Advancements in Diagnosis, Follow-up, and Management of Solitary Pulmonary Nodules: A Comprehensive Review
Jubran Al Balushi1, Nadia Nishat2, José C Rosario-Curcio3, Carlos E. Díaz Ortiz3, Alba Vidal Ronchas3, Pubali Biswas4, Rakshita Bonu4, Rodrigo Sebastian Maldonado5, Fekadu Belay Ayalew6, Endalkachew Belayneh Melese7, Sanjana Patil and Felipe Velasquez8*
1University College Dublin, Ireland
2Mandya Institute of Medical Sciences, India
3University of Medicine and Health Sciences, St. Kitts
4Vydehi Institute of Medical Sciences and Research Centre, India
5Universidad Nacional de Córdoba, Argentina
6Johns Hopkins University Bloomberg School of Public Health, USA
7Department of Internal Medicine, College of Medicine and Health Science, University of Gondar, Ethiopia
8CES University, Colombia
Submission: March 22, 2024; Published: April 04, 2024
*Corresponding author: Felipe Velasquez, CES University, 154 Samson Rd, Frisco, TX, 76081, Colombia
How to cite this article: Jubran Al B, Nadia N, José C R-C, Carlos E. Díaz O, Alba Vidal R, et al. Advancements in Diagnosis, Follow-up, and Management of Solitary Pulmonary Nodules: A Comprehensive Review. Int J Pul & Res Sci. 2024; 7(2): 555708. DOI: 10.19080/IJOPRS.2024.07.555708
Abstract
Solitary pulmonary nodules are a frequent finding in chest imaging, presenting as a diagnostic challenge due to the possibility of both benign and malignant etiologies, requiring a complete guide for a more accurate, updated, and standardized guide for clinicians. This narrative review comprehensively explores SPNs, encompassing their definition, epidemiology, etiology and pathogenesis, imaging findings, follow-up strategies, and treatment options. SPNs are incidentally detected in 0.1-0.2% of chest X-rays and 13% of CT scans in the general population. However, the prevalence increases significantly (17-53%) among those with high-risk factors like smoking. Age and smoking history are the most significant risk factors, with men having a slightly higher incidence than women. A wide range of conditions can cause SPNs, including benign processes like granulomas, hamartomas, and inflammatory nodules and malignant etiologies such as primary lung cancer and metastatic disease. Infectious agents like aspergillus and tuberculosis can also manifest as SPNs. Additionally, autoimmune diseases and congenital malformations can be presented as SPNs. Chest X-ray can detect SPNs, but CT scans provide detailed anatomical information crucial for characterization. Imaging features like size, shape, margins, calcifications, and enhancement patterns are vital in differentiating benign from malignant nodules. PET scans may assess metabolic activity and aid in the evaluation of malignancy. Effective follow-up involves clinical assessment, risk stratification, and serial imaging (primarily CT scans). The frequency of follow-up imaging depends on the risk of malignancy and stability of the SPN. A multidisciplinary approach involving radiologists, pulmonologists, thoracic surgeons, and oncologists is crucial for optimal management. Management of SPNs depends on the underlying etiology. Benign nodules may require no intervention or surgical resection for symptomatic lesions. Malignant nodules typically require surgical resection, chemotherapy, radiation therapy, or a combination of these modalities. The role of PET scans and biopsy techniques in evaluating SPNs continues to evolve. Future advancements may provide more accurate and less invasive diagnostic tools. SPNs pose a diagnostic challenge due to their diverse etiology. A comprehensive evaluation is essential for accurate diagnosis and optimal patient management.
