Cobalt Toxicity, An overlooked Cause of Hypothyroidism
Run Yu*
Department of Endocrinology, UCLA David Geffen School of Medicine, USA
Submission: February 16, 2017; Published: March 23, 2017
*Corresponding author: Run Yu, MD, PhD, Department of Endocrinology, UCLA David Geffen School of Medicine, 200 Medical Plaza Dr, Suite 530, Los Angeles, CA 90095, USA, Tel: 310-825-7922; Fax: 310-267-1899; Email: runyu@mednet.ucla.edu
How to cite this article: Yu R. Cobalt Toxicity, An Overlooked Cause of Hypothyroidism. J Endocrinol Thyroid Res. 2017; 1(3): 555563. DOI:10.19080/JETR.2017.01.555563
Abstract
The search for a specific cause of hypothyroidism, a common endocrine disease, is often not enthusiastic in clinical care. Here a case of hypothyroidism caused by cobalt toxicity is presented. A 64-year-old male with a history of revision hip arthroplasty presented with visual and hearing loss and hypothyroidism. His thyroglobulin level was very high and he exhibited features of destruction thyroiditis on CT. His serum cobalt levels were remarkably elevated. No other specific diagnoses could be reached for the neurological symptoms. He ultimately succumbed to heart failure. This case illustrates that seeking specific etiology of hypothyroidism is important when hypothyroidism is concurrent with other abnormalities of unclear causes. Earlier recognition of cobalt toxicity may avoid excessive work-up of the myriad of seemingly unrelated symptoms and may facilitate timely intervention. This case further demonstrates that cobalt toxicity on the thyroid may manifest in destruction thyroiditis and impaired thyroid hormone synthesis.
Keywords:Cobalt toxicity; Hypothyroidism; Destruction thyroiditis; Hip arthroplasty
Introduction
Hypothyroidism is a common endocrine disease. The frequent causes of hypothyroidism include autoimmune thyroid disease, thyroid ablation, thyroidectomy, and medications such as amiodarone and immune modulating drugs [1]. As hypothyroidism is usually straightforward to treat with thyroid hormone replacement, the search for a specific cause of hypothyroidism is often not enthusiastic in clinical care. Here a case of hypothyroidism caused by cobalt toxicity is presented.
Case Report
A 64-year-old male was referred to endocrine service for management of hypothyroidism. In the preceding 6 weeks, he had had progressive and bilateral hearing impairment, visual loss, and visual hallucinations. He went to emergency room for those symptoms. Neurological examination revealed bilateral decreased vision acuity (20/80 on right eye, 20/200 on left eye) but grossly normal hearing. Laboratory tests showed normal hemogram, glucose, electrolytes, renal and hepatic functions, rapid plasma regain (RPR), B12, folate, rheumatoid factor, and paraneoplastic panel. Erythrocyte sedimentation rate (ESR) was 50mm/hr (normal≤12). Hypothyroidism was found through screening. His TSH level was 63.0mcIU/mL (normal 0.3-4.7), free T4 0.2ng/dL (normal 0.8-1.6), free T3 85pg/dL (normal 222-383). Further thyroid testing showed that thyroid peroxidase antibody level was 24.2IU/mL (normal≤20), thyroglobulinantibody <0.9IU/mL (normal <4.0) and thyroglobulin 3060ng/mL (normal 3-40). Extensive neuroimaging did not find abnormalities.
His past medical history included hypertension, osteoarthritis, skin cancer, and aortic aneurysm. He never had a goiter or been exposed to neck radiation. Past surgical history included multiple left knee surgery due to trauma nearly 50 years before, lumbar spine surgery nearly 20 years before, total right hip arthroplasty (hip replacement) (with ceramic-on-ceramic bearing) 7 years before and total left hip arthroplasty (with ceramic-on-polyethylene bearing) 5 years before. While the left hip functioned well after replacement, the right hip did not, and it had been painful, produced noise, and been prone to subluxation. Hip X-ray film had showed extensive metallosis around the right hip2 years before (Figure 1). He had undergone revision of total right hip acetabular component with ceramic-on-polyethylene bearing 2 years before. Intraoperatively, large amount of metal debris was noted and cleaned. Medications taken at the time of presentation to emergency room included atorvastatin, bupropion, gabapentin, loratadine, losartan and metoprolol. Family history included prostate cancer in father and an unknown cancer in mother, and heart disease in father. No family members had known thyroid disease. He was divorced and lived alone and did not smoke or drink. He used marijuana daily for 20 years. Review of systems noted weight fluctuation withbaseline weight of 230 pounds and weight gain of 30 pounds due to immobility from right hip revision and then intentional weight loss of 50 pounds by diet over the 2 preceding years. He had had poor energy levels in the previous few years and poor appetite and nausea in the past week. He had normal concentration and denied cold intolerance, constipation, hair loss, skin changes or changes in bowel habit.
