Detection and Genotype Identification of Pneumocystis Jirovecii In COVID-19 Patients with Severe Pneumonia
Heydari A1,2, Mobaien A3, Ghodrati S4 and Fazaeli A2*
1Social Determinants of Health Research Center, Health and Metabolic Diseases Research Institute, Zanjan, Iran
2Department of Medical Parasitology & Mycology, Medical School, Zanjan University of Medical Sciences, Zanjan, Iran
3Department of Infectious Diseases, Medical School, Zanjan University of Medical Sciences, Zanjan, Iran
4Department of Internal Medicine, Medical School, Zanjan University of Medical Sciences, Zanjan, Iran
Submission: October 02, 2024; Published: October 07, 2024
*Corresponding author: Asghar Fazaeli, Department of Medical Parasitology & Mycology, School of Medicine, Zanjan University of Medical Sciences, Zanjan, Iran
How to cite this article: Heydari A, Mobaien A, Ghodrati S, Fazaeli A. Detection and Genotype Identification of Pneumocystis Jirovecii In COVID-19 Patients with Severe Pneumonia. Int J Pul & Res Sci. 2024; 7(4): 555716. DOI: 10.19080/IJOPRS.2024.07.555716
Abstract
Pneumocystis jirovecii is a widespread and important opportunistic fungal or parasitic organisms causing alveolar infection that may affect groups of patients with underlying diseases, particularly those with immunodeficiency. It may coexist with other respiratory infections and increase the severity of the clinical consequences. This study aimed to investigate the prevalence of P. jirovecii and its genotypes in patients with severe pneumonia admitted to intensive care unit in a COVID-19 referral center. A total of 93 mini bronchoalveolar lavage (mini BAL) specimens of COVID-19 patients with severe pneumonia and underlying diseases admitted to intensive care unit, were analyzed by cytology staining and PCR method. In addition, the positive P. jirovecii were genotyped by mitochondrial large subunit rRNA locus sequencing. PCR screening of the mini BAL specimens showed that 5 patients (5.38%) had P. jirovecii co-infection with COVID-19. The genotype analysis showed that 3 isolates were P. jirovecii genotype 1 (wild) and 2 isolates were genotype 2. The genotype 3 of this organism was not found in the isolates. The present study showed that the infection rate of P. jirovecii was relatively low in the patients with severe pneumonia. Meanwhile, they were detected in the diabetics or cardiovascular patients. The P. jirovecii isolates consisted of two genotypes I and II.
Keywords:Pneumocystis jirovecii, Genotype, Pneumocystis pneumonia, Severe pneumonia, COVID-19
Abbreviations:AIDS: Acquired Immunodeficiency Syndrome; HIV: Human Immunedefiency Virus; ICU: Intensive Care Unit; BAL: Bronchoalveolar Lavage; GMS: Gomori Methenamine Silver; LDH: Lactate Dehydrogenase
Introduction
Pneumocystis jirovecii is unicellular and opportunistic fungus, which can cause plasma cell pneumonitis, particularly in the high risk groups and neonates [1,2]. The prevalence of P. jirovecii increased significantly in patients with acquired immunodeficiency syndrome (AIDS), kidney transplant recipients, and even immunocompetent individuals [1,3]. In a retrospective multicenter study, considering characteristics and prognosis factors of Pneumocystis jirovecii Pneumonia, long-term corticosteroid therapy was found to be independently associated with increased mortality, specifically in patients with immune-mediated inflammatory diseases [4]. The clinical symptoms of the pneumocystis pneumonia are nonspecific, including respiratory distress, nonproductive cough, cyanosis, low grade fever, and, in severe cases, respiratory failure, which are similar to symptoms of other viral and bacterial infections [1,5] .
Meanwhile, the presence of P. jirovecii in the lungs are not always symptomatic. A review study showed that the mortality of pneumocystis pneumonia ranged between 20% to 53% (30.6% overall) in non-HIV patients and 5-30% in patients live with HIV (human immunedefiency virus) even with treatment [6,7]. This difference may be attributed to host responses to the infection. The immune response to pneumonia caused by P. jirovecii in AIDS patients is suppressed; this results in decreased inflammation in the alveoli [1]. Clinical pneumonia caused by P. jirovecii can be developed by mononuclear cell response and filling the alveoli with proteinaceous debris and fluid in the immunocompromised patients, especially patients with T-cell immunodepression [1,8]. Adjunctive immunomodulatory and/or steroids therapies may develop lymphocytopenia in COVID-19 patients, which is of susceptibility factors for developing pneumocystosis [9-11]. There are some literature demonstrated that COVID-19 can become complicated with bacterial or fungal superinfection such as aspergilosis and pneumocystosis [12,13].
