Bronchial Thermoplasty: An Unutilized Therapy for Severe Asthma Between Caution and Prospects
Saeed Albogami1*, Kholoud Hambishi2, Ali Almutawa2, Horia Aboushosha2, Ohoud Aljohani2, Fayza Alghanmi3, Walaa Abulola4, Mohammed Alabyad4, Mohammed Basnawi4, Fahad Alrajhi5 and Sarah Alotaibi5
1Head Division of Pulmonology, Allergy & Immunology, King Fahad Hospital, Jeddah 2nd Health Cluster, Saudi Arabia.
2Consultant Pulmonologist, King Fahad Hospital, Jeddah 2nd Health Cluster, Saudi Arabia
3Allergy Specialist, King Fahad Hospital, Jeddah 2nd Health Cluster, Saudi Arabia
4Pulmonology Fellow, King Fahad Hospital, Jeddah 2nd Health Cluster, Saudi Arabia
5Internal Medicine Resident, King Fahad Hospital, Jeddah 2nd Health Cluster, Saudi Arabia
Submission: April 01, 2024; Published: April 15, 2024
*Corresponding author: Saeed Albogami,Consultant Pulmonologist, and Internist, Head ivision of Pulmonology, Allergy and Immunology, and Bronchoscopy Unit, Director of Adult Respirology Fellowship Training Program, King Fahad Hospital. Leader of Pulmonology Specialty Services at 2nd Jeddah Health Cluster, Jeddah, Saudi Arabia
How to cite this article: Saeed A, Kholoud H, Ali A, Horia A, Ohoud A, et al. Bronchial Thermoplasty: An Unutilized Therapy for Severe Asthma Between Caution and Prospects. Int J Pul & Res Sci. 2024; 7(2): 555709. DOI: 10.19080/IJOPRS.2024.07.555709
Abstract
Bronchial thermoplasty is a non-drug interventional treatment of severe uncontrolled asthma. Although, it has been described for more than two decades and approved by FDA but never been clearly recommended in any clinical guidelines from of the major pulmonology societies. Because of this and the unfamiliarity with the procedure and the fear of its complications by many pulmonologists, bronchial thermoplasty continued to be unutilized. Several well-designed multicenter trials have documented its effectiveness and safety and that it has led to an improvement in quality of life, reduction in exacerbations and hospitalizations in severe asthma patients. In addition, BT is a cost-effective option if peri-procedural costs are outweighed by costs related to hospitalization, ED visits and other standard therapies. In view of this, we tried in this review to redirect the focus toward this underestimated kind of treatment especially after the advancements in the procedure technique, tools, and preparations. We thought it might represent a hope for carefully selected patients with severe asthma. However, further studies are needed to elucidate the hidden points in the mechanism of action of BT and which asthma subtype would benefit more from this kind of therapy.
Keywords:Alair system; Airway smooth muscle; Bronchial thermoplasty; Radiofrequency energy; Severe asthma
Abbreviations: AIR: Asthma Intervention Research; ACQ: Asthma Control Questionnaire; AQLQ: Asthma Quality of Life Questionnaire; ASM: Airway Smooth Muscle; BT: Bronchial Thermoplasty; DB: Double Blind; ICS: Inhaled Corticosteroid; LABA: Long-Acting Beta-Agonist; OCS: Oral Corticosteroid; PAS2: Post-FDA Approval Clinical Trial Evaluating Bronchial Thermoplasty in Severe Persistent Asthma; RCT: Randomized Control Trial; RISA: Research in Severe Asthma; RF: Radiofrequency energy; TASMA: Unravelling Targets of Therapy in Bronchial Thermoplasty in Severe Asthma.
Introduction
One of the important components of the airways structure is the airway smooth muscle (ASM) which plays an important role in the pathophysiology of asthma including immune modulation, asthma exacerbations and long-term airway remodeling [1-3]. Thus, targeting ASM has been recognized as an important therapeutic strategy, either by pharmacological or non-pharmacological measures. Bronchial thermoplasty (BT) is a non-pharmacological therapy that has been used for decades in this issue. In BT, using bronchoscopy, a radiofrequency (RF) thermal energy via the Alair catheter electrode is systemically applied to the airway wall in aim to reduce the quantity and function of the hypertrophied ASM that contributes to airway hyperreactivity in severe asthma patients [4-7]. BT is indicated in patients with severe asthma who remain symptomatic despite adequate treatment.
In 2007, an expert’s opinion addressing the clinical use of BT by Mayse et al. [8] was published, since that, BT has been used in many parts of the world and its long-term efficacy has been well established [8]. Although it has been approved by the US Food and Drug Administration (FDA) in 2010, the implementation of BT in regular practice remains unsatisfactory because of the invasive nature of the procedure, its possible complications and questionable long-term benefit. In this review article, we tried to redirect the focus toward this kind of treatment especially after the advancements in BT technique, tools, and preparations. We thought BT is underestimated and might represent a hope for carefully selected patients with severe asthma poorly controlled by medications to improve their quality of life. We also, highlighted the essential principles of BT procedure, indications and contraindications, safety and efficacy, side effects, and complications, and cost effectiveness.
