A Rare Case of Thyrotoxic Periodic Paralysis Caused by TSH-Secreting Pituitary Adenoma: Case Report and Review of the Literature
Monami Koga1, Takashi Fukuda1, Kunitaka Murase1, Ryoko Motonaga1, Makito Tanabe1, Takashi Nomiyama1, Toshirou Katsuta2, Tooru Inoue2, Hiroyuki Hayashi3, Kazuki Nabeshima3 and Toshihiko Yanase1*
1Department of Endocrinology and Diabetes Mellitus, Fukuoka University, Japan
2Department of Neurosurgery, Fukuoka University, Japan
3Department of Pathology, Fukuoka University, Japan
Submission: January 28, 2017; Published: February 08, 2017
*Corresponding author: Toshihiko Yanase, Department of Endocrinology and Diabetes Mellitus, School of Medicine, Fukuoka University, 7-45-1 Nanakuma, Jonan-ku, Fukuoka 814-0180, Japan, Tel:+81-92-801-1011 ; Fax: +81-92-865-5163; Email; tyanase@fukuoka-u.ac.jp
How to cite this article: Monami K, Takashi F, Kunitaka M, Ryoko M, Makito T, Takashi N, et al. A Rare Case of Thyrotoxic Periodic Paralysis Caused by TSH-Secreting Pituitary Adenoma: Case Report and Review of the Literature. J Endocrinol Thyroid Res. 2017; 1(1): 555551. DOI:10.19080/JETR.2017.01.555551
Abstract
A 28-year-old man manifested thyrotoxicosis and periodic quadriplegia with hypokalemia. Further examination showed that he had inappropriate TSH secretion and a pituitary tumor. He was suspected as having thyrotoxic periodic paralysis (TPP) due to TSH-secreting pituitary adenoma. Therefore, transsphenoidal surgery was performed. The adenoma only stained positive for TSH. TPP is mostly associated with Basedow’s disease. Our case is rare and the 6th reported TPP case caused by TSH-secreting pituitary adenoma. Our results suggest that TPP is not caused by an autoimmune mechanism, but thyroid hormones instead. We review five other rare cases of TPP caused by TSH-secreting pituitary adenoma.
Keywords: Thyrotoxic periodic paralysis; Inappropriate TSH secretion; TSH-secreting pituitary adenoma
Abbreviations: TPP: Thyrotoxic Periodic Paralysis; BMI: Body Mass Index; IRI: Immunoreactive Insulin; OGTT: Oral Glucose Tolerance test; IGT: Impaired Glucose Tolerance; T1WI: T1 Weighted Image; T2WI: T2 Weighted Image; HLA: Human Leukocyte Antigen
Introduction
Periodic quadriplegia presents with a transient quadriplegia attack, which lasts for several hours or for a few days. Periodic quadriplegia is generally categorized into three types of low, normal, or high potassium. Low potassium (hypokalemia) is the consequence of a rapid and massive shift of potassium from the extracellular into the intracellular compartment, mainly into the muscles. The low potassium type of periodic quadriplegia is frequently found as a type of familial periodic quadriplegia in Western countries. However, the low potassium type of periodic quadriplegia is frequently observed as thyrotoxic periodic paralysis (TPP) in Asian countries, including Japan [1,2], and is rare in Western countries [3,4]. The overall incidence of TPP in Japanese thyrotoxic patients is 1.9% and 8%, respectively [1,2]. In North America, the incidence rate of TPP is approximately 0.1–0.2% in thyrotoxic patients [4,5]. In addition, despite a much higher incidence of thyrotoxicosis in women, TPP predominantly affects men [1-5]. TPP comprises approximately 50% of the total causes of periodic quadriplegia in Asia [2].