Approximately 5-10% of patients with COVID-19 required intensive care unit (ICU) management [14]. The hospitalized patients of COVID-19 in ICU might be at risk of infection caused by P. jirovecii due to the existence of cytokine storm, mechanical ventilation and use of corticosteroid therapy. Several cases of superinfection with severe acute respiratory syndrome were reported in patients with COVID-19 but the prevalence of P. jirovecii in these patients was unknown. Only a few reports are presented in this regard. Gerber et al. [12] found 4 cases of Pneumocystis pneumonia among 57 COVID-19 patients; Blaize et al. [15] found 1.4% positive cases of the patients in ICU and Alanio et al. [16] found 9.3% positive cases using PCR in sputum, bronchoalveolar lavage (BAL) and tracheal aspirate. To do further investigation, we aimed to survey the prevalence of P. jirovecii and its genotypes in COVID-19 patients admitted to ICU in referral centers in Zanjan province in 2021.
Materials and Methods
Specimen Collection
A total of 93 mini BAL specimens were collected from COVID-19 patients with severe pneumonia and underlying diseases, admitted to ICU in Valiasr teaching hospital, Zanjan, Iran, from February to August 2021. The samples were also previously evaluated for Lophomonas blattarum infection in another study [17]. The patients’ records, including demographic information and medical history, as well as clinical, microbiological, and radiological data were collected to be considered.
P. jirovecii Detection and Genotype Analysis

The specimens were stained with Giemsa, Papanicolaou and Gomori methenamine silver (GMS) staining methods. Total DNA were extracted using a DNA extraction kit (Addbio, Korea). Nested PCR was performed by two primer pairs of mitochondrial large subunit ribosomal RNA (mtLSU rRNA) locus shown in Table 1. The PCR products were analyzed by 1.5% agarose gel and visualized using standard UV trans-illuminator. The positive PCR products were sequenced with Sanger sequencing (ABI 3500, Applied Biosystems Inc., USA). All post sequencing analysis was performed using Multiple Sequence Comparison by Log- Expectation (MUSCLE) of EMBL-EBI (https://www.ebi.ac.uk/Tools/msa/muscle/). Each sequence was compared with a reference sequence of mtLSU rRNA of P. jirovecii (Genbank accession number: M58605.1) [18].
Results
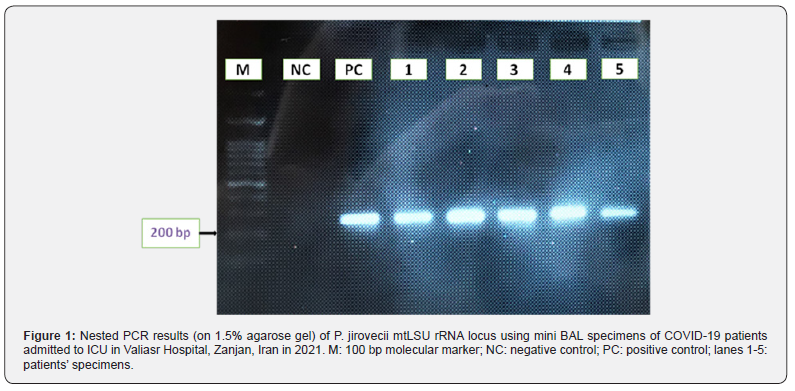
The patients subjected to the study, included 49 males and 44 females with the mean age of 68.8 ± 14.8, ranged between 20 to 90 years old. Over 80% of the patients showed to have underlying diseases, including cardiovascular disease and hypertension in 29 (31.18%), diabetes in 21 (22.58%), pulmonary disease in 13 (13.98%), different types of cancer in 4 (4.30%), other diseases in 9 (9.68%) cases, and 2 patients were kidney transplant recipients (2.15%). All patients received dexamethasone and broad-spectrum antibiotics such as imipenem, ceftriaxone, cefotaxime. The BAL cytology, using GMS, Papanicolaou, and Giemsa staining detected P. jirovecii in 6 specimens (6.45%). PCR amplification of the 93 mini BAL specimens detected P. jirovecii DNA in 5 patients (5.38%) Figure 1. The genotypic analysis of P. jirovecii showed that 3 isolates were genotype 1 (wild) and 2 isolates were genotype 2 Figure 2. The genotype 3 of this organism was not found within the isolates. The demographic, laboratory and clinical data of the patients with co-infection of COVID-19 and P. jirovecii are shown in Table 2.