Methods
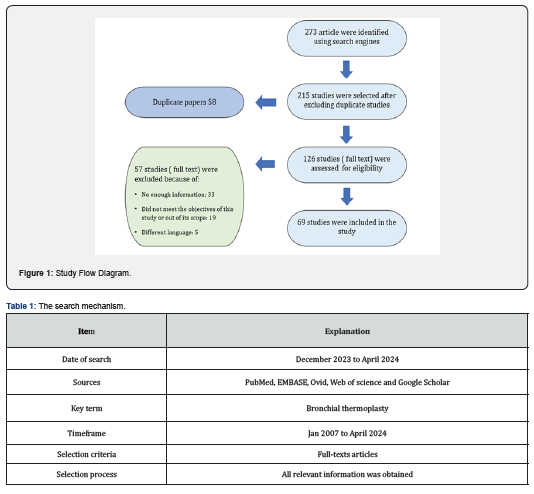
We did a comprehensive literature search using some of available search engines such as but not limited to PubMed, EMBASE, Ovid, Web of science and Google Scholar, using the term “bronchial thermoplasty”. Relevant articles were collected, and all relevant information was extracted and reviewed (Table 1). At first, more than 273 articles related to BT were identified, and after close observation we have selected 69 full text articles that fall within the scope of this study (i.e., studies that report the use of BT in adults and its consequences) (Figure 1).Outcomes of these studies including major clinical trials and full text articles were reviewed, analyzed, and compared to each other (Table 2). Important aspects related to the use of BT in clinical practice (the essential principles of BT procedure, indications and contraindications, safety and efficacy, side effects and complications and cost effectiveness were highlighted. Finaly, limitations of BT utilization and prospects were discussed in the view of these findings.
Results
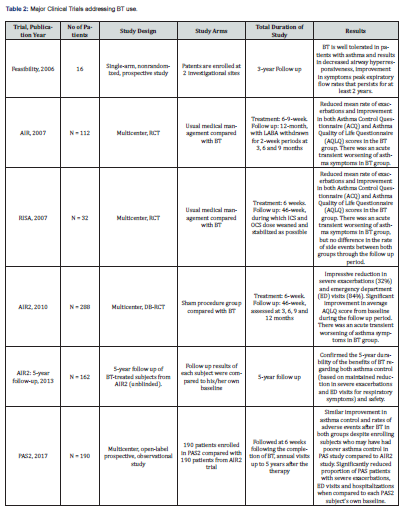
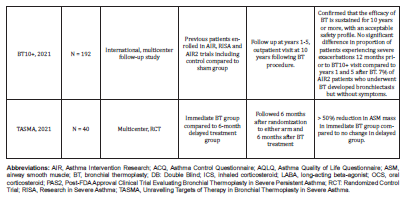
The results of the major clinical trials addressing the role and the effects of BT in severe asthma treatment were variable, however, they were encouraging and showed significant improvements in the studied variables in the BT arm compared to the control arm (Table 2). The Feasibility Study by Cox G et al. [9] was the first study published (2006) exploring the safety and clinical effects of BT use in asthma patients. This was a single-arm nonrandomized, prospective study in which 16 subjects with stable asthma were enrolled at two investigational sites between October 2000 and June 2002 and followed through October 2004. It concluded that BT is well tolerated in patients with asthma and results in decreased airway hyperresponsiveness, improvement in symptoms and peak expiratory flow rates that persists for at least 2 years. Limitations of this study include the lack of a control group, the small number of subjects studied, and the high number of patients lost to follow-up, especially in the untreated group, however, this study was designed to evaluate the feasibility and safety of BT, not its efficacy [9].
The AIR (Asthma Intervention Research) study is a multicenter RCT that was published in 2007. The participants (112 patients) with moderate to severe asthma were randomized to either BT or usual management care. The result showed reduced mean rate of mild exacerbations and improvement in both Asthma Control Questionnaire (ACQ) and Asthma Quality of Life Questionnaire (AQLQ) scores in the BT arm. However, there was a transient worsening of asthma symptoms soon after undergoing BT. In fact, there was no sham control and no blinding in this study thus the placebo effect is expected, and this represents a major limitation [10]. In the same year, another clinical trial the RISA (Research in Severe Asthma) with a similar design to the AIR study but with lesser number of participants (32 patients but with more severe asthma) was published and the results were like that in AIR study. However, the rate of adverse events was similar between treatment and control groups during the follow up period. Once again, this study has the same reasons of limitation as AIR study [11].
The AIR2 study which was published in 2010 has overtook the major limitation of the preceding two studies, it avoided the placebo effect using double-blinding and sham control. The control group underwent bronchoscopy but without performing BT. The number of participants was 228 patients. Treatment group had much fewer severe exacerbations (32%), emergency department (ED) visits (84%) and significant improvement in AQLQ scores. However, it was unclear whether these impressive gains would last beyond 1 year [7,12]. Later, Wechsler ME et al. [13] published a 5-year follow up results of 162 patients of the 190 BT-treated subjects from the AIR2 trial who completed 5 years of follow-up. There was a reduction in severe exacerbations and emergency department (ED) 44% and 78% respectively. This has confirmed the 5-year durability of the benefits of BT regarding both effectiveness and safety [13].