The biggest cause of thyrotoxicosis causing TPP is Basedow’s disease, which accounts for 83% of the total cases [3]. Thyrotropin (TSH)-producing pituitary tumor is a rare pituitary tumor. This tumor only comprises 0.5–3% of functioning pituitary adenoma, and is the cause of Thyrotoxicosis at a rate of less than 1% [6,7]. We experienced an extremely a rare case of TPP in association with a TSH-producing pituitary tumor. To the best of our knowledge, only five other cases similar to our case have been reported worldwide [8-12]. We present our rare case in this report and review a total of six (including our case) case reports of TPP in association with a TSH-producing pituitary tumor
Case Report
A 28 year-old man first noted fatigue of the limbs and muscle weakness of both femoral regions, especially when he got up in the morning. He also noticed loss of pectoral muscle strength, palpitation, slight dyspnea, diarrhea, and excessive sweating at approximately one month. One morning, he experienced an attack of quadriplegia when he tried to get out of bed, and then he was urgently transferred to a nearby doctor. A blood test showed that the serum level of TSH was 2.5μIU/mL, FT4 was 2.7 ng/dL, and K was 2.5 mEq/L. Because of the severe hypokalemia and thyrotoxicosis, he was suspected as having the low potassium type of TPP. He was then immediately administered 10 mEq of KCL. His symptoms improved several hours after treatment. To improve thyrotoxicosis, medication of 15 mg/day of thiamazole was started the next day. However, because of the appearance of skin eruption, treatment with thiamazole was stopped and replaced with propylthiouracil at a dose of 150 mg/day. However, thyrotoxicosis was not improved. He visited our department for further examinations. He had no important past medical history. In his family history, his aunt was affected by Basedow’s disease.
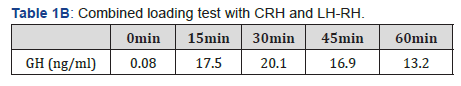
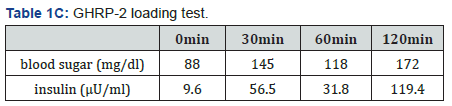
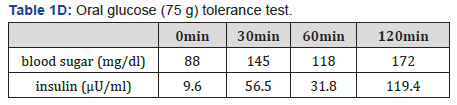
The patient’s physical examination showed a height of 183.2cm, body weight of 73.6 kg body mass index (BMI) of 22.19 kg/m2, blood pressure of 117/65 mmHg, pulse of 74 beats/ min, no exophthalmoses, no abnormality of eye movement, and no visual field disturbance. In the neck, a diffusely enlarged and elastic hard thyroid gland was palpable, but showed no tenderness. No abnormalities of the head, chest abdomen, muscle strength, and nervous system were observed. The patient showed no Cushingoid or acromegalic features. General laboratory data of a peripheral blood cell count and serum chemistry showed no specific abnormalities, including serum potassium levels (4.4 mEq/L). A thyroid function test showed the following: TSH, 6.98 μIU/mL (reference range 0.5-5.0); FT4, 2.56 ng/dL (0.9-1.7) and FT3, 7.95 pg/mL (2.3-4.0). These findings suggested the presence of the syndrome of inappropriate secretion of TSH, as also demonstrated by previous data that were measured by the first doctor. All of the thyroidal autoantibodies were negative as follows: anti-TPO antibody, 9I U/mL; anti-thyroglobulin antibody, <10 IU/mL, TRAb <1.0 IU/mL and TSAb, 102%. Importantly, the α subunit/TSH ratio, which was measured by an immunochem. iluminometric assay (Mayo Clinic, USA), was greater than the usual cut-off value of 1, namely 1.7. This finding also suggested the presence of a TSH-secreting pituitary tumor [7]. The basal values of other pituitary, adrenal, and gonadal hormones were as follows: GH, 0.08 ng/ml (reference range ≤ 2.47); ACTH, 20.6pg/ ml (7.2-63.3); prolactin, 15.6 ng/mL (3.5-19.4), LH, 2.6mIU/ ml (0.6-12.1); FSH, 3.1mIU/ml (1.0-12.0); IGF-1, 202 ng/mL (114-315); cortisol, 18.8μg/dL (6.2-19.4); and testosterone, 815.2 ng/mL (225.0-1039.0). All of these values were within the normal range. Table 1A-1C shows the hormonal responses of TSH and prolactin (PRL) to a thyrotropin-releasing hormone (TRH) loading test, responses of ACTH and cortisol to a CRH test, responses of LH and FSH to a LH-RH test, and the response of GH to a GHRP-2 test.
