Body Composition Analysis by Dual-Energy X-Ray Absorptiometry in Women Aged 20-75 Years
Mihail A Boyanov*
Department Internal Medicine, Medical University of Sofia, Bulgaria
Submission: April 05, 2017; Published: May 30, 2017
*Corresponding author: Mihail A Boyanov, Professor, Clinic of Endocrinology and Metabolic Diseases, University Hospital Alexandrovska, Department Internal Medicine, Faculty of Medicine, Medical University Sofia, 1, G. Sofiyski street, 1431 Sofia, Bulgaria, Tel: +3592-9230-784; Fax: +3592-9230-779; Email: mihailboyanov@yahoo.com
How to cite this article: Mihail A B. Body Composition Analysis by Dual-Energy X-Ray Absorptiometry in Women Aged 20-75 Years. J Endocrinol Thyroid Res . 2017; 2(1): 555578.. DOI:10.19080/JETR.2017.02.555578
Abstract
Purpose: To measure the total and regional fat and lean mass (FM and LM) in women of different age and to build an age-adjusted dataset using Dual X-ray absorptiometry (DXA).
Methods: This is a retrospective post hoc analysis of a whole body DXA study. 140 women (aged 20-75 years) referred for DXA were eligible. They were subdivided in age groups: 20-44 years (30 premenopausal women), 45-59 years (80 postmenopausal women), and 60-75 years (30 women). DXA was performed on a Hologic QDR 4500 A bone densitometer (Hologic Inc., Bedford MA) with software version 8.26:3. Total body and regional BMC, FM and LM were measured. Regional analysis included arms and legs (the sum of left and right), and the trunk. Statistical analysis was performed by IBM SPSS Statistics 19.0 for Windows.
Results: Comparing the 45-59 versus the 20-44 year-aged groups, FM and LM showed a parallel decrease, while in the elderly group (60-75 years), the decrease of FM was greater than that of LM. The body fat percentage was lowest in the oldest (39.6±6.2) and highest in the middle-aged group (41.8±5.8), p=0.04. Comparing the 45-59 versus the 20-44 year-aged group, FM and LM showed a parallel decrease in the legs, while in the arms and trunk, FM showed a greater age-related decrease than LM.
Conclusion: Aging has a more pronounced effect on FM than LM. The reduction of both body compartments is different according to the region studied. BMI-adjusted data on body composition in different populations are needed.
Keywords: DXA; Whole body; Regional analysis; Fat mass; Lean mass
List of abbreviations: ALM: Appendicular Lean Mass; BMI: Body Mass Index; DXA: Dual-Energy X-Ray Absorptiometry; FM: Fat Mass; % FM: Percentage of Fat Mass; LM: Lean Mass
Introduction
Obesity has become a worldwide epidemic [1]. Its consequences include severely increased morbidity and mortality, as well as impaired quality of life. According to recent publications, 38.8% of the men and 28.3% of the women in a random Bulgarian population (1046 women and 912 men) have overweight and obesity [2]. Common measures of overweight and obesity are the body weight, body mass index (BMI), waist circumference, waist-to-hip ratio and some others. They show, however, a number of limitations in differentiating visceral from subcutaneous fat accumulation and increases in lean mass (LM) from those in fat mass (FM).
Body composition analysis provides useful information in epidemiological studies of obesity and overweight, as well as of sarcopenia and frailty [3-5].“Gold standards” for body composition analysis are computed tomography (CT), magnetic resonance imaging (MRI), hydro-densitometry, deuterium oxide dilution techniques and air displacement plethismography, but they are expensive and rather sophisticated for everyday use. Dual-energy X-ray absorptiometry (DXA) is a reliable method for body composition analysis,combining both simplicity of use and objectivity in measuring physically the different body compartments [6,7]. It can also provide precise regional data for different body parts and regions.
The International Society for Clinical Densitometry (ISCD)2013 Conference advocated the use of whole body scans and totalbody composition with regional analysis particularlyin threesituations: 1/in obese patients undergoing bariatric surgery (ormedical, diet or weight loss regimens with significant weightloss); 2/in patients with muscle weakness or poor physicalfunctioning (to improve the diagnosis of sarcopenia); and 3/ inHIV patients onanti-retroviral therapy, to assess fat distributionand possible lipoatrophy [8]. An emerging trend is the use of DXAfor the estimation of total and appendicular lean mass (ALM)in the setting of sarcopenia [9,10]. However, the interpretationof body compartment estimates necessitates some referencevalues. A number of studies have published data from variousreference populations- Europeans, Asians and Americans [11-20]. The DXA manufacturers incorporate different referencedatabases, primarily for bone mineral density and content (BMDand BMC). The NHANES 1999-2004 database is an effort toprovide reliable and internationally validated reference valuesfor several indices in the body composition analysis [11,21].The ISCD 2013 Conference recommends using them insteadof manufacturer-provided reference populations [22]. ThreeEuropean organizations (ESCEO/IOF, ESPEN and EUGMS) issuedan appeal for local data on body composition to be included in anInternational Sarcopenia Cohort Study (ISCS).