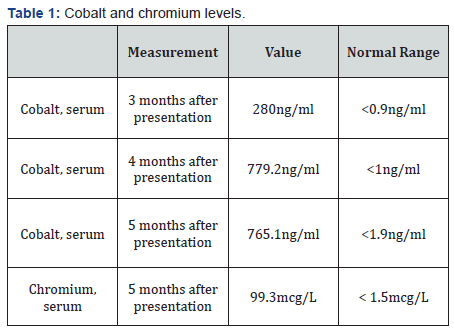
At the time he was referred to endocrine service, he had not had a clear neurological diagnosis or received specific treatments for the neurological symptoms. The newly found hypothyroidism was initially thought to be due to Hashimoto’s thyroiditis for which he started levothyroxine 75mcg daily. During the next few days, his vision improved but subjective hearing impairment persisted. He was discharged after several days of hospitalization. In the following 2 months, his visual and hearing impairment worsened; he had a weight loss of 7 pounds and fatigue with exertion. His TSH level was 33.94 and free T4 0.5 2 months after presentation. The levothyroxine dose was increased to 100mcg daily. He was readmitted for worsening visual and hearing impairment. MRI of the orbits showed new, bilateral optic nerve enhancement but infectious and autoimmune tests still returned negative results. A tentative diagnosis of optic neuritis was made. He was treated with methylprednisolone 1g daily for 7 days and plasma exchange for 5 cycles but his vision and hearing did not improve. At that time, cobalt toxicity became a concern and very high cobalt levels were found (Table 1). The patient soon developed congestive heart failure and died 6 months after presentation.
Discussion
The cause of hypothyroidism in an individual patient is usually apparently obvious [1]. A casual diagnosis on the etiology of a patient’s hyperthyroidism is often sufficient for practical purposes of hypothyroidism treatment and follow-up. When hypothyroidism occurs in association with other potentially related abnormalities, a specific etiology of hypothyroidism needs to be identified to comprehensively assess the patient’sunderlying condition, to guide treatment, and to make prognosis. For example, simultaneous hypothyroidism and hypogonadism suggest hypopituitarism, a unifying diagnosis that may require further diagnostic work-up and even surgical treatment. When newly found hypothyroidism and sub acute visual and hearing impairment of unclear causes are present at the same time, as in this patient, there can be multiple relationships between hypothyroidism and the latter dysfunctions. Hypothyroidism is very common so it is possible that the patient’s hypothyroidism had been chronic and was not diagnosed until he presented with the visual and hearing loss. It is hard to imagine, however, with his severe hypothyroidism, he would have been asymptomatic for hypothyroidism and not have sought medical attention [2]. His disease course suggests that that his hypothyroidism was also sub acute and coincidental with the visual and hearing loss. Although severe hypothyroidism can cause cognitive and affective disorders, it is not known to cause focal neurological symptoms other than peripheral neuropathy and ataxia [3,4]. More likely, hypothyroidism and visual and hearing loss are caused by a common etiology in this patient. Autoimmune thyroiditis and optic neuritis, especially neuromyelitisoptica, often coexist, but imaging evidence of optic nerve inflammation is usually clear and prominent in optic neuritis, and optic neuritis is rarely associated with hearing loss [5]. This patient’s lack of clear imaging evidence of optic nerve involvement and prominent hearing loss are atypical for optic neuritis.
The most plausible diagnosis of this patient’s puzzling condition is cobalt toxicity. In retrospect, the patient’s presentation of hypothyroidism and visual and hearing loss are typical for cobalt systemic toxicity. Hip arthroplasty is a commonly performed procedure. The artificial femoral head and acetabular lining are made of cobalt-chromium alloy without or with coating of ceramic or polyethylene. The wear and tear of the metal parts release metal particles and ions. Chromium particles and ions are not toxic but cobalt particles and ions are and can produce local and systemic toxic effects [6,7]. Metallosis refers to inflammatory changes instigated by cobalt around the hip joint and seen as opacities around the hip on X-ray film (Figure 1). Systemic cobalt toxicity becomes a concern after the cobalt serum level reaches 7ng/ml [8]. Peripheral neuropathy, sensorineural hearing loss, visual loss due to retinal-optic nerve damage, hypothyroidism, and cardiomyopathy are the most commonly reported features of systemic cobalt toxicity [6-8]. So far, cobalt toxicity has only been reported in patient’s months to years after revision for failed hip arthroplasty. All artificial joint bearing types have been reported in cobalt toxicity. Cobalt toxicity remains a clinical diagnosis. The diagnosis criteria for prosthetic hip-associated cobalt toxicity are: elevated cobalt levels due to a prosthetic hip, at least two confirmed features consistent with cobalt toxicity, and exclusion of other causes [8]. This patient’s history of revision hip arthroplasty, his very high cobalt levels (up to 700sng/ml), visual loss, hearing loss, hypothyroidism,cardiomyopathy, and the exclusion of other causes of the constellation of abnormalities make the diagnosis of systemic cobalt toxicity very solid. The treatment of cobalt toxicity has not been standardized; removal of the culprit hardware may be ultimately needed [6,7]. As the risk of hip arthroplasty failure and revision is about 1% per year [9], endocrinologists should expect to diagnose a significant number of patients with cobalt toxicity on the thyroid.