The PAS2 (Post-FDA Approval Clinical Trial Evaluating Bronchial Thermoplasty in Severe Persistent Asthma) study is a multicenter prospective observational study that compared outcomes in BT subjects from the PAS study (190 patients) with those (190 patients) from the AIR2 trial at the same interval post-BT (3 years of follow-up) [14]. Notably, The PAS2 subjects were older, more obese, took higher doses of inhaled corticosteroids and had experienced severe exacerbations in the 12 months prior to BT. By the end of the 3-year follow up period, there was a similar improvement in asthma control after BT in both groups despite enrolling subjects who may have had poorer asthma control in PAS study compared to AIR2 study. In addition, there were no differences noted in the rates of respiratory-related adverse events between both groups. However, the percentage of PAS2 subjects with severe exacerbations, emergency department visits and hospitalizations significantly decreased by 45%, 55% and 40%, respectively when compared to each PAS2 subject’s own baseline.
An extended follow-up of the PAS2 cohort demonstrated that these gains lasted for 5 years [15,16]. BT10+ study was an international, multicenter, follow-up study of participants who were previously enrolled in the AIR, RISA, and AIR2 trials and who had 10 or more years of follow-up since BT treatment. The results showed an Improvements in mean AQLQ and ACQ scores after BT that were sustained beyond 10 years. There was no significant difference in proportion of patients experiencing severe exacerbations 12 months prior to BT10+ visit compared to years 1 and 5 after BT. Some of AIR2 patients who underwent BT developed bronchiectasis (7%) but without symptoms. This study confirmed that the efficacy of bronchial thermoplasty is sustained for 10 years or more, with an acceptable safety profile [17].
The TASMA (Unravelling Targets of Therapy in Bronchial Thermoplasty in Severe Asthma) study assessed the effect of BT on ASM mass in patients with severe asthma. The study had no sham control but used blinding in the outcomes assessment. The results showed that the median ASM mass decreased by >50% in the immediate BT group versus no change in the delayed control group. Subsequently, the delayed control group also underwent BT and had similar improvements at 6 months. Treatment response was associated with serum IgE and eosinophil levels but not with ASM mass. The study has a major limitation in that there were no sham control and no long-term follow up plan [18]. A Cochrane systematic review of the BT trials (AIR, RISA, AIR2) by Torrego A et al. [19] concluded that there was a modest clinical benefit in asthma quality of life and a reduction in exacerbation rates 12 months after bronchial thermoplasty [19]. Many other observational studies and case series also reported the benefits of BT in treatment of severe asthma [4,20-22].
Discussion
Essential principles and effects of BT
BT is a non-drug option for the treatment of severe asthma. During BT procedure, thermal ablation energy is applied to the airway wall, which led to a reduction in ASM quantity, thereby relieving symptoms, decreasing exacerbations, and improving the quality of life of asthmatic patients [4,5,6,7,18]. It has been hypothesized that BT thermal ablation causes inter-structural alterations in the airways wall including changes in muscular, Epithelial, and neuronal layers [4,6,13,23] (Table 3). However, some studies mentioned that the effect of BT cannot be explained by these changes alone and the mechanism of action of BT may be not fully understood [18,24,25]. Notably, the downstream effects of ASM reduction may better correlate with clinical outcomes than an absolute reduction in ASM mass. This was illustrated in one study, as computer-based simulation was applied to an ex vivo human lung specimens to demonstrate improvement in airflow through distal small airways brought about by a 75% reduction in larger airway ASM following BT [26].
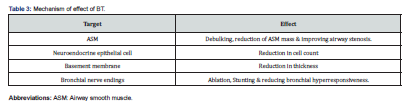
ASM plays an important role in airway narrowing and remodeling. With frequent exacerbations and undertreatment of severe asthma episodes, ASM starts to proliferate till hypertrophied. This ASM hypertrophy not only resulted in intense contraction and narrowing of airways but also contributed to angiogenesis and extracellular matrix formation, leading to further airway resistance [27]. Furthermore, in response to broncho constrictive stimuli, ASM contraction and secretion of inflammatory cytokines play an important role in airway hyperresponsiveness [28-31]. In addition, neuronal stimulation, and neuromodulation (nerve endings in ASM and epithelial layers) exaggerate the airway hyperresponsiveness process. In fact, these mechanical, cytological, and neuronal mechanisms offered a strong base to the use BT in the treatment of severe asthma [4,5,6,7,13,32].
BT procedure and technique
To ensure successful BT procedure, there are some requirements and preparations (Table 4). Screening bronchoscopy with airway examination is performed prior to the treatment and mucous should be suctioned to ensure adequate visualization and airway wall contact during the treatments. In addition, it is recommended to give OCS (50mg daily) for 3 days prior to the procedure, the day of the procedure, and 1 day following the procedure to minimize post-procedure airway inflammation. The experienced bronchoscopist sent the Alair flexible catheter through the bronchoscope’s working channel until showed in the airway, then open the expandable electrodes, which dilate to form a basket that become in physical contact with the airway wall (Figure 2). The RF thermal energy is applied via a footswitch pedal through the basket to the bronchial wall causing heating and ablation (65°C for 10 seconds) [33,34].