Discussion
In this study, 5 out of 93 (5.38%) COVID-19 patients admitted to ICU were found to be co-infected with P. jirovecii using microscopic and PCR methods. Individuals with healthy immune system can play the role of asymptomatic carriers and sources of P. jirovecii infection. This infection may lead to the stimulation of inflammatory responses and lung tissue damage [2,19]. The results demonstrated that 2 cytology positive mini BAL specimens were negative by PCR test; they were diagnosed by Papanicolaou and GMS staining. In addition, one cytology negative isolate was positive by PCR test. These might be due to microscopic false positivity. It can also be attributed to the skill and expertise of the researcher in identifying the organism, the error during work, as well as the high sensitivity and specificity of the PCR method. The sensitivity of different staining methods depends on various factors such as type and quality of the sample, the number of organisms present and the experience of the laboratory staff [20]. PCR test of P. jirovecii could not distinct between pneumocystis pneumonia and colonization, especially in immunocompetent patients involved with COVID-19 [21,22]. Direct examination due to the estimated risk of aerosolization is usually not performed in COVID-19 patients, therefore the diagnosis of pneumocystis pneumonia in these patients are very challenging [15]. The existence of a cytokine storm, use of corticosteroid therapy or mechanical ventilation might be at risk of pneumocystis pneumonia in COVID-19 patients hospitalized in the ICU [23], which was found in all 5 patients reported in this study. In addition, COVID-19 pneumonia and pneumocystis pneumonia have a similar pattern in terms of clinical symptoms and chest radiology (CT scan) findings (24). In this regard, Menon et al. [22] recommended to use the mycological criteria, on a set of arguments including clinical worsening, serum (1,3)-b-D-glucan, lactate dehydrogenase (LDH) assays, deep lymphocytopenia, immunosuppression, and response to treatment for the diagnosis of pneumocystis pneumonia.

Abbreviations: CT-scan: computed tomography scanning; GGO: ground-glass opacities; ICU: intensive care unit; Pred: prednisolone; IFN: interferon; Dex: dexametazon HCT: hydrocortisone.
There are several case reports that describe co-infections of SARS-CoV-2 with P. jirovecii. Mang et al. [24] described a HIV patient with COVID-19, who treated with trimethoprim-sulfamethoxazole and presented an elevated level of LDH, fine reticular changes in chest radiology and a severe depletion of CD4 T-cells. CD4+ T and CD8+ T cell levels could be remarkably decrease in severe COVID-19 cases [11,15]. Blaize et al. [15] suggested that there are no association between the occurrence of Pneumocystis pneumonia and the lymphocytopenia encountered in severe COVID-19 cases. Therefore, we did not assess the lymphocytopenia in the patients. Limited studies have been conducted to investigate P. jirovecii and COVID-19 in different patients at the same time. Alanio et al. [25] reported the co-infection of P. jirovecii in patients with COVID-19 hospitalized in the ICU. It was positive in 5 out of 27 (17%) BAL samples and 2 aspirate fluid samples. Gerber et al. [12] reported 7.1% co-infection of P. jirovecii in the patients with COVID-19. The impact of Pneumocystis contribution leading to pneumonia depends on several factors, including the studied population, the existence of clinical background and other diseases, geographical area, administered drugs (i.e. a broad spectrum antibiotics received by COVID-19 patients, as mentioned in this study). One of the possible reasons for relatively low infection rate of P. jirovecii in the examined patients in the present study, may be due to the effects of antibiotics being used to treat COVID-19. Two samples were positive by microscopic examination but were not detected by PCR amplification. On the other hand, a sample with microscopically negative result was positive with PCR method. This may be due to lower sensitivity and/or low specificity of microscopic observation. The different staining methods also showed variable microscopic results.