The aim of the present study was to perform whole bodyand regional body composition analysis in women aged 20-75years and to establish age-adjusted data for FM and LM and thepercentage of fat mass (% FM).
Material and Methods
Subjects
This is a retrospective post hoc analysis of data coming froma cross-sectional observational study, performed in a hospitalbasedoutpatient DXA setting. The primary goal of the study wasto explore the agreement between the body composition analysisby leg-to-leg BIA and by fan-beam DXA, with the assumption thatsevere obesity might negatively affect the agreement betweenthe two techniques. The results of the primary analysis havebeen published elsewhere [23]. A total of 159 women (meanage 49.1±10.0 years) were involved in the original study, butafter reviewing the available data, only 140 subjects were foundsuitable for proper analysis of regional body composition.Bearing in mind that several body composition indices reachtheir peak after the age of 40 years, the subjects were assignedinto three groups: 30 women aged 20-44 years, 80 women aged45-59 years and 30 women aged 60-75 years [21]. The subjectscame from an urban background. Seventy two (72) of them werereferred for bone density testing (65 postmenopausal and 7premenopausal women), 56 were referred for body compositionanalysis because of overweight or obesity (22 premenopausaland 34 postmenopausal women) and 12 were healthy volunteers(1 premenopausal and 11 postmenopausal women).
All women had given their informed consents prior to anyprocedure. The study was approved by the responsible ethicauthorities at the University Hospital and was performed inaccordance with the ethical standards as laid down in theHelsinki Declaration (1964) and its later amendments. Theinclusion criteria were the patient’s informed consent and theavailability of readable total and regional body compositiondata from DXA. Subjects with any medical conditions ormedications, known to cause excessive obesity, dehydration andwater retention or electrolyte disturbance affecting the bodycomposition measurements, had been excluded from this study.The exclusion criteria included also the presence of multipledeformed or fractured lumbar vertebrae, severe scoliosis (>15°)and other conditions, which might interfere the proper analysisof BMD scans.
Age (in yrs), height (in cm, measured on a Harpenderstadiometer), weight (in kg, measured on a standard weightbalance) and age at menopause (if menopausal), were recordedprior to the whole body DXA scans. Body mass index (BMI) wascalculated from weight and height in kg/m2.
Body composition analysis
The whole body DXA was performed in the early morningafter an overnight fasting for at least 12 hours. The subjectswere required to adhere to standard body composition testingguidelines, wearing light clothes [24]. They were positionedlying supine with the entire body, including all soft tissues, withinthe table margins. The arms were positioned palm down with aspace straight at the patient’s sides; the legs were kept togetherwith the feet relaxed. Fan-beam dual-energy X-ray (DXA) bodycomposition analysis was performed on a Hologic QDR 4500A bone densitometer (Hologic Inc., Bedford, MA 02154, USA).All DXA scans were read by the same technologist in a semiautomaticway, including manual modifications of the regionsof interest. The software (version 8.26:3) assumed that brain fatrepresented 17% of the body fat. Body composition data werepresented by the software as FM in grams, LM in grams, andbone mineral content (BMC) in grams. The percentage of FM (%FM) was also calculated. Data were calculated separately for thedifferent body sub-regions (arms, legs and trunk), as well as totalvalues according to the ISCD 2013 guideline [8].
Precision study
The manufacturer-provided standard Hologic tissue barwas scanned once weekly for whole body quality control. The invivo coefficient of variation (CV %) of the whole body DXA wascalculated from duplicate measurements in 30 female subjectsaccording to Glüer et al. (CV% = root-mean-squares/mean x 100)[25]. These subjects were randomly selected from the femalestudy population. All retests were performed on the same daywithin a very short time. The in vivo CV % of DXA was 0.79% for% FM, 760 grams for FM (corresponding to 2.05%), 710 gramsfor LM (1.46%), and 40 grams for BMC (2.01%), which is withinthe limits advocated by the ISCD 2013 conference [24].