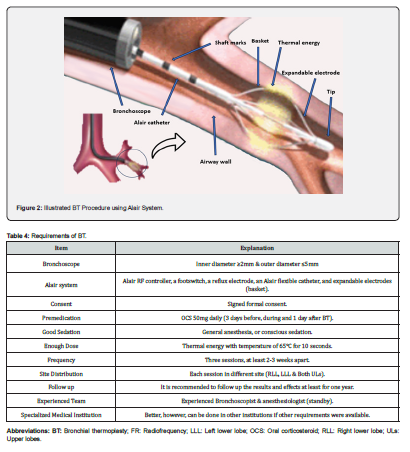
Each BT session takes 30-45 minutes and consists of a series of RF activations (30-70 activations) targeting each bronchus stepwise by 5mm sequence along its entire visible length, beginning distally, and moving proximally [35]. However, the duration and number of activations can vary depending on the experience level of the bronchoscopist and the patient’s airway anatomy. Treated areas should not be retreated, however, it is important to make sure that all assigned areas are treated as the effectiveness of the treatment may depend on how thoroughly the procedure is performed; if a segment is left untreated it may decrease the benefit of the treatment [36]. Transient blanching and whitening of the mucosa are expected after RF activations. It is recommended to do the BT procedure three times, each at least 2-3 weeks apart. This allows shorter procedure time and minimizes the risks associated with diffuse airway irritation. Each session should be done for different lobe including, the right lower lobe, the left lower lobe, and both upper lobes, no matter which one is the first [35,37].
However, one pilot randomized controlled clinical trial demonstrated an equivalent clinical efficacy at 1 year and more than 50% reduction in short-term adverse events when limiting treatment to a single BT session compared with the standard three-session BT [38]. The Right middle lobe should be left untreated in BT procedures because it has a relatively elongated and narrow bronchus, thus the risk of chronic injury, stenosis and subsequent collapse is high although some experts argued that applying BT to the right middle lobe bronchus, could also improve lung function and symptoms in patients with severe asthma without clearly impacting either overall safety or overall efficacy [39,40,41].
Indications and contraindications of BT
Generally, patients over 18 years old who have severe asthma that remain uncontrolled despite adequate treatment of high-dose ICS and LABA are candidates for BT (Table 5). Although, those who are at risk should not undergo BT, however, several recent observational studies have demonstrated largely comparable safety of BT among patients with severe asthma (FEV1<30%) with similar clinical improvements [42,43]. BT is meanly offered for patients with severe non-eosinophilic asthma and may be as a second-line option for patients with severe eosinophilic asthma who have failed to respond to biologic therapies. In a recent study, endobronchial biopsies performed post-BT showed that effect of BT varied considerably with the underlying asthma subtype with more reduction in ASM was among patients with eosinophilic asthma, and more epithelial cell proliferation was among patients with non-eosinophilic asthma [44]. Interestingly, all subgroups in the study demonstrated similar degrees of clinical improvement (p<0.001).
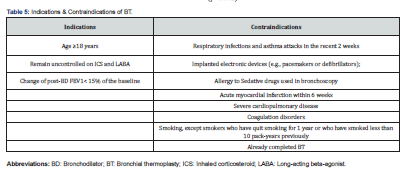
Other parameters that may predict better response to BT are shorter duration of asthma, higher baseline serum eosinophil counts, higher serum IgE levels, higher baseline OCS, atopic asthma, young age and more severe disease [45-49]. Contraindications of BT include patients who have respiratory infections and asthma attacks in the recent 2 weeks, implanted electronic devices (e.g., pacemakers or defibrillators), allergy to sedative drugs used in bronchoscopy, acute myocardial infarction within 6 weeks, severe cardiopulmonary disease, and coagulation disorders. Smokers also should be excluded, except smokers who have quit smoking for 1 year or who have smoked less than 10 pack-years [33,50,51]. As mentioned previously, peri-procedure systemic corticosteroid therapy, hospitalization for monitoring and/or bronchoscopy may be necessary. At the end of the treatment period, the clinical benefit should ideally be reassessed. Drug treatments should be adjusted to their lowest possible dosages if the asthma remains well controlled [52].
Effectiveness and safety of BT
The long-term effectiveness and safety of BT have been demonstrated by many studies in all aspects including clinical, functional, radiological, and pathological aspects. The reduction in ASM mass caused by BT lead to an increase in airways luminal dimension and volume and reduction in airways stenosis and air trapping which were proven pathologically and radiologically and this led to an improvement in the symptoms [6,53,54]. Unsurprisingly, functional residual capacity (FRC) and total lung capacity (TLC) measured using HRCT imaging were noted to be significantly increased following BT, as early as at 1 month and were sustained at 12 months [55]. Many Studies reported a significant improvement in the symptoms post-BT as assessed by the ACQ score and a significant improvement in the Mini Asthma Quality of Life Questionnaire (mini-AQLQ) and a reduction in drug use 1 year after BT treatment [7,10-12,55-58]. The long-term effectiveness and safety of BT were also verified in the follow up of patients which extend up to 5-10 years after BT [13-17,59].
Side effects and complications of BT
BT is not a completely safe procedure, some side effects and complications ranging from mild to severe were reported. Overall, the complications rate is 11.2% and mostly were mild and did not require invasive intervention and largely represent transient worsening of baseline symptoms [60]. The complications of BT include acute exacerbation of bronchial asthma, hypoxemia, chest pain, bleeding, pneumonia, and lobar collapse [7,60-63]. Rare complications include pneumothorax, plastic bronchitis, and bronchial artery pseudoaneurysm [64,65]. d'Hooghe et al. [66] reported some acute transient radiological complications after BT that have resolved with no long-term outcomes. These include peribronchial consolidations (94%), partial bronchial occlusions (63%), atelectasis (38%), and bronchial dilatations (19%) [66].