P. jirovecii has genotypic diversity that may differently affect the development of pneumocystis pneumonia, colonization, and drug resistance. Sequencing of several gene loci of P. jirovecii, especially the mtLSU rRNA locus, is a standard method for genetic diversity analysis. Based on this target, genotypes 2 and 3 are globally reported to be associated with severe Pneumocystis pneumonia and are claimed to be very fatal [26]. The molecular results of the present study using sequence comparison showed that out of 5 P. jirovecii isolates, 3 samples were related to genotype 1 and 2 samples belonged to genotype 2; no genotype 3 was observed in sequence analysis. Genotype 2 was observed in a diabetic patient and in a lymphoma cancer patient; genotype 1 was observed in patients with diabetes and/or cardiovascular disease. However, it is not possible to confirm the distinct connection of P. jirovecii infection with the type of underlying disease due to the small number of positive cases in the studied samples.
Conclusion
This study showed that P. jirovecii infection has a relatively low rate in the patients with COVID-19, but it was found in the patients with severe condition. Therefore, Pneumocystis pneumonia should be considered in the patients with severe COVID-19 and similar pulmonary infections. Determining the sequence of the amplified DNA fragment showed that genotypes 1 and 2 constituted the Pneumocystis genotypes in the COVID-19 patients with underlying diseases. We suggest additional studies on colonization, distribution, diagnostics, and interaction of P. jirovecii with regards to other respiratory coexisted infections and disorders.
Acknowledgement
This study was approved by the Research Council and financially supported by the Deputy for Research at Zanjan University of Medical Sciences (code: A-12-245-11). It is part of A. Heidari’s thesis for awarding a MSc degree. The scientific and administrative supports of the Social Determinants of Health Research Center, Zanjan University of Medical Sciences, Zanjan, are greatly appreciated.
Ethical consideration
All ethical principles are considered in this research. The project was confirmed by the Research Ethics Committee of Zanjan University of Medical Sciences (code: IR.ZUMS.REC.1399.014).
References
- Weyant RB, Kabbani D, Doucette K, Lau C, Cervera C (2021) Pneumocystis jirovecii: a review with a focus on prevention and treatment. Expert Opin Pharmacother 22(12): 1579-1592.
- Sokulska M, Kicia M, Wesołowska M, Hendrich AB (2015) Pneumocystis jirovecii-from a commensal to pathogen: clinical and diagnostic review. Parasitol Res 114(10): 3577-3585.
- De Francesco MA, Alberici F, Bossini N, Scolari F, Pascucci F, et al. (2020) Pneumocystis jirevocii and SARS-CoV-2 co-infection: a common feature in transplant recipients? Vaccines 8(3): 544.
- Lécuye R, Issa N, Camou F, Lavergne R, Gabriel F, et al. (2024) Characteristics and prognosis factors of Pneumocystis jirovecii pneumonia according to underlying disease, a retrospective multicenter study. CHEST 165(6): 1319-1329.
- Truong J, Ashurst JV (2024) Pneumocystis jirovecii Pneumonia. In: StatPearls [Internet]. Treasure Island (FL): StatPearls Publishing.
- Liu Y, Su L, Jiang S-J, Qu H (2017) Risk factors for mortality from pneumocystis carinii pneumonia (PCP) in non-HIV patients: a meta-analysis. Oncotarget 8(35): 59729.
- Ma L, Chen Z, Huang DW, Kutty G, Ishihara M, et al. (2016) Genome analysis of three Pneumocystis species reveals adaptation mechanisms to life exclusively in mammalian hosts. Nat Commun 7(1): 1-14.
- Alanio A, Hauser PM, Lagrou K, Melchers WJ, Helweg-Larsen J, et al. (2016) ECIL guidelines for the diagnosis of Pneumocystis jirovecii pneumonia in patients with haematological malignancies and stem cell transplant recipients. J Antimicrob Chemother 71(9): 2386-2396.
