- WHO, UNICEF, ICCIDD (2007) A guide for programme managers. Assessment of Iodine Deficiency Disorders and Monitoring their Elimination. Geneva, Switzerland, p. 98.
- UNICEF (2008) Sustainable Elimination of Iodine Deficiency; Progress since the 1990 World Summit for Children. New York, USA.
- Dunn JT, Delange F (2011) Damaged reproduction: the most important consequence of iodine deficiency. J Clin Endocrinol Metab 86(6): 2360- 2363.
- Pathak P, Singh P, Kapil U, Raghuvanshi RS (2003) Prevalence of iron, vit A, and iodine deficiencies amongst adolescent pregnant mothers. Indian J Pediatr 70(4): 299-301.
- Mittal M, Tandon M, Raghuvanshi RS (2000) Iodine status of children and use of iodized salt in Tarai region of North India. J Trop Pediatr 46(5): 300-302.
- Kapil U, Pathak P, Singh C, Tandon M, Pradhan R (1999) Micronutrient deficiency disorder amongst pregnant women in three urban slum communities of Delhi. Indian Pediatr 36(10): 983-989.
- National Family Health Survey (NFHS-4) (2016) International Institute for Population Sciences (IIPS) and Macro International. National Family Health Survey (NFHS-4), 2005-06, Volume II, Mumbai: IIPS, India.
- Dunn JT, Crutchfield HE, Gutekunst R, Dunn AD (1993) Two simple methods for measuring iodine in urine. Thyroid 3(2): 119-123.
- Karmarkar MG, Pandav CS, Krishnamachari KAVR (1986) Principle and Procedure for Iodine Estimation- A Laboratory Manual. ICMR Press, New Delhi, India.
- Westgard JO, Barry PL, Hunt MR, Groth T (1981) A multi-rule Shewhart chart for quality control in clinical chemistry. Clin Chem 27(3): 493- 501.
- Slazyk WE, Hannon WH (1993) In: Therrell BL (Ed.), Laboratory methods for Neonatal Screening. American Public Health Association, Washington, DC, USA, p. 23.
- Westgard JO, Klee GG (1999) In: Burtis CA, Ashwood R (Eds.), Tietz textbook of clinical chemistry. (3rd edn), WB Saunders Co., Philadelphia, USA, 45(6): 384-388.
- Spencer CA, Takeuchi M, Kazarosyan M, MacKenzie F, Beckett GJ, et al. (1995) Inter laboratory/inter method differences in functional sensitivity of immuno- metric assays of thyrotropin (TSH) and impact on reliability of measurement of subnormal concentrations of TSH. Clin Chem 41(3): 367-374.
- Majumder A, Jaiswal A, Chatterjee S (2014) Prevalence of iodine deficiency among pregnant and lactating women: Experience in Kolkata. Indian J Endocrinol Metab 18(4): 486-490.
- Dodd NS, Madan J (1993) Iodine status in Pregnancy. Asia Pacific J Clin Nutr 2(3): 119-23.
- Chakraborty I, Chatterjee S, Bhadra D, Mukhopadhaya B, Dasgupta A, et al. (2006) Iodine deficiency disorders among the pregnant women in a rural hospital of West Bengal. Indian J Med Res 123(6): 825-829.
- Singh MB, Fotedar R, Lakshminarayan J (2009) Micronutrient deficiency status among women of desert areas of western Rajasthan, India. Public Health Nutr 12(5): 624-629.
- Bath SC, Steer CD, Golding J, Emmett P, Rayman MP (2013) Effect of inadequate iodine status in UK pregnant women on cognitive outcomes in their children: results from the Avon Longitudinal Study of Parents and Children (ALSPAC). Lancet 382(9889): 331-337.
- Shamim AA, Christian P, Schulze KJ, Ali H, Kabir A, et al. (2012) Iodine status in pregnancy and household salt iodine content in rural Bangladesh. Matern Child Nutr 8(2): 162-173.
- Mehdi T, Hoque M, Nasreen Z, Farzana Shirin, Maqsudul Hakim Khan (2009) Maternal iodine status and thyroid function during pregnancy. J medicine 10: 56-59.
- Glinoer D, de Nayer P, Bourdoux P, Lemone M, Robyn C, et al. (1990) Regulation of maternal thyroid during pregnancy. J Clin Endocrinol Metab 71(2): 276-287.
- Grewal E, Khadgawat R, Gupta N, Desai A, Tandon N (2013) Assessment of iodine nutrition in pregnant north Indian subjects in three trimesters. Indian J Endocrinol Metab 17(2): 289-289.
- Ehrenkranz J, Fualal J, Ndizihiwe A, Clarke I, Alder S (2011) Neonatal age and point of care TSH testing in the monitoring of iodine deficiency disorders: findings from western Uganda. Thyroid 21(2): 183-188.
- Mikelsaar RV, Viikmaa M (1999) Neonatal thyroid stimulating hormone screening as an indirect method for the assessment of iodine deficiency in Estonia. Horm Res 52(6): 284-286.
- Costante G, Grasso L, Ludovico O, Marasco MF, Nocera M, et al. (1997) The statistical analysis of neonatal TSH results from congenital hypothyroidism screening programs provides a useful tool for the characterization of moderate iodine deficiency regions. J Endocrinol Invest 20(5): 251-256.
- Peris RB, Calvo RF, Tenias Burillo JM, Merchante AA, Presencia RG, et al. (2009) Iodine deficiency and pregnancy. Current situation. Endocrinol Nutr 56(1): 9-12.
- Jaruratanasirikul S, Sangsupawanich P, Koranantakul O, Chanvitan P, Ruaengrairatanaroj P, et al. (2009) Maternal iodine status and neonatal thyroid-stimulating hormone concentration: a community survey in Songkhla, southern Thailand. Public Health Nutr 12(12): 2279-2284.
- Rahman A, Savige GS, Deacon NJ, Francis I, Chesters JE (2010) Increased iodine deficiency in Victoria, Australia: analysis of neonatal thyroidstimulating hormone data, 2001 to 2006. Med J Aust 193(9): 503-505.
- Ołtarzewski M, Szymborski J (2003) Neonatal hypothyroid screening in monitoring of iodine deficiency and iodine supplementation in Poland. J Endocrinol Invest 26(2 suppl): 27-31.
- Najafi M, Khodaee GH, Bahari M, Sabah M, Farsi MM, et al. (2008) Neonatal thyroid screening in a mild iodine deficiency endemic area in Iran. Indian J Med Sci 62(3): 113-116.






