Cost effectiveness of BT
The BT is considered to be a high-cost procedure. The 9 initial increase in the cost during the first year of treatment which limits its utilization especially in the absence of insurance coverage. However, BT cumulative cost and cost per patient per year decreases within the subsequent years of treatment [67]. In one study, cost effectiveness of BT (with standard therapy) was evaluated and compared to standard therapy alone. The study reported cost effectiveness of BT to be US$5,459 per quality-adjusted life year [68]. Another study assessed the 10-year cost-effectiveness of BT for patients with severe uncontrolled asthma to be US$29,821 per quality-adjusted life year [69]. Despite of this, BT is more cost effective when compared to standard therapy especially when taking in the account the duration of treatment. BT cost was compared to the anti-IgE antibody therapy (omalizumab) and BT showed to be more cost effective [70]. In general, BT is a cost-effective option if peri-procedural costs are outweighed by costs related to hospitalization, ED visits and other standard therapies.
BT utilization between caution and prospects
Although the BT has been described for the first time for more than two decades but unfortunately still not widely utilized. The landmark pulmonology societies’ clinical guidelines regarding the implementation of BT were not convincing and the evidence assessments were divergent. Many pulmonologists have some concerns and hesitate to refer their patients to undergo BT. One of these concerns is that the exact mechanism of BT effect (beyond ASM mass reduction and neuronal pathway ablation) is not yet fully understood. Some patients did not show significant clinical improvement compared to the pathological improvement. In addition, the expected complications after BT procedure although have low incidence rate but still important to be taken in account. The availability of BT procedure and its other facilities are limited to some centers in most countries, and it is not covered by insurance companies in other countries.
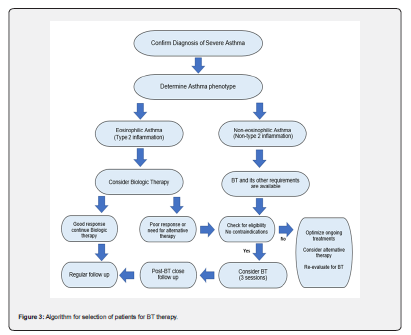
In fact, there is no clear consensus for which asthma subtypes should BT offered although it is currently offered for patients with severe non-eosinophilic asthma and as a second-line option for patients with severe eosinophilic asthma who have failed to respond to biologic therapies (Figure 3). There is controversy in some issues related to the dose and the site of the thermal energy given during BT. Some studies mentioned that the increase in the RF thermal activations is associated with more clinical and pathological improvements and not associated with severe side effects while other studies mentioned that the increase in RF activations has not led to significant clinical or pathological changes, on the contrary has led to more complications [6,71-73]. It is still unclear yet whether the right middle lobe of the lung can be treated with BT or not as mentioned previously. The absence of direct comparison of BT with novel biologic therapies mandate the performance of randomized trials entailing head-to-head comparison [74].
Indeed, the developments in the BT procedure technique and tools and the availability of other related facilities have led to better results and lesser side effects. In addition, the increase in the awareness among pulmonologists and bronchoscopist about the usefulness, safety and effectiveness of the BT will increase the referrals to BT. Many studies and experts’ opinions have recommended the utilization of BT when indicated and described it as an important treatment option that should not be neglected. I the view of this, we would expect that the future may carry more about the implementation and utilization of BT with better treatment strategies.
Conclusion
BT is an unutilized therapy of severe asthma and its effectiveness and safety have been documented in several well-designed multicenter trials. BT had led to an improvement in quality of life, reduction in exacerbations and hospitalizations in severe asthma patients. Although some adverse events and complications were reported but most of them are mild, transient and in the early stages. In the presence of indications and suitable requirements and the absence of contradictions, BT should be considered in carefully selected patients. Actually, selection of asthma patients for BT needs further studies to elucidate the hidden points in the mechanism of action of BT and which asthma subtype would benefit more from this kind of therapy.
Acknowledgment
The authors would like to thank the Department of Medicine and the staff of Pulmonology, Allergy, and Immunology division for their continued support.
References
- Khalfaoui L, Pabelick CM (2023) Airway smooth muscle in contractility and remodeling of asthma: potential drug target mechanisms. Expert Opin Ther Targets 27(1): 19-29.
- Panettieri RA Jr, Kotlikoff MI, Gerthoffer WT, Marc BH, Prescott GW, et al. (2008) Airway smooth muscle in bronchial tone, inflammation, and remodeling: basic knowledge to clinical relevance. Am J Respir Crit Care Med 177(3): 248-252.
- Chung KF, Wenzel SE, Brozek JL, Andrew B, Mario C, et al. (2014) International ERS/ATS guidelines on definition, evaluation, and treatment of severe asthma. Eur Respir J 43(2): 343-373.
- Chakir J, Haj-Salem I, Gras D, Philippe J, Sabrina B, et al. (2015) Effects of bronchial thermoplasty on airway smooth muscle and collagen deposition in asthma. Ann Am Thorac Soc 12(11): 1612-1618.
- Pretolani M, Dombret MC, Thabut G, Dominique K, Fatima H, et al. (2014) Reduction of airway smooth muscle mass by bronchial thermoplasty in patients with severe asthma. Am J Respir Crit Care Med 190(12): 1452-1454.