Statistical analysis
An IBM SPSS 19.0 for WINDOWS package (Chicago, IL, USA)was used for processing the numerical data. The Kolmogorov-Smirnov test for normal distribution was first performed,followed by tests for homogeneity of variance, and t-tests.Statistical significance was set at p0.05. Whole body BMDT-scores were calculated by the software and were expressedin units of SD’s above or below the mean of the manufacturerprovidedhealthy reference for adult pre-menopausal populationaged 20-29 years (PS reference data base issued 25 Oct 1991).
Results
The anthropometric and whole body composition dataofthe subjectsare presented in Table 1. Total body FM washighest in the youngest age-group, with a steeper decline in theelderly group. Total body LM, on the contrary, showed a morepronounced difference between the 20-44 and 45-59 year agedgroups than between the 45-59 and 60-75-year aged groups.This discrepancy between the lean and fat mass led to % FMthat was highest in the middle-aged group (45-59 years). TotalBMC and BMD declined with advancing age and the intergroupdifference was highest in the elderly- 45-59 versus 60-75-yearaged group.
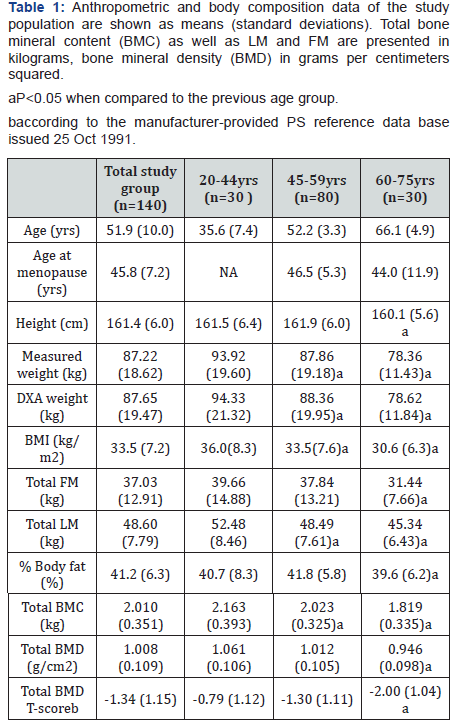
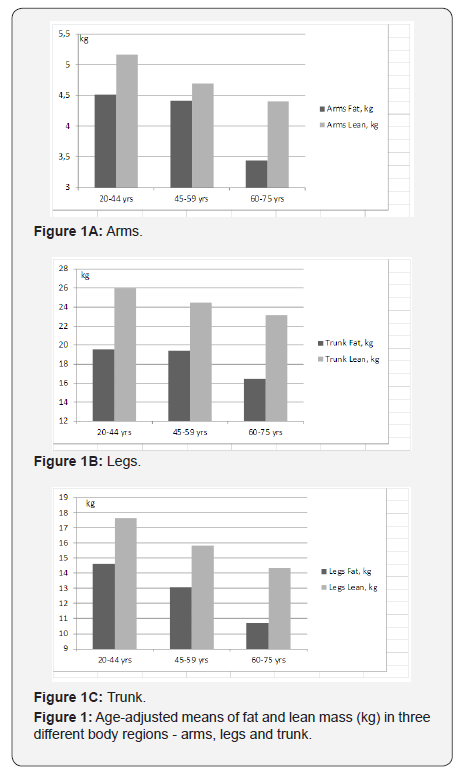
Figures 1a-1c show the age-adjusted means of FM and LMin three different body regions- arms, legs and the trunk. In thearms, LM shows a greater difference between the ages of 20-44 and 45-59 years than between the ages of 45-59 and 60-75years, while the difference in FM is greater between the ages of45-59 and 60-75 years (Figure 1a). The same tendency of FM,declining more steeply in older ages, can be seen for the trunk(Figure 1c), while in the legs, FM and LM show an almost paralleldecline with aging (Figure 1b). The mean percentages of totaland regional FM are displayed on Figure 2. These are highest inthe middle-aged group (45-59 years).