Keywords: Solitary lung nodules; Etiology; Imaging findings; Benign lung nodules; Malignant lung nodules; PET scan
Abbreviations: SPN: Solitary Pulmonary Nodule; CT: Computed Tomography; COPD: Chronic Obstructive Pulmonary Disease; PTEN: Phosphatase and Tensin Homolog; FDG-PET: Fluorodeoxyglucose Positron Emission Tomography; ACCP: American College of Chest Physicians; PET: Positron Emission Tomography; ROC: Receiver Operating Characteristic; MRI: Magnetic Resonance Imaging; VDT: Volume-Doubling Time; SLN: Solitary Lung Nodules
Introduction
Solitary pulmonary nodules (SPNs) are defined as discrete, well-circumscribed lesions within the lung parenchyma, measuring up to 3cm in diameter, surrounded by lung parenchyma, and not associated with lymphadenopathy or atelectasis [1]. These nodules are incidentally discovered in imaging studies, such as chest radiography or computed tomography (CT). They may present as asymptomatic findings or as part of an evaluation for respiratory symptoms [1,2]. The epidemiology of SPNs varies depending on population characteristics and imaging techniques, but they are commonly encountered in clinical practice, with an estimated prevalence of 8-51% in chest imaging studies [3]. SPNs are more frequently identified in older individuals and are associated with a higher prevalence among smokers. Additionally, the prevalence of malignancy among SPNs increases with age and smoking history [4].
The etiology and pathogenesis of SPNs are multifactorial and can include benign processes such as granulomas, hamartomas, and inflammatory nodules, as well as malignant etiologies such as primary lung cancer and metastases from extrapulmonary malignancies [1,4]. Risk factors for malignancy include older age, smoking history, size of the nodule, and imaging characteristics suggestive of malignancy [2,5]. Imaging findings of SPNs on CT include size, shape, margins, and internal characteristics such as calcifications, cavitation, and enhancement patterns. Benign features include well-defined margins, central calcifications, and fat attenuation, while malignant features include irregular margins, spiculated appearance, and pleural or vascular invasion [1,5]. The follow-up of SPNs involves risk stratification based on clinical and imaging characteristics to determine the need for further evaluation or surveillance.
Management options range from observation with serial imaging for stable, low-risk nodules to tissue sampling via biopsy or surgical resection for suspicious or high-risk nodules [4,6]. Treatment strategies for SPNs depend on the underlying etiology and the risk of malignancy. Benign nodules may require no intervention or surgical resection for symptomatic lesions, while malignant nodules typically require surgical resection, chemotherapy, radiation therapy, or a combination of these modalities [3]. This article aims to provide a comprehensive overview of solitary pulmonary nodules, including their definition, epidemiology, etiology and pathogenesis, imaging findings, benign and malignant features, follow-up strategies, and treatment options.
Epidemiology
Solitary pulmonary nodules (SPN) are found incidentally in 0.1% to 0.2% of chest x-rays and 13% on CT scans in the general population compared to those with a high risk for malignancy who have a higher incidental finding of SPN on 9% of chest X-rays and 33% on low dose CT scans. The estimated prevalence of SPN in the general population and those screened due to high risk for malignancy is between 2% to 24% and 17% to 53%, respectively. Some risk factors associated with increased incidence of SPN include current or past smoking history, chronic obstructive pulmonary disease, and old age [7]. A recent study found that the incidence of clinically relevant SPN increased in each decade of life [8]. Studies show that SPN is found more often in men than in women, although women have a higher incidence of SPN in nonsmokers [7]. A recent retrospective study showed an increased rate of SPN detection with CT scans. However, the rate of lung cancer diagnosis did not increase with the rise in the number of SPN detections [9]. The increased incidence of SPN is attributed to the enhanced sensitivity of CT scans.
Etiology
Lung opacities can be caused by a variety of conditions, including cancer, infection, congenital or even autoimmune conditions. Identifying the condition's etiology is vital to prescribing the appropriate treatment plan. Most solitary pulmonary nodules are benign and have characteristic features such as smaller size, smooth border, and central calcification, usually located in the lower lobe [10,11]. A classic example is Hamartomas, benign mesenchymal neoplasms that account for 15% of benign SPN and 8% of all lung tumors. They are abnormal, non-encapsulated normal tissue growths due to a developmental error or genetic mutation, the most studied being PTEN loss [12,13]. Other benign tumors that cause SPN include fibroma and lipoma [14]. Due to the accessible nature of the lungs, they are vulnerable to various infections, many of which present with solitary pulmonary nodules. Infections represent 15% of SPN cases, with the two most common infectious agents being aspergillus and tuberculosis [12]. Similar to other fungal infections, aspergillus presents as a consolidation with crescent or halo signs, which results from colonizing pre-existing cavities and forming an aspergilloma [11].