- Pretolani M, Bergqvist A, Thabut G, Marie-Christine D, Dominique K, et al. (2017) Effectiveness of bronchial thermoplasty in patients with severe refractory asthma: clinical and histopathologic correlations. J Allergy Clin Immunol 139(4): 1176-1185.
- Castro M, Rubin AS, Laviolette M, Jussara F, Marina DAL, et al. (2010) Effectiveness and safety of bronchial thermoplasty in the treatment of severe asthma: a multicenter, randomized, double-blind, sham-controlled clinical trial. Am J Respir Crit Care Med 181(2): 116-124.
- Mayse M, Laviolette M, Rubin AS, Noel L, Michael S, et al. (2007) Clinical pearls for bronchial thermoplasty. J Bronchology Interv Pulmonol 14(2): 115-123.
- Cox G, Miller JD, McWilliams A, J Mark F, Stephen L (2006) Bronchial thermoplasty for asthma. Am J Respir Crit Care Med 173(9): 965-969.
- Cox G, Thomson NC, Rubin AS, Robert MN, Paul AC, et al. (2007) Asthma control during the year after bronchial thermoplasty. N Engl J Med 356(13): 1327-1337.
- Pavord ID, Cox G, Thomson NC, Adalberto SR, Paul AC, et al. (2007) Safety and efficacy of bronchial thermoplasty in symptomatic, severe asthma. Am J Respir Crit Care Med 176(12): 1185-1191.
- Thomson NC (2019) Recent developments in bronchial thermoplasty for severe asthma. J Asthma Allergy 12: 375-387.
- Wechsler ME, Laviolette M, Rubin AS, Jussara F, Jose RLS, et al. (2013) BT: Long-term safety and effectiveness in patients with severe persistent asthma. J Allergy Clin Immunol 132(6): 1295-1302.
- Chupp G, Laviolette M, Cohn L, Charlene M, Sandeep B, et al. (2017) Long-term outcomes of bronchial thermoplasty in subjects with severe asthma: a comparison of 3-year follow-up results from two prospective multicenter studies. Eur Respir J 50(2): 1700017.
- Chupp G, Kline J, Khatri S, Charlene M, Adrian S, et al. (2020) Long-term efficacy and safety of BT: 5-year follow-up results from a large-scale prospective study. Chest 158(4): A12-A16.
- Chupp G, Kline JN, Khatri S, Charlene M, Genard AS, et al. (2022) Bronchial thermoplasty in patients with severe asthma at 5 years: the PAS2 study. Chest 161(3): 614-628.
- Chaudhuri R, Rubin A, Sumino K, Jose RLS, Robert N, et al. (2021) Safety and effectiveness of bronchial thermoplasty after 10 years in patients with persistent asthma (BT10+): a follow-up of three randomized controlled trials. Lancet Respir Med 9(5): 457-466.
- Goorsenberg AW, d’Hooghe JNS, Srikanthan K, Nick HTTH, EJM Weersink, et al. (2021) Bronchial thermoplasty induced airway smooth muscle reduction and clinical response in severe asthma. The TASMA randomized trial. Am J Respir Crit Care Med 203(2): 175-184.
- Torrego A, Solà I, Munoz A, Marta RIF, J Yepes-Nuñez J, et al. (2014) Bronchial thermoplasty for moderate or severe persistent asthma in adults. Cochrane Database Syst Rev 2014(3): CD009910.
- Bicknell S, Chaudhuri R, Lee N, Shepherd M, Spears M, et al. (2015) Effectiveness of Bronchial thermoplasty in severe asthma in real life patients compared with those recruited to clinical trials in the same center. Ther Adv Respir Dis 9(6): 267-271.
- Langton D, Sha J, Ing A, David F, Erica W (2017) Bronchial thermoplasty in severe asthma in Australia. Intern Med J 47(5): 536-541.
- Denner DR, Doeing DC, Hogarth DK, Karen D, Edward TN, et al. (2015) Airway inflammation after bronchial thermoplasty for severe asthma. Ann Am Thorac Soc 12(9): 1302-1309.
- Salem IH, Boulet LP, Biardel S, Noel L, Simon M, et al. (2016) Long-Term Effects of Bronchial Thermoplasty on Airway Smooth Muscle and Reticular Basement Membrane Thickness in Severe Asthma. Ann Am Thorac Soc 13(8): 1426-1428.
- Dyrda P, Tazzeo T, DoHarris L, Berndt N, Horia NR, et al. (2011) Acute response of airway muscle to extreme temperature includes disruption of actin-myosin interaction. Am J Respir Cell Mol Biol 44(2): 213-221.
- Thomson NC, Bicknell S, Chaudhuri R (2012) Bronchial thermoplasty for severe asthma. Curr Opin Allergy Clin Immunol 12(3): 241-248.
- Donovan GM, Elliot JG, Green FHY, Alan LJ, Peter BN (2018) Unraveling a clinical paradox: why does bronchial thermoplasty work in asthma? Am J Respir Cell Mol Biol 59(3): 355-362.
- James AL, Elliot JG, Jones RL, Mark LC, Thais M, et al. (2012) Airway smooth muscle hypertrophy and hyperplasia in asthma. Am J Respir Crit Care Med 185(10): 1058-1064.