- Beumer M, Koch R, Van Beuningen D, OudeLashof A, Van de Veerdonk F, et al. (2019) Influenza virus and factors that are associated with ICU admission, pulmonary co-infections and ICU mortality. J Critic Care 50: 59-65.
- Dakowitz M, Korus J, Mazanowska O, Krajewska M, Kamińska D (2022) Co-Infection of COVID-19 and Pneumocystosis Following Rituximab Infusion & mdash; A Case Report. Transplantol 3(1): 83-90.
- Gholizadeh P, Safari R, Marofi P, Zeinalzadeh E, Pagliano P, et al. (2020) Alteration of liver biomarkers in patients with SARS-CoV-2 (COVID-19). J Inflammation Res 13: 285-292.
- Gerber V, Ruch Y, Chamaraux-Tran T-N, Oulehri W, Schneider F, et al. (2021) Detection of Pneumocystis jirovecii in patients with severe COVID-19: diagnostic and therapeutic challenges. J Fungi 7(8): 585.
- Baddley JW (2022) Coronavirus disease 2019-associated pulmonary aspergillosis: Do we have the Capacity to improve outcomes? Clin Infect Dis 74(1): 92-94.
- Thibault R, Seguin P, Tamion F, Pichard C, Singer P (2020) Nutrition of the COVID-19 patient in the intensive care unit (ICU): a practical guidance. Critical Care 24(1): 447.
- Blaize M, Mayaux J, Luyt C-E, Lampros A, Fekkar A (2020) COVID-19–related respiratory failure and lymphopenia do not seem associated with pneumocystosis. Am J Respi Critl Care Med 202(12): 1734-1736.
- Alanio A, Dellière S, Voicu S, Bretagne S, Mégarbane B (2021) The presence of Pneumocystis jirovecii in critically ill patients with COVID-19. J Infect 82(4): 84-123.
- Heydari A, Mobaien A, Ghodrati S, Fazaeli A (2024) Pulmonary Infection of Lophomonas blattarum as a Co-infection with COVID-19 in Patients with Severe Pneumonia. J Hum Environ Health Promot 10(2): 79-82.
- Monroy-Vaca EX, de Armas Y, Illnait-Zaragozí MT, Diaz R, Toraño G, et al. (2014) Genetic diversity of Pneumocystis jirovecii in colonized Cuban infants and toddlers. Infect Genet Evol 22: 60-66.
- Medrano FJ, Montes-Cano M, Conde M, De La Horra C, Respaldiza N, et al. (2005) Pneumocystis jirovecii in general population. Emerg Infect Dis 11(2): 245.
- Carmona EM, Limper AH (2011) Update on the diagnosis and treatment of Pneumocystis pneumonia. Therap Adv Respir Dis 5(1): 41-59.
- Menon AA, Berg DD, Brea EJ, Deutsch AJ, Kidia KK, et al. (2020) A case of COVID-19 and Pneumocystis jirovecii coinfection. Am J Respir Crit Care Med 202(1): 136-138.
- Menon AA, Berg DD, Gay EB, Fredenburgh LE, Reply to Blaize, et al. (2020) COVID-19–related Respiratory Failure and Lymphopenia Do Not Seem Associated with Pneumocystosis. Am J Respir Crit Care Med 202(12): 1736-1737.
- Gangneux J-P, Bougnoux M-E, Dannaoui E, Cornet M, Zahar J (2020) Invasive fungal diseases during COVID-19: we should be prepared. J Mycol Med 30(2): 100971.
- Mang S, Kaddu-MD, Metz C, Becker A, Seiler F, et al. (2020) Pneumocystis jirovecii Pneumonia and SARS-CoV-2 Co-Infection in newly diagnosed HIV-1 infection. Clin Infect Dis 72(8): 1487-1489.
- Alanio A, Voicu S, Dellière S, Mégarbane B, Bretagne S (2020) Do COVID-19 patients admitted to the ICU require anti-Pneumocystis jirovecii prophylaxis? medRxiv.
- Singh Y, Mirdha BR, Guleria R, Kabra SK, Mohan A, et al. (2019) Genetic polymorphisms associated with treatment failure and mortality in pediatric pneumocystosis. Sci Rep 9(1): 1-10.






