In contrast, tuberculosis presents with a characteristic cavitary lesion with a central necrotic nodule on CT. In addition, granulomatous infections, including fungal agents such as coccidioidomycosis and histoplasmosis, as well as non-granulomatous infections such as echinococcus, pose a substantial infectious burden also leading to the formation of SPN. Furthermore, it is worth noting that the scars remaining post-infection may display as SPNs indefinitely. Various autoimmune diseases present as SPN in the lungs, including granulomatosis, polyangiitis, and rheumatoid arthritis [11]. The most notable is sarcoidosis, an inflammatory disease mainly targeting the lungs with a characteristic honey-comb cyst presentation. granulomas form as a result of an unknown antigen triggering immune cells into forming clusters seen as SPNs on CT. Although the lungs are not the primary target, amyloidosis has been shown to involve the lungs and can also form SPNs. An autopsy study discovered lung involvement in 30% of patients with amyloidosis, and a post-mortem series by Browning et al. reports histological findings of pulmonary involvement in 90% of primary amyloidosis cases and 33% of secondary amyloidosis cases [15,16].
A small number of benign SPNs are incidental findings of congenital malformations. Bronchogenic cysts are rare foregut-derived cystic malformations in the lungs [17]. Another noteworthy idiopathic abnormality is pulmonary artery malformation, which presents with a mono-focal form and can be found in the lower lung lobe and the subpleural area [18]. Malignant presentations are of more significant concern and account for 2-23% of SPN [11]. Adenocarcinomas represent 47% of malignant SPN findings, and KRAS is the primary proto-oncogenic mutation that initiates its pathogenesis. Meanwhile, EML4-ALK tyrosine kinase mutation and upregulation of EGFR account for many non-small cell carcinomas, representing 7% of malignant SPNs. 22% of malignant SPNs are squamous cell carcinoma, in which P53 mutation is the major pathogenic cause. Environmental factors also play a significant role in lung cancer [19]. This is especially evident in small cell carcinomas being exclusive to those exposed to tobacco smoke and rarely found in non-smokers. Furthermore, the lung is one of the most affected organs in metastatic cancer, with an incidence rate of 20.46 in males and 15.95 in females, representing 8% of malignant SPN findings [12,19].
Imaging Findings
Solitary pulmonary nodules (SPNs) are focal, discrete, well-demarcated lesions within the lung parenchyma, measuring less than or equal to 3cm in diameter. Imaging findings of SPNs are crucial for characterization, differential diagnosis, and management decisions. Chest X-rays are the most commonly performed imaging modality to pick up a SPN. They can identify a large number of asymptomatic patients with an SPN [20]. A solitary lung nodule is a rounded opacity, well or poorly defined on a conventional radiograph, measuring up to 3cm in diameter [21]. SPNs are typically found incidentally on imaging studies unrelated to the respiratory system in 0.09-0.2% of all chest radiographs [22]. On chest X-ray, SPNs may appear as round or oval opacities with smooth or irregular margins. They can be located centrally or peripherally within the lung parenchyma. Calcifications, if present, may manifest as punctate, popcorn-like, or diffuse patterns within the nodule. Moreover, SPNs may exhibit signs of cavitation, which can indicate necrosis or infectious etiologies [23,24].
CT imaging provides detailed anatomical information and is essential for further characterization of SPNs. SPNs can display various attenuation patterns on CT, including solid, ground-glass, part-solid, or calcified components. A solid nodule appears homogeneously dense with well-defined margins, whereas a ground-glass nodule demonstrates hazy opacity without obscuration of underlying structures. Part-solid nodules exhibit both solid and ground-glass components, often suggestive of an indolent malignancy like adenocarcinoma in situ [25,26]. Additionally, calcified nodules reveal hyperdense foci within the lesion, indicative of dystrophic or psammomatous calcifications. Commonly, a chest CT scan is a modality that is most likely to pick up a solitary pulmonary nodule as it can detect changes in the size of 1 to 2mm, which is often an integral part of determining nodule etiology [20]. If the nodule is cystic or ground glass in appearance, then the MRI may be of more use [22]. Electromagnetic navigation bronchoscopy offers a noninvasive means of evaluation but is limited by its cost and is usually only used if other means are impossible or unsuccessful [23].