The TRH loading test indicated a poor response of TSH and PRL. This result suggested the presence of a TSH-secreting pituitary tumor because TSH secretion from a TSH-secreting pituitary tumor does not usually increase much in response to TRH [7]. In the CRH loading test, ACTH showed a poor response, but normal cortisol response. In the LH-RH loading test, FSH showed a normal response, but LH showed a poor response. In the GHRP-2 loading test, GH showed a normal response. While fasting blood glucose (96 mg/dL) and HbA1c (5.5%) were within the normal range, the IRI level (11.4 μU/mL) was slightly high. The 75-g OGTT showed 172 mg/dL of blood sugar level at 120 min, corresponding to IGT type. The insulin response was enhanced considering that he was not obese (BMI 22.2 kg/m2) (Table 1D). Magnetic resonance imaging of the pituitary showed a macroadenoma (31.4×18.3 mm), which was a low signal in T1WI (Figure 1A) and a low-high signal in T2WI (Figure 1B). There was relatively uniform enhancement by a contrast agent, gadolinium, in T1W1 (Figure 1C). Progression of the pituitary tumor to the spheroidal sinus and the left cavernous sinus, and compression to the upper part of both sides of the optic nerve and the optic chiasm were suspected. However, Goldman’s test, which is used to examine the visual field, showed no abnormalities. Thyroid echography demonstrated a diffuse swelling of the thyroid gland with a small amount of increased blood flow in the total gland. We did not observe a thyroid tumor or lymph node swelling (data not shown). Based on these findings, the patient was highly suspected to have thyrotoxicosis by a TSH-secreting pituitary tumor.

Table 1: Hormonal response to TRH, CRH, LH-RH, GHRP-2 and glucose loading.

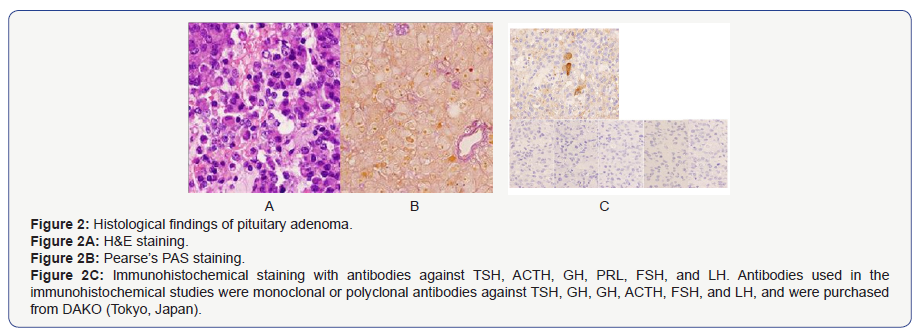
As a result, endoscopic transsphenoidal surgery to remove the pituitary tumor was performed. The pathological finding of the resected tumor was pituitary adenoma (Figure 2A), which consisted of chromophobic cells as shown by Pearse’s PAS staining (Figure 2B). A PAS-stained small body was also partly seen. Immunohistochemically, the adenoma was positive for TSH and negative for GH, PRL, ACTH, FSH, and LH (Figure 2C). Pathologically, there was no progression to the spheroidal sinus, but invasion to the cavernous sinus was observed. Based on these findings, TSH-secreting pituitary adenoma was finally diagnosed. After the surgery, he had no episode of periodic paralysis so far (Figure 3).