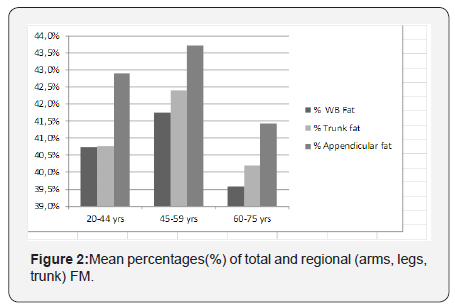
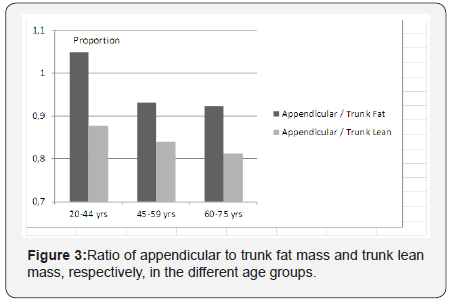
Figure 3 shows the ratio of appendicular to trunk fat massand trunk lean mass, respectively, in the different age groups.The proportion of appendicular fat and lean mass is decreasingsteadily with aging, showing a more centripetal body masspredisposition. The most marked redistribution can be seenbetween the ages of 20-44 and 45-59 years, involving the fatmass.
Discussion
This is the first study in our country that reports DXA-derivedestimates of whole and regional body composition analysis. TotalBMC and BMD of the studied women were consistently lowerthan the Hologic and NHANES 1999-2004 reference databasesand were lower with advancing age. A similar age-relateddifference was seen in regional LM and FM. FM decreased atdifferent rates in the trunk, legs and arms and predominantlyat older ages, whereas LM followed an almost linear patternof decline with aging. This resulted in % FM that was highestin the middle-aged group (45-59 years). Trunk fat remainedalmost similar in the 20-44 and 45-59-year aged groups, whichled to a significant difference in the appendicular/trunk fatratio. We made an attempt to explain these findings with theinfluence of at least three factors: 1/ increase of chronologicalage; 2/ perimenopausal and postmenopausal transition, sinceall women aged under 45 years were premenopausal, while theelderly were postmenopausal; 3/ decreasing BMI in the elderlyage group that results in smaller body proportions affecting theFM and LM and their ratios in the trunk and limbs. In brief, ourresults show that LM and FM do not follow the same pattern ofage-related differences in the various body regions.
A number of studies have published body compositionestimates coming from Asia, Europe and North America [11-20]. Few among them have examined the relationships of FMand LM with BMD [13,14,16], while others have suggested localreference values [11,12,15,17,18,20]. Comparing our data tothese of a Spanish study, we found a major difference concerningthe changes in total FM and % FM with aging [20]. While theSpanish women showed a consistent increase in total body FMand % FM throughout life, our data indicate a steady decline ofabsolute FM and a peak of % FM around the perimenopausal andearly postmenopausal ages (45-59 years). However, data of bothstudies are similar for the total body LM, which is decreasingwith advancing age. A similar trend for increasing the total bodyFM and % FM throughout life was observed in Italian subjects[18,19]. On the contrary, a study in healthy Brazilian womenregistered a bimodal distribution of body fat- increasing until50-59, with a slight subsequent decrease [17]. The lowest valuesof FM and %FM in the elderly group of our study populationmight be explained by the decreasing BMI-starting at a mean of36.0kg/m2 in the youngest age group and reaching 30.4kg/m2in the oldest one. This discrepancy underlines the need for BMIadjusted reference values. Such BMI-adjusted reference valueshave already been published for other populations [11], but ourstudy group was under powered for this kind of analysis.
Our data are different from the published body compositionindices in a 20-to-80-year-old healthy Italian population [18].In this particular study, trunk FM values were higher in oldersubjects, while the leg FM was similar in women at different ages.In another publication, based on the same data set, women’sarm FM increased with aging, their arm fat-free mass remainedstable, while decreasing in their legs [19]. These studies, alongwith our findings, may raise the hypothesis for the differentweight-bearing roles of the upper and lower limbs, resulting indifferential FM and LM changes with aging.
We also made an attempt to compare our data with theNHANES 1999-2004 reference population, as published by theISCD [22]. Almost all body composition indices were higher in thestudied women, showing the presence of a systematic difference.It may be explained by genetic and ethnic factors, but also by thepredominantly high BMI in our study population. The impact ofrace-ethnicity can easily be seen in the publications based on the1999-2004 NHANES data [21]. Most of our participants had overweight or obesity. As a consequence, our study underlines theneed for national-based body composition data from subjectswith different levels of BMI, reflecting the nation-specific levelsof over weight and obesity.