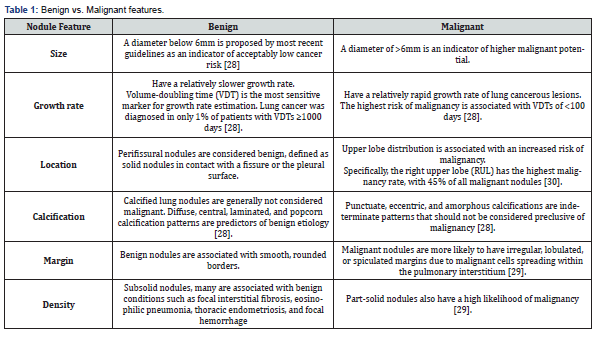
Regarding enhancement characteristics, SPNs can demonstrate heterogeneous enhancement patterns on contrast-enhanced CT scans. Peripheral or eccentric enhancement may suggest an inflammatory or granulomatous etiology, while homogeneous enhancement is more typical of vascular or benign neoplastic lesions. Furthermore, the presence of necrosis within the nodule may result in non-enhancing areas suggestive of aggressive or infectious processes [24,26]. Ancillary imaging modalities such as positron emission tomography (PET) scans can aid in evaluating SPNs by assessing metabolic activity. Malignant nodules often exhibit increased fluorodeoxyglucose (FDG) uptake, reflecting higher metabolic activity. Conversely, benign nodules typically demonstrate lower FDG uptake, although certain benign conditions, such as granulomas or hamartomas, may exhibit variable uptake patterns [25-27]. Malignant vs benign features are described in (Table 1) [28-30].
Imaging findings of solitary pulmonary nodules encompass a spectrum of morphological, attenuation, enhancement, and metabolic characteristics. A comprehensive evaluation of these features, clinical correlation, and follow-up imaging studies are essential for accurate diagnosis and management planning.
Several risk factors contribute to the likelihood of developing lung cancer. These include current or past history of tobacco smoking, with the risk escalating alongside both the quantity of tobacco consumed daily and the duration of active smoking. Additionally, aging is associated with a heightened probability of malignancy in individuals with lung nodules. Occupational exposure to carcinogenic agents, particularly when coupled with cigarette smoke, synergistically amplifies the risk of lung cancer. Those with a history of previous lung cancer are at an increased risk for a second primary lung cancer. Furthermore, comorbid chronic lung diseases, such as chronic obstructive pulmonary disease (COPD), have been strongly linked to lung cancer, indicating a correlation beyond the common underlying cause of smoking [28].
Follow-up Strategies
An effective follow-up strategy for solitary pulmonary nodules (SPNs) involves clinical assessment, risk stratification, and imaging surveillance. The management algorithm aims to differentiate benign from malignant nodules, determine the appropriate interval and modality for follow-up imaging, and integrate clinical data for optimal patient care [31,32]. Following the initial detection of an SPN on imaging, a comprehensive clinical evaluation is conducted, including patient history, risk factors for malignancy such as smoking history, occupational exposures, and physical examination findings. This initial assessment guides subsequent management decisions and establishes a baseline for follow-up monitoring [33]. The timing and intervals for follow-up imaging depend on the risk of malignancy and stability of the SPN. Low-risk nodules, typically defined as those <6mm in diameter and presenting in individuals with no significant risk factors, may undergo surveillance at longer intervals, such as 12 to 24 months. In contrast, higher-risk nodules, including those >8mm in diameter or exhibiting suspicious radiological features, require more frequent imaging surveillance, typically every 6 to 12 months [33,34]. Serial imaging studies, mainly CT scans, are essential for monitoring the progression of SPNs over time and assessing changes in size, morphology, and internal characteristics.
For stable nodules, interval imaging intervals may be extended, whereas suspicious or high-risk nodules may require more frequent surveillance. Serial CT scans allow for assessing nodule stability, growth rate, and evolution of radiological features over time [34]. Comparison of sequential images helps identify changes in nodule size, morphology, and internal characteristics, which can aid in risk stratification and decision-making regarding further intervention. Response assessment during follow-up imaging involves evaluating changes in nodule size and characteristics compared to baseline or prior scans. Stable or decreasing nodule size and absence of new suspicious features are considered benignity indicators. In contrast, progressive growth or development of concerning features may prompt further evaluation or intervention [34,35]. The imaging studies needed for follow-up primarily involve high-resolution CT scans due to their superior resolution and ability to characterize pulmonary nodules accurately. CT scans provide detailed information about nodule size, morphology, margins, presence of calcifications, and growth rate [33,34].