Discussion
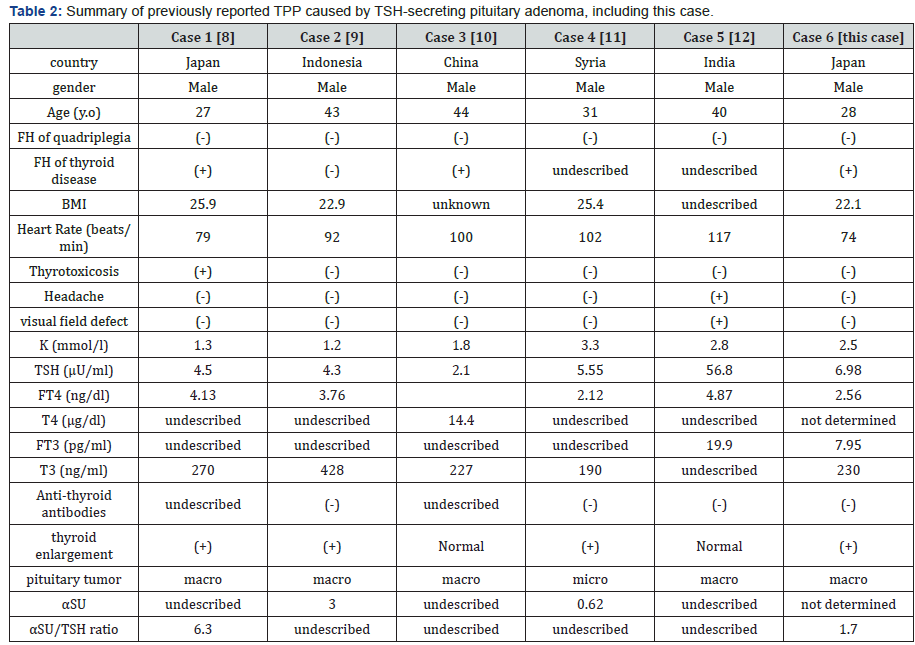
TPP is mostly observed in patients with Basedow’s disease [1-5]. Our case is rare in that TPP was not caused by Basedow’s disease, but by TSH-secreting pituitary adenoma instead. Only five cases of a TSH-secreting tumor that manifested as TPP have been reported to date. The characteristics of all of the six cases, including our case, are shown in Table 2. All of the patients were men from Asia to the East Asian region, and their age ranged from the twenties to the forties. Sex, area, and age of our case were similar to those of usual TPP cases [1-5]. No family history of quadriplegia was noted. BMI of these patients was 22-25 kg/m2, suggesting that no excessive thyrotoxicosis causing emaciation was present. All of the six patients had mildly elevated serum thyroid hormone levels and manifested few symptoms, except for case 1. These findings are also compatible with a previous report on TPP, which described that because of a mild elevation in thyroid hormones, only 10% of patients showed mild thyrotoxic symptoms [13].
The subtlety or silence of the features of hyperthyroidism [5] together with the rarity of TPP may make it difficult to diagnose this condition. All six patients showed hypokalemia, but the extent of hypokalemia was not correlated with the severity of hyperthyroidism (i.e., levels of serum FT4 and TSH). Thyroid antibodies were negative in patients in whom anti-thyroid antibodies were measured. The thyroidal gland varied from a normal size to diffuse swelling. The finding of negative antithyroid antibodies in the five previously reported cases suggests that an autoimmune mechanism is probably not important for the mechanism of TPP, even though TPP is mostly observed in Basedow’s disease. With regard to the size of the pituitary tumor, macroadenoma was observed in five of six cases, which is also consistent with the fact that TSH-secreting pituitary adenomas are relatively large [7].