- Nair P, Martin JG, Cockcroft DC, Myrna D, Catherine L, et al. (2017) Airway Hyperresponsiveness in Asthma: Measurement and Clinical Relevance. J Allergy Clin Immunol Pract 5(3): 649-659.
- Chang PJ, Bhavsar PK, Michaeloudes C, Nadia K, Kian FC (2012) Corticosteroid insensitivity of chemokine expression in airway smooth muscle of patients with severe asthma. J Allergy Clin Immunol 130(4): 877-885.
- Koziol-White CJ, Panettieri RA (2011) Airway smooth muscle and immunomodulation in acute exacerbations of airway disease. Immunol Rev 242(1): 178-185.
- Black JL, Panettieri RA, Banerjee A, Patrick B (2012) Airway smooth muscle in asthma: just a target for bronchodilation? Clin Chest Med 33(3): 543-558.
- Facciolongo N, Di Stefano A, Pietrini V, Galeone C, Bellanova F, et al. (2018) Nerve ablation after bronchial thermoplasty and sustained improvement in severe asthma. BMC Pulm Med 18(1): 29.
- Lin J, Nong Y, Yang D, Shiyue L, Guangfa W, et al. (2017) Chinese consensus statement on standard procedure and perioperative management of bronchial thermoplasty. J Thorac Dis 9(12): 5507-5514.
- Bonta PI, Chanez P, Annema JT, Pallav LS, Robert N (2018) Bronchial thermoplasty in severe asthma: best practice recommendations from an expert panel. Respiration 95(5): 289-300.
- Dombret MC, Alagha K, Boulet LP, Pierre YB, Guy J, et al. (2014) Bronchial thermoplasty: a new therapeutic option for the treatment of severe, uncontrolled asthma in adults. Eur Respir Rev 23(134): 510-518.
- Castro M, Musani AI, Mayse ML, Narinder SS (2010) Bronchial thermoplasty: A novel technique in the treatment of severe asthma. Ther Adv Respir Dis 4(2): 101-116.
- Cox G, Miller JD, McWilliams A, Marc FJ, Stephen L (2006) Bronchial thermoplasty for asthma. Am J Respir Crit Care Med 173(9): 965-969.
- Hall CS, Quirk JD, Goss CW, Daphne L, Kozloski J, et al. (2020) Single-session BT guided by 129Xe magnetic resonance imaging. A pilot randomized controlled clinical trial. Am J Respir Crit Care Med 202(4): 524-534.
- Eisenmann S, Schütte W, Funke F, Filiz O, Shaheen I, et al. (2019) Bronchial Thermoplasty Including the Middle Lobe Bronchus Significantly Improves Lung Function and Quality of Life in Patients Suffering from Severe Asthma. Lung 197(4): 493-499.
- O’Reilly A, Lane S (2018) What is the role of bronchial thermoplasty in the management of severe asthma? Ther Adv Respir Dis 12: 1753466618792410.
- Wiese T, Kondapaneni M (2013) The safety of treating the right middle lobe with bronchial thermoplasty. Eur Respir J 42(Suppl 57): P2299.
- Langton D, Ing A, Fielding D, Nichole H, Joy S, et al. (2020) Safety & effectiveness of bronchial thermoplasty when FEV1 is less than 50%. Chest 157(3): 509-515.
- Doeing DC, Mahajan AK, White SR, Edward TN, Jerry AK, et al. (2013) Safety and feasibility of bronchial thermoplasty in asthma patients with very severe fixed airflow obstruction: a case series. J Asthma 50(2): 215-218.
- Papakonstantinou E, Koletsa T, Zhou L, Lie F, Micheal R, et al. (2021) Bronchial thermoplasty in asthma: an exploratory histopathological evaluation in distinct asthma endotypes/phenotypes. Respir Res 22(1): 186.
- Ladjemi MZ, di Candia L, Heddebaut N, Camille T, Eloise A, et al. (2021) Clinical and histopathologic predictors of therapeutic response to bronchial thermoplasty in severe refractory asthma. J Allergy Clin Immunol 148(5): 1227-1235.
- Postigo M, Hall CS, Castro M (2020) Predicting the response to bronchial thermoplasty: the needier, the better. J Allergy Clin Immunol Pract 8(4): 1261-1262.
- Sarikonda K, Sheshadri A, Koch T, et al. (2014) Predictors of bronchial thermoplasty response in patients with severe refractory asthma, in B13. Mechanisms and treatment considerations for severe asthma. Am J Respir Crit Care Med 189: A2429.
- Ano S, Kikuchi N, Matsuyama M, Masayuki N, Yuzuru K, et al. (2022) Transcriptome genetic differences between responders and non-responders before bronchial thermoplasty. J Asthma 59(8): 1641-1651.
- Sarikonda K, Sheshadri A, Koch T, Kozlowski J, Bradley SW, et al. (2014) Predictors of bronchial thermoplasty response in patients with severe refractory asthma. Am J Respir Crit Care Med 189: A2429.
- Senquan Wu, Shaomei Li, Ping Zhang, Nianxin F, Chen Q, (2022) Recent advances in bronchial thermoplasty for severe asthma: a narrative review. Ann Transl Med 10(6): 370.
- Singh SK, Tiwari KK (2015) Bronchial Thermoplasty a non-pharmacological approach Clin Respir J 11(1): 13-20.