Additionally, positron emission tomography (PET) scans may be considered in select cases, particularly when evaluating for malignancy or assessing nodal and distant metastases. Integrating PET-CT imaging into the follow-up strategy can provide valuable metabolic information and aid in assessing nodule malignancy [32]. Follow-up imaging should be integrated with clinical data, including patient history, risk factors for malignancy, and symptoms. Clinical assessment may influence the frequency and timing of follow-up imaging, especially in patients with significant comorbidities or limited life expectancy [33,35]. It is crucial to emphasize the importance of a multidisciplinary approach to follow-up strategies for SPNs, involving collaboration among radiologists, pulmonologists, thoracic surgeons, and oncologists [36,37]. This interdisciplinary collaboration facilitates comprehensive evaluation, risk stratification, and decision-making regarding further management. Patient education and counseling regarding the rationale, risks, and benefits of follow-up imaging are also essential to the management strategy, ensuring patient understanding and adherence to the follow-up plan [32,35].
Role of PET Scan in Solitary Nodules
The use of 18F-fluorodeoxyglucose positron-emission tomography (18F-FDG PET) has been extensively evaluated in patients with an indeterminate solitary pulmonary nodule [38]. The American College of Chest Physicians (ACCP) recommends using thoracic CT scans as one of the main modalities and using PET as a conjunction in specific cases where the nodule is above 8mm and has a moderate pretest probability of malignancy. (Figure 1) [39]. Gould et al. [40] conducted a comprehensive meta-analysis comprising 40 studies to estimate the diagnostic accuracy of FDG-PET for malignant focal pulmonary lesions. Their investigation, encompassing 450 pulmonary nodules, revealed a sensitivity of 94.2% (95% CI: 89.1-97.0%) at a specificity point of 83.3% on the ROC curve. Their findings led them to conclude that 18F-FDG PET is a precise noninvasive imaging modality for diagnosing pulmonary nodules and larger mass lesions [41]. Kim et al. [41] conducted a retrospective study involving 42 patients, assessing the diagnostic performance of CT, PET, and PET/CT. Their findings revealed sensitivity and specificity values for CT, PET, and PET/CT: 93%/31%, 69%/85%, and 97%/85%, respectively. Significant disparities (P<0.05) were observed between PET/CT and PET regarding accuracy, sensitivity, and specificity. PET/CT emerged as exceptionally adept at distinguishing between benign and malignant solitary pulmonary nodules (SPNs). The integration of anatomical and metabolic imaging in PET/CT synergistically retained CT's sensitivity and PET's specificity, resulting in a notably enhanced overall accuracy. Notably, visual interpretation sufficed for SPN characterization, with quantitative analysis failing to enhance PET/CT accuracy [42].

In their research, Schrevens et al. [42] highlight PET's pivotal role in diagnosing and staging non-small cell lung cancer. PET stands out as an attractive staging tool due to its capacity to precisely delineate the primary tumor and local and distant metastases in a single noninvasive examination. Compared to conventional imaging methods, PET offers superior accuracy, thus potentially reshaping stage designation and therapeutic strategies. By providing more precise staging, PET minimizes the necessity for invasive procedures. Additionally, FDG-PET influences radiation therapy planning by optimizing target volume delineation, resulting in reduced radiation exposure to healthy tissues (Table 2) [38]. The American College of Chest Physicians (ACCP) recommends using thoracic PET scans as one of the main modalities in screening lung cancer in high-risk populations [43,44] (Figure 1). The ACCP also advises performing PET in conjunction with chest CT in some specific cases. PET is not indicated for Solitary Pulmonary Nodules of less than 8mm in diameter in the ACCP guidelines or less than 10mm in the French guidelines [39,40,43, 45]. This 8-10mm threshold was used to consider the spatial resolution of PET systems due to the high risk of false-negative findings for small lesions.