The pathogenesis of TPP remains unclear. Hypokalemia is thought to be related to increased s o d i u m - p o t a s s i u m - adenosine triphosphatase (Na/K-ATPase) pump activity. Na/KATPase pump activity determined in peripheral tissues, such as red blood cells, leukocytes, and platelets, shows a higher activity in patients with thyrotoxicosis and TPP than in thyrotoxic patients without TPP [14-16]. Importantly, when thyrotoxicosis is controlled, Na/K-ATPase activity returns to the level of healthy controls. Thyroid hormones can increase Na/K-ATPase activity in various tissues, including skeletal muscle, thus inducing an influx of potassium into the intracellular space [17-19]. These findings suggest that elevation of thyroid hormones appears be a central mechanism of TPP, regardless of the cause (e.g. Basedow’s disease or TSH-secreting pituitary adenoma). Catecholamines can also increase Na/K-ATPase activity in skeletal muscle [20]. Therefore, an enhanced β-adrenergic response in thyrotoxicosis may further increase Na/K-ATPase activity. In addition, patients with TPP have an exaggerated insulin response during an oral glucose challenge compared with thyrotoxic patients without TPP [21,22]. Insulin-response sequences are present in the upstream region of Na/K-ATPase genes, and insulin has been shown to stimulate Na/K-ATPase activity [23]. Therefore, insulin can play a permissive role in the potassium shift in patients with TPP [24]. A hyper insulinemic response is reasonable to explain the association of TPP with carbohydrate-rich meals. While a high carbohydrate meal intake was not clear in our patient, he showed hyperinsulinemia in a 75-g OGTT, despite his normal BMI. Therefore, hyperinsulinemia could be a potential factor for the susceptibility to TPP in this patient. Exercise releases potassium from the skeletal muscles, whereas rest promotes influx of potassium. This explains why paralytic attacks occur only during recovery from exercise [4]. The reason for male predominance in TPP is unclear, but is suggested to be due to increased Na-K ATPase activity by hyper androgenism [25].
There is a relatively high prevalence of some types of human leukocyte antigens (HLAs) in Asian patients with TPP [26,27]. However, specificities of such HLA genes in TPP is not clear because these HLA associations are also observed in Basedow’s’ disease. Further investigations are needed for clarifying the total mechanism of TPP. Overall, patients with TPP appear to have an underlying predisposition for activation of Na/K-ATPase activity, either directly by thyroid hormones, or indirectly via sex, unknown constitution, adrenergic stimulation, insulin or exercise.
Conclusion
In conclusion, we report a case of TPP by TSH-producing pituitary adenoma. The mechanism appears to be related to mild elevation of thyroid hormones and possibly hyper insulinemia after carbohydrate loading.
References
- Okinaka S, Shizume K, Iino S, Watanabe A, Irie M, et al. (1957) The association of periodic paralysis and hyperthyroidism in Japan. J Clin Endocrinol Metab 17(12): 1454-1459.
- Satoyoshi E, Murakami K, Kowa H, Kinoshita M, Nishiyama Y (1963) Periodic paralysis in hyperthyroidism. Neurology 13: 746-752.
- Pothiwala P, Levine SN (2010) Analytic review: thyrotoxic periodic paralysis: a review. J Intensive Care Med 25(2): 71-77.
- Ober KP (1992) Thyrotoxic periodic paralysis in the United States. Report of 7 cases and review of the literature. Medicine (Baltimore) 71(3): 109-120.
- Kelley DE, Gharib H, Kennedy FP, Duda RJ, McManis MB (1989) Thyrotoxic periodic paralysis. Report of 10 cases and review of electromyographic findings. Arch Intern Med 149(11): 2597-2600.
- Ónnestam L, Berinder K, Burman P, Dahlqvist P, Engström BE, et al. (2013) National incidence and prevalence of TSH-secreting pituitary adenomas in Sweden. J Clin Endocrinol Metab 98(2): 626-635.
- Beck-Peccoz P, Lania A, Beckers A, Chatterjee K, Wemeau JL (2013) European thyroid association guidelines for the diagnosis and treatment of thyrotropin-secreting pituitary tumors. Eur Thyroid J 2(2): 76-82.
- Kiso Y, Yoshida K, Kaise K, Kaise N, Masuda T, et al. (1990) A case of thyrotropin-secreting tumor complicated by periodic paralysis. Jpn J Med 29(4): 399-404.
- Alings AM, Fliers E, de Herder WW, Hofland LJ, Sluiter HE, et al. (1998) A thyrotropin-secreting pituitary adenoma as a cause of thyrotoxic periodic paralysis. J Endocrinol Invest 21(10): 703-706.