- Boulet LP, FitzGerald JM, Levy ML, Alvaro AC, Soren P, et al. (2012) A guide to the translation of the Global Initiative for Asthma (GINA) strategy into improved care. Eur Respir J 39(5): 1220-1229.
- Langton D, Sloan G, Banks C, Bennetts K, Plummer V, et al. (2019) Bronchial thermoplasty increases airway volume measured by functional respiratory imaging. Respir Res 20(1): 157.
- Konietzke P, Weinheimer O, Wielpütz MO, Willi LW, Philine K, et al. (2018) Quantitative CT detects changes in airway dimensions and air-trapping after BT for severe asthma. Eur J Radiol 107: 33-38.
- Langton D, Banks C, Noble PB, Virginia P, Francis T, et al. (2020) The effect of bronchial thermoplasty on airway volume measured 12 months post-procedure. ERJ Open Res 6(4): 00300-2020.
- Langton D, Bennetts K, Noble P, Virginia P, Francis T (2020) Bronchial thermoplasty reduces airway resistance. Respir Res 21(1): 76.
- Langton D, Ing A, Bennetts K, Wang W, Claude F, et al. (2018) Bronchial thermoplasty reduces gas trapping in severe asthma. BMC Pulm Med 18(1): 1-7.
- Seeley EJ, Alshelli I, Canfield J, Mendy L, Ganesh K (2019) The Impact of Bronchial Thermoplasty on Asthma-Related Quality of Life and Controller Medication Use. Respiration 98(2): 165-170.
- O'Reilly A, Browne I, Watchorn D, Egan JJ, Lane S (2018) The efficacy and safety of bronchial thermoplasty in severe persistent asthma on extended follow-up. QJM 111(3): 155-159.
- Burn J, Sims AJ, Keltie K, Hannah P, Sally AW, et al. (2017) Procedural and short-term safety of bronchial thermoplasty in clinical practice: evidence from a national registry and Hospital Episode Statistics. J Asthma 54(8): 872-879.
- Koshy K, Sha J, Bennetts K, David L (2021) Safety of delivering bronchial thermoplasty in two treatment sessions. Respir Res 22(1): 307.
- Madan K, Suri TM, Mittal S, Venkata NM, Pattabhiraman VR, et al. (2021) A multicenter study on the safety and efficacy of bronchial thermoplasty in adults with severe asthma. Lung India 38(6): 524-528.
- Vijayan K, Karakattu SM, Bansal A, Akesh T, Ahmad A, et al. (2022) Immediate complications, and flow volume changes during treatment phases of bronchial thermoplasty: a single-center descriptive study. J Asthma 59(7): 1433-1437.
- Barbarroja-Escudero J, Sánchez-González MJ, Ruíz-Peña A, Pérez-Labour RA, Rodríguez-Rodríguez M, et al. (2019) A Rare Case of Plastic Bronchitis Following BT. J Investig Allergol Clin Immunol 29(4): 331-332.
- Nguyen DV, Murin S (2016) Bronchial Artery Pseudoaneurysm with Major Hemorrhage After Bronchial Thermoplasty. Chest 149(4): e95-e97.
- d'Hooghe JNS, Van Den Berk IAH, Annema JT, Peter IB (2017) Acute Radiological Abnormalities after Bronchial Thermoplasty: A Prospective Cohort Trial. Respiration 94(3): 258-262.
- Menzella F, Zucchi L, Piro R, Carla G, Claudia C, et al. (2014) A budget impact analysis of bronchial thermoplasty for severe asthma in clinical practice. Adv Ther 31(7): 751-761.
- Cangelosi MJ, Ortendahl JD, Meckley LM, Tanya GKB, Ayanna MA, et al. (2015) Cost effectiveness of bronchial thermoplasty in commercially insured patients with poorly controlled, severe, persistent asthma. Expert Rev Pharmacoecon Outcomes Res 15(2): 357-364.
- Zein JG, Menegay MC, Singer ME, Serpil CE, Thomas RG, et al. (2016) Cost effectiveness of bronchial thermoplasty in patients with severe uncontrolled asthma. J Asthma 53(2): 194-200.
- Zafari Z, Sadatsafavi M, Marra CA, Wenjia C, Mark FitzGerald J (2016) Cost Effectiveness of Bronchial Thermoplasty, Omalizumab, and Standard Therapy for Moderate-to-Severe Allergic Asthma. PLoS One 11(1): e0146003.
- Langton D, Sha J, Ing A, David F, Francis T, et al. (2017) Bronchial thermoplasty: activations predict response. Respir Res 18(1): 134.
- Yamamoto S, Iikura M, Kakuwa T, Yoshie T, Sachi M, et al. (2019) Can the Number of Radiofrequency Activations Predict Serious Adverse Events after BT? A Retrospective Case-Control Study. Pulm Ther 5(2): 221-233.
- Wang T, Long F, Huang Z, Liang L, Wenting H, et al. (2022) Correlation of Activation Site and Number with the Clinical Response to Bronchial Thermoplasty. J Asthma Allergy 15: 437-452.
- Menzella F, Fontana M, Galeone C, Maria D, Giorgio WC, et al. (2021) A real-world evaluation of clinical outcomes of biologicals and bronchial thermoplasty for severe refractory asthma (BIOTERM). J Asthma Allergy 14: 1019-1031.






