PET can be avoided in nonsolid nodules (ground glass opacity or mixed nodules) and replaced by thin-section CT of the lungs, which performs better in these circumstances [46,47]. Thus, one of the primary sources of false-negative (carcinoma in situ and other forms previously called “bronchioloalveolar carcinoma”) and false-positive findings associated with PET (inflammatory episodes or infection) is eliminated. Such nodules are monitored using CT [48,49]. PET is, therefore, mainly used as a complementary modality in the investigation of solid nodules ≥ 8mm. Nevertheless, PET should be included in a global strategy for characterizing solitary pulmonary nodules that consider their size, doubling time, morphology, density, and clinical likelihood of malignancy. When chest radiography or CT reveals a solid SPN, prior images of the same patient must be reviewed to determine whether the nodule was present earlier to assess its progression and doubling time [50]. If the nodule is found on previous images and has not grown in size over two 2-year, then it is probably benign. Thin-section chest CT also provides specific information on the morphology of the SPN [50,51].
Role of Biopsy in Solitary Nodules
In the world of an earlier diagnosis leading to a better prognosis, and with the high mortality of lung cancer, new advances are constantly worked on in order to help future patients. The role of biopsy in solitary lung nodules (SLN) depends on the risk category that the nodule is placed in, for which several risk calculator formulas have been created in order to determine how much of a risk a nodule has so that the patient gets the less invasive and more effective diagnostic tool. An important one is the Brock Risk Calculator, which has been reported to be more accurate than the Lung Imaging Reporting and Data System (Lung-RADS), as the latter underrepresented the risk for malignancy in two categories [52]. The Brock Risk Calculator can use several features to determine risk, which is not limited to just size, type, and location but also other factors such as age, sex, and family history. Some features that suggest malignancy in an SLN are size >10mm, irregular borders, nonsolid or ground glass density, noncalcified nodules, and doubling size within one month to a year. There are several guidelines regarding how to manage SLN. However, most agree with the past American College of Chest Physicians (ACCP) guidelines that indicate that SLN between 8 to 30mm with high pretest probability should get a direct biopsy to exclude malignancy, whereas low or moderate can be managed initially with imaging.
These guidelines indicate that nodules less than 8mm can be managed with imaging for surveillance with different imaging periods depending on their risk. Nodules determined low risk can also be biopsied if there is evidence of notable growth or a positive PDG-PET scan on follow-up [53,54]. Three main types of nodules are recommended for biopsy depending on their size: part-solid, nonsolid, and ground glass nodules, as all these are features of possible malignancy. If part-solid nodules are >5mm after 3 months of surveillance, they are recommended to get biopsied due to the risk of malignancy; if nonsolid nodules are >10mm after 3 months of surveillance are recommended to biopsy and ground glass nodules >5mm that persist for 3 years, a biopsy is recommended as well [55]. Lung biopsies can be done in different ways, including fine needle aspiration, core needle, transbronchial, thoracoscopic, open, and others. However, it has been found in some studies that CT-guided core needle biopsies have the highest most overall sensitive, specific, and accurate technique and also improve definitive diagnosis of benign lesions as compared to Fine Needle Aspiration from 52 to 91%, demonstrating it is an excellent technique for both ruling in and ruling out malignancy [56].
Conclusion
Solitary pulmonary nodules represent a diagnostic challenge in clinical practice, often requiring a comprehensive evaluation to differentiate between benign and malignant etiologies. Their incidental discovery in imaging studies underscores the importance of understanding their epidemiology, etiology, and imaging characteristics. Risk factors such as age, smoking history, and occupational exposures contribute to the likelihood of malignancy, necessitating careful consideration in clinical decision-making. Imaging modalities, particularly CT scans, are crucial in characterizing SPNs and guiding management strategies. Follow-up strategies based on risk stratification and clinical assessment are essential for monitoring SPNs over time and determining the need for further intervention. PET scans offer valuable metabolic information in select cases, aiding in diagnostic accuracy and staging of SPNs. Biopsy remains a cornerstone in the diagnostic approach to suspicious nodules, with advancements in techniques such as CT-guided core needle biopsy enhancing diagnostic yield and accuracy. Overall, a multidisciplinary approach involving collaboration among radiologists, pulmonologists, thoracic surgeons, and oncologists is essential for optimizing the management of SPNs and improving patient outcomes.