Our study has a number of limitations. First, the number ofparticipants is rather modest, which prevented us from separateanalyses based on BMI. Second, we were unable to study separatelythe contribution of menopause to changes in body composition,since the younger age group included premenopausal, whilethe middle-aged and elderly group- postmenopausal womenonly. In a study assessing the relative contribution of aging andmenopause to changes in LM and FM in segmental regions, thedecrease in LM was found to be more menopause-related, whilethe shift toward upper body fat distribution - more age-related[26,27]. Third, this is a post hoc analysis in women referred forDXA for a variety of indications. Our data cannot be generalizedto the whole female population. And fourth, the study designis cross-sectional, allowing rough estimates of real age-relatedchanges in body composition only. Until now, there are nostudies reporting longitudinal body composition changes in agiven population for longer time periods (e.g. >10yrs).
One of the major strengths of our body composition studyis that it is the first DXA-based study in our country. DXA is anon-expensive and very precise method for studying the bodycomposition, being critically validated in a variety of studies[6,7,28,29]. The mean difference between the body weight valuescoming from DXA and from the digital scale balance in our dataset was less than 0.5kg, meaning that DXA has quite accuratelyestimated the real weight.
Conclusion
Aging has a more pronounced effect on fat than lean mass.The reductions of fat and lean mass are not parallel in thedifferent body regions. Nationally representative data on bodycomposition adjusted to BMI are needed.
Acknowledgment
The author would like to thank Assoc. Prof. Dr. Zhivka Boneva-Asiova (Head of the Endocrine Unit, MVR Institute- CentralHospital, Sofia) and the DXA technologist Darina Antonova fromthe same Endocrine Unit for their technical support and devotionto this study.
Conflicts of Interest Statement
The author declares no direct or indirect conflicts of interestrelated to this study.
This research did not receive any specific grant from fundingagencies in the public, commercial, or not-for-profit sectors.The author has received speaker honoraria from a number ofpharmaceutical companies such as Amgen Inc., F. Hoffmann-LaRoche Ltd., Eli Lilly and Company, Novo Nordisk, Sanofi-Aventis,Laboratoires Servier, Novartis AG, Merck-Sharpe and Dohme,Merck GMBH, InoTech and others.
References
- Villareal DT, Apovian CM, Kushner RF, Klein S, American Society forNutrition (2005) Obesity in older adults: technical review and positionstatement of the American Society for Nutrition and NAASO, TheObesity Society. Am J Clin Nutr 82(5): 923-934.
- Borissova AM, Shinkov AD, Vlahov JD, Dakovska LN, Todorov TC (2015)Survey on the prevalence of obesity in Bulgarian population in 2012year. J Endocrinologia 20: 82-92.
- Rotella CM, Dicembrini I (2015) Measurement of body compositionas a surrogate evaluation of energy balance in obese patients. WorldJ Methodol 5(1): 1-9.
- Graf CE, Karsegard VL, Spoerri A, Makhlouf AM, Ho S, et al. (2015) Bodycomposition and all-cause mortality in subjects older than 65 y. Am JClin Nutr 101(4): 760-767.
- Falsarella GR, Gasparotto LP, Barcelos CC, Coimbra IB, Moretto MC,et al. (2015) Body composition as a frailty marker for the elderlycommunity. Clin Interv Aging 10: 1661-1666.
- Van Loan MD, Mayclin PL (1992) Body composition assessment: dualenergyX-ray absorptiometry (DEXA) compared to reference methods.Eur J Clin Nutr 46(2): 125-130.
- Van Loan MD (1998) Is dual-energy X-ray absorptiometry ready forprime time in the clinical evaluation of body composition. Am J ClinNutr 68(6): 1155-1156.
- Kendler DL, Borges JLC, Fielding RA, Itabashi A, Krueger D, et al.(2013) The Official Positions of the International Society for ClinicalDensitometry: indications of use and reporting of DXA for bodycomposition. J Clin Densitom 16(4): 496-507.
- Müller MJ, Geisler C, Pourhassan M, Glüer CC, Bosy-Westphal A (2014)Assessment and definition of lean body mass deficiency in the elderly.Eur J Clin Nutr 68(11): 1220-1227.
- Coin A, Sarti S, Ruggiero E, Giannini S, Pedrazzoni M, et al. (2013)Prevalence of sarcopenia based on different diagnostic criteria usingDEXA and appendicular skeletal muscle mass reference values in anItalian population aged 20 to 80. J Am Med Dir Assoc 14(7): 507-512.
- US Department of Health and Human Services (2010) Body compositiondata for individuals 8 years of age and older: U.S. Population 1999-2004. Vital and Health Statistics 250(series 11): 95.