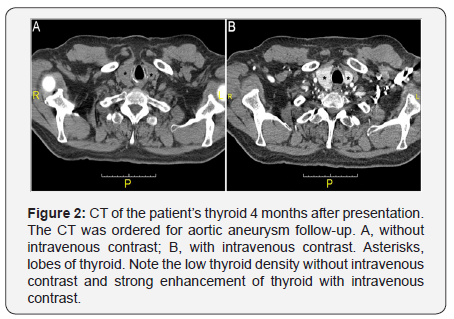
The mechanism for cobalt toxicity is direct cell injury [6,7]. The specific thyroid damage by cobalt has not been directly addressed. Our patient’s very high thyroglobulin level was consistent with destruction thyroiditis [10]. Unlike in sub acute thyroiditis, also a form of destruction thyroiditis, the high thyroglobulin level was associated with hypothyroidism, rather than hyperthyroidism, in this patient, suggesting that thyroid hormone synthesis may be impaired by cobalt, in addition to follicular cell injury. CT of the patient’s thyroid 4 months after presentation also demonstrates imaging features of destructionthyroiditis (Figure 2). The Hounsfield units (HU) of strap muscle were 51 without and with contrast, while that of the thyroid was 52 and 170-200, respectively. In comparison, 9 months before presentation, when he was feeling well, the HU of strap muscle was 49 and 56 without and with contrast, while that of thyroid was 105 and 140-160, respectively. The low thyroid HU without contrast after he developed cobalt toxicity symptoms implies that the thyroid had low iodine content, while the high thyroid HU with contrast indicates that the thyroid had high blood flow, both are features of destruction thyroiditis [11]. Thus biochemical and imaging findings both demonstrate that he had destruction thyroiditis and impaired thyroid hormone synthesis.
Conclusion
In summary, this case illustrates that seeking specific etiology of hypothyroidism is important when hypothyroidism is concurrent with other abnormalities of unclear causes. Earlier recognition of cobalt toxicity may avoid excessive work-up of the myriad of seemingly unrelated symptoms and may facilitate timely intervention. This case further demonstrates that cobalt toxicity on the thyroid may manifest in destruction thyroiditis and impaired thyroid hormone synthesis.
References
- Roberts CG, Ladenson PW (2004) Hypothyroidism. Lancet 363(9411): 793-803.
- Mitrou P, Raptis SA, Dimitriadis G (2011) Thyroid disease in older people. Maturitas 70(1): 5-9.
- Farhad K, Traub R, Ruzhansky KM, Brannagan TH (2016) Causes of neuropathy in patientsreferred as “idiopathicneuropathy”. Muscle Nerve 53(6): 856-861.
- Kotwal SK, Kotwal S, Gupta R, Singh JB, Mahajan A (2016) Cerebellar ataxia as presenting feature of hypothyroidism. Arch Endocrinol Metab 60(2):183-185.
- Iyer A, Elsone L, Appleton R, Jacob A (2014) A review of the current literature and a guide to the early diagnosis of autoinmune disorders associated with neuro myelitisoptica. Autoimmunity 47(3): 154-161.
- Devlin JJ, Pomerleau AC, Brent J, Morgan BW, Deitchman S, et al. (2013) Clinical features, testing and management of patients with suspected prosthetic hip-associated cobalt toxicity: a systematic review of cases. J Med Toxicol 9(4): 405-415.
- Bradberry SM, Wilkinson JM, Ferner RE (2014) Systemic toxicity related to metal hip prostheses. Clin Toxicol (Phila) 52(8): 837-847.
- Pizon AF, Abesamis M, King AM, Menke N (2013) Prosthetichipassociated cobalt toxicity. J Med Toxicol 9(4): 416-417.
- Crawford RW, Murray DW (1997) Total hip replacement: indications for surgery and risk factors for failure. Ann Rheum Dis 56(8): 455-457.
- Madeddu G, Casu AR, Costanza C, Marras G, Arras ML, et al. (1985) Serum thyroglobulin levels in the diagnosis and follow-up of subacute ‘painful’ thyroiditis. A sequential study. Arch Intern Med 145(2): 243- 247.
- Rho MH, Kim DW (2014) Computed tomography features of incidentally detected diffuse thyroid disease. Int J Endocrinol 2014: 921934.






