References
- Donington J, Lynch WR, Peter JM, David EM, David PN, et al. (2013) Evaluation of individuals with pulmonary nodules: when is it lung cancer? Diagnosis and management of lung cancer, (3rd edn): American College of Chest Physicians evidence-based clinical practice guidelines. Chest 143(5 Suppl): e93S-e120S.
- Naidich DP, Goo JM, Jin MG, Kyung SL, Ann NL, et al. (2017) Guidelines for management of incidental pulmonary nodules detected on CT images: from the Fleischner Society 2017. Radiology 284(1): 228-243.
- Ost DE, Gould MK (2012) Decision making in patients with pulmonary nodules. Am J Respir Crit Care Med 185(4): 363-372.
- Ruparel M, Quaife SL, Navani N, Wardle J, Janes SM, et al. (2016) Pulmonary nodules and CT screening: the past, present and future. Thorax 71(4): 367-375.
- William DT, Brambilla E, Nicholson AG, Yasushi Y, John HMA, et al. (2015) The 2015 World Health Organization Classification of Lung Tumors: Impact of Genetic, Clinical and Radiologic Advances Since the 2004 Classification. J Thorac Oncol 10(9): 1243-1260.
- Wahidi MM, Govert JA, Goudar RK, Michael KG, Douglas CM, et al. (2007) Evidence for the treatment of patients with pulmonary nodules: when is it lung cancer? ACCP evidence-based clinical practice guidelines (2nd edn). Chest 132(3 Suppl): 94S-107S.
- Wyker A, William WH (2022) Solitary pulmonary nodule. In: StatPearls [Internet].
- Kuckelman J, Dezube A, Jacobson F, Learn PA, Miller D, et al. (2023) Incidence of clinically relevant solitary pulmonary nodules utilizing a universal health care system. Mil Med 188(11-12): e3635-e3640.
- Loverdos K, Fotiadis A, Kontogianni C, Iliopoulou M, Gaga M (2019) Lung Nodules: A comprehensive review on current approach and Management. Ann Thorac Med 14(4): 226-238.
- Cruickshank A, Stieler G, Ameer F (2019) Evaluation of the solitary pulmonary nodule. Internal Med J 49(3): 306-315.
- Albert RH, Russell JJ (2009) Evaluation of the solitary pulmonary nodule. Am Fam Physician 80(8): 827-831.
- Ali SA, Mulita F (2023) Hamartoma. In: StatPearls [Internet].
- Dezube R (2023) Solitary Pulmonary Nodule.
- Smith RR, Hutchins GM, Moore GW, Humphrey RL (1979) Type and distribution of pulmonary parenchymal and vascular amyloid. Am J Med 66(1): 96-104.
- Browning MJ, Banks RA, Tribe CR. Hollingworth P, Kingswood C, et al. (1985) Ten Years’ experience of an amyloid clinic--A clinicopathological survey. QJ Med 54(215): 213-227.
- Limaiem F, Mlika M (2023) Bronchogenic Cyst. In: StatPearls [Internet].
- Cong CV, Luong DV, Anh TT, Minh NN, Ly TT, et al. (2022) Pulmonary arteriovenous malformation and inherent complications with solitary lung nodule biopsy-literature overview and Case report. Radiol Case Rep 17(7): 2353-2361.
- Yolanda S (2021) Lung Cancer Pathogenesis.
- Chen H, Stoltzfus KC, Lehrer EJ, Samantha RH, Shankar S, et al. (2021) The Epidemiology of Lung Metastases. Front Med (Lausanne) 8: 723396.
- Andrew W, Henderson WW (2022) Solitary Pulmonary Nodule. In: StatPearls [Internet].
- Khan AN, Al-Jahdali HH, Irion KL, Arabi M, Koteyar SS (2011) Solitary pulmonary nodule: A diagnostic algorithm in the light of current imaging technique. Avicenna J Med (2): 39-51.
- Stern JB, Vieira T, Perrot L, Lefevre M, Sayah MI, et al. (2019) The role of electromagnetic navigation bronchoscopy in the diagnosis of peripheral pulmonary lesions. Rev Mal Respir 36(8): 946-954.
- Simon M, Zukotynski K, Naeger DM (2018) Pulmonary nodules as incidental findings. CMAJ 190(6): E167.