- Marwaha RK, Garg MK, Bhadra K, Mithal A, Tandon N (2014)Assessment of lean (muscle) mass and its distribution by dual energyX-ray absorptiometry in healthy Indian females. Arch Osteoporos 9:186.
- Marwaha RK, Garg MK, Tandon N, Mehan N, Sastry A, et al. (2013)Relationship of body fat and its distribution with bone mineral densityin Indian population. J Clin Densitom16(3): 353-359.
- Zhang J, Jin Y, Xu S, Zheng J, Zhang Q, et al. (2015) Associations of fatmass and fat distribution with bone mineral density in Chinese obesepopulation. J Clin Densitom18(1): 44-49.
- Cheng Q, Zhu X, Zhang X, Li H, Du Y, et al. (2014) A cross-sectional studyof loss of muscle mass corresponding to sarcopenia in healthy Chinesemen and women: reference values, prevalence, and association withbone mass. J Bone Miner Metab 32(1): 78-88.
- Lee K (2013) Regional percent fat and bone mineral density inKorean adolescents: the Fourth Korea National Health and NutritionExamination Survey (KNHANES IV-3), 2009. Asia Pac J Clin Nutr 22(1):69-73.
- Sousa MD, Pinheiro MM, Szejnfeld VL, Castro CH (2013) Bodycomposition parameters in healthy Brazilian women differ from white,black, and Hispanic American women reference range. J Clin Densitom16(3): 360-367.
- Coin A, Ruggiero E, Giannini S, Pedrazzoni M, Minisola S, et al. (2012)Trunk and lower limb fat mass evaluated by dual-energy X-rayabsorptiometry in a 20- to 80-year-old healthy Italian population. AnnNutr Metab 61(12): 151-159.
- Coin A, Giannini S, Minicuci N, Rinaldi G, Pedrazzoni M, et al. (2012)Limb fat-free mass and fat mass reference values by dual-energy X-rayabsorptiometry (DEXA) in a 20-80 year-old Italian population. ClinNutr 31(4): 506-511.
- Henche SA, Torres RR, Pellico LG (2008) An evaluation of patterns ofchange in total and regional body fat mass in healthy Spanish subjectsusing dual-energy X-ray absorptiometry (DXA). Eur J Clin Nutr 62(12):1440-1448.
- Heo M, Faith MS, Pietrobelli A, Heymsfield SB (2012) Percentageof body fat cutoffs by sex, age, and race-ethnicity in the US adultpopulation from NHANES 1999-2004. Am J Clin Nutr 95(3): 594-602
- Petak S, Barbu CG, Yu EW, Fielding R, Mulligan K, Sabowitz B, etal. (2013) The Official Positions of the International Society forClinical Densitometry: body composition analysis reporting. J ClinDensitom16(4): 508-519.
- Boneva-AZ, Boyanov MA (2008) Body composition analysis by leg-tolegbioelectrical impedance and dual-energy X-ray absorptiometry innon-obese and obese individuals. Diab Obes Metab 10(11): 1012-1018.
- Hangartner TN, Warner S, Braillon P, Jankowski L, Shepherd J(2013) The Official Positions of the International Society for ClinicalDensitometry: acquisition of dual-energy X-ray absorptiometry bodycomposition and considerations regarding analysis and repeatabilityof measures. J Clin Densitom16(4): 520-536.
- Glüer C, Blake G, Lu Y, Blunt BA, Jergas M, Genant HK (1995) Accurateassessment of precision errors: How to measure the reproducibility ofbone densitometry techniques. Osteoporos Int 5(4): 262-270.
- Cruz-JAJ, Baeyens JP, Bauer JM, Boirie Y, Cederholm T, et al. (2010)Sarcopenia: European consensus on definition and diagnosis: Reportof the European Working Group on Sarcopenia in Older People. AgeAgeing 39(4): 412-423.
- Douchi T, Yamamoto S, Yoshimitsu N, Andoh T, Matsuo T, Nagata Y(2002) Relative contribution of aging and menopause to changes inlean and fat mass in segmental regions. Maturitas 42(4): 301-306.
- La Forgia J, Dollman J, Dale MJ, Withers RT, Hill AM (2009) Validationof DXA body composition estimates in obese men and women. Obesity(Silver Spring) 17(4): 821-826.
- Lohman M, Tallroth K, Kettunen JA, Marttinen MT (2009)Reproducibility of dual-energy x-ray absorptiometry total and regionalbody composition measurements using different scanning positionsand definitions of regions. Metabolism 58(11): 1663-1668.






