- Hsu FS, Tsai WS, Chau T, Chen HH, Chen YC, et al. (2003) Thyrotropin- Secreting Pituitary Adenoma Presenting as Hypokalemic Periodic Paralysis. Am J Med Sci 325(1): 48-50.
- Pappa T, Papanastasiou L, Markou A, Androulakis I, Kontogeorgos G, et al. (2010) Thyrotoxic periodic paralysis as the first manifestation of a thyrotropin-secreting pituitary adenoma. Hormones (Athens) 9(1): 82-86.
- Ghosh S, Sahoo R, Rao S, Rath D (2013) Pituitary macroadenoma: a rare cause of thyrotoxic hypokalaemic periodic paralysis. BMJ Case Rep pii: bcr2012008411.
- Ko GT, Chow CC, Yeung VT, Chan HH, Li JK, et al. (1996) Thyrotoxic periodic paralysis in a Chinese population. QJM 89(6): 463-468.
- Khan FA, Baron DN (1987) Ion flux and Na+, K+-ATPase activity of erythrocytes and leucocytes in thyroid disease. Clin Sci (Lond) 72(2): 171-179.
- Chan A, Shinde R, Chow CC, Cockram CS, Swaminathan R (1991) In vivo and in vitro sodium pump activity in subjects with thyrotoxic periodic paralysis. BMJ 303(6810): 1096-1099.
- Lam KS, Yeung RT, Benson EA, Wang C (1989) Erythrocyte sodiumpotassium pump in thyrotoxic periodic paralysis. Aust N Z J Med 19(1): 6-10.
- Lo CS, Edelman IS (1976) Effect of triiodothyronine on the synthesis and degradation of renal cortical Na+ K+ adenosine triphosphatase. J Biol Chem 251(24): 7834-7840.
- Curfman GD, Crowley TJ, Smith TW (1977) Thyroid-induced alterations in myocardial sodium-potassium-activated adenosine triphosphatase, monovalent cation active transport, and cardiac glycoside binding. J Clin Invest 59(3): 586-590.
- Lin MH, Akera T (1978) Increased Na+, K+-ATPase concentrations in various tissues of rats caused by thyroid hormone treatment. J Biol Chem 253(3): 723-726.
- Layzer RB (1982) Periodic paralysis and the sodium-potassium pump. Ann Neurol 11(6): 547-552.
- Shishiba Y, Shimuzu T, Saito T, Shizuma K (1972) Elevated immunoreactive insulin concentration during spontaneous attacks in thyrotoxic periodic paralysis. Metabolism 21(4): 285-290.
- Lee KO, Taylor EA, Oh VMS, Cheah JS, Aw SE (1991) Hyperinsulinaemia in thyrotoxic hypokalaemic periodic paralysis. Lancet 337(8749): 1063-1064.
- Hundal HS, Marette A, Mitsumoto Y, Ramlal T, Blostein R, et al. (1992) Insulin induces translocation of the α2 and β1 subunits of the Na+/K+- ATPase from intracellular compartments to the plasma membrane in mammalian skeletal muscle. J Biol Chem 267(8): 5040-5043.
- Chan A, Shinde R, Chow CC, Cockram CS, Swaminathan R (1994) Hyperinsulinaemia and Na+, K+-ATPase activity in thyrotoxic periodic paralysis. Clin Endocrinol (Oxf) 41(2): 213-216.
- Yao Y, Fan L, Zhang X, Xiao Z, Long Y, et al. (2013) Episodes of paralysis in Chinese men with thyrotoxic periodic paralysis are associated with elevated serum testosterone. Thyroid 23(4): 420-427.
- Hawkins BR, Ma JT, Lam KS, Wang CC, Yeung RT (1985) Association of HLA antigens with thyrotoxic Graves’ disease and periodic paralysis in Hong Kong Chinese. Clin Endocrinol (Oxf) 23(3): 245-252.
- Yeo PP, Chan SH, Lui KF, Wu GB, Lim P, et al. (1978) HLA and thyrotoxic periodic paralysis. Br Med J 2(6142): 930






