- Lynch WR, Jessica D, William RL, Peter JM, David EM, et al. (2013) Evaluation of Individuals with Pulmonary Nodules: When Is It Lung Cancer? Diagnosis and Management of Lung Cancer, 3rd ed: American College of Chest Physicians Evidence-Based Clinical Practice Guidelines. Chest 143(5 Suppl): e93S-e120S.
- MacMahon H, Naidich DP, Goo JM, Kyung SL, Ann NL, et al. (2017) Guidelines for Management of Incidental Pulmonary Nodules Detected on CT Images: From the Fleischner Society 2017. Radiology 284(1): 228-243.
- David SE, Douglas EW, Dara LA, Wallace A, Jessica RB, et al. (2022) Non-Small Cell Lung Cancer, Version 6.2022, NCCN Clinical Practice Guidelines in Oncology. J Natl Compr Canc Netw 20(5): 497-530.
- (2023) American College of Radiology. ACR Appropriateness Criteria® Suspected Pulmonary Nodule.
- Loverdos K, Fotiadis A, Kontogianni C, Iliopoulou M, Gaga M (2019) Lung nodules: A comprehensive review on current approach and management. Ann Thorac Med 14(4): 226-238.
- Cruickshank A, Stieler G, Ameer F (2019) Evaluation of the solitary pulmonary nodule. Intern Med J 49(3): 306-315.
- Schmid-Bindert G, Vogel-Claussen J, Gütz S, Fink J, Hoffmann H, et al. (2022) Incidental Pulmonary Nodules - What Do We Know in 2022. Respiration 101(11): 1024-1034.
- McWilliams A, Martin CT, John RM, Heidi R, Geoffrey L, et al. (2013) Probability of cancer in pulmonary nodules detected on first screening CT. The New England J Med 369(10): 910-919.
- MacMahon H, Naidich DP, Goo JM, Kyung SL, Ann NL, et al. (2017) Guidelines for management of incidental pulmonary nodules detected on CT images: from the Fleischner Society 2017. Radiology 284(1): 228-243.
- Ost DE, Gould MK (2012) Decision making in patients with pulmonary nodules. Am J Respir Crit Care Med 185(4): 363-372.
- Gould MK, Donington J, Lynch WR, Peter JM, David EM, et al. (2013) Evaluation of individuals with pulmonary nodules: when is it lung cancer? Diagnosis and management of lung cancer, (3rd edn): American College of Chest Physicians evidence-based clinical practice guidelines. Chest 143(5 Suppl): e93S-e120S.
- Nair A, Klusmann MJ, Jogeesvaran KH, et al. (2017) Pulmonary nodules and CT screening: the past, present and future. Thorax 72(4): 367-375.
- (2023) National Comprehensive Cancer Network. NCCN Clinical Practice Guidelines in Oncology (NCCN Guidelines®) – Non-Small Cell Lung Cancer. Version 1.2023.
- Wahidi MM, Govert JA, Goudar RK, Michael KG, Douglas C, et al. (2007) Evidence for the treatment of patients with pulmonary nodules: when is it lung cancer? ACCP evidence-based clinical practice guidelines (2nd edn). Chest 132(3 Suppl): 94S-107S.
- Mosmann MP, Borba MA, de Macedo FP, Liguori AA, Villarim NA, et al. (2016) Solitary pulmonary nodule and (18)F-FDG PET/CT. Part 2: accuracy, cost-effectiveness, and current recommendations. Radiol Bras 49(2): 104-111.
- Groheux D, Quéré G, Blanc E, Lemarignier C, Vercellino L, et al. (2016) FDGPET-CT for solitary pulmonary nodule and lung cancer: Literature review. Diagn Interv Imaging 97(10):1003-1017.
- Gould MK, Maclean CC, Kuschner WG, Rydzak CE, Owens DK (2001) Accuracy of positron emission tomography for diagnosis of pulmonary nodules and mass lesions: a meta-analysis. JAMA 285(7): 914-924.
- Kim SK, Allen-Auerbach M, Goldin J, Fueger BJ, Dahlbom M, et al. (2007) Accuracy of PET/CT in characterization of solitary pulmonary lesions. J Nucl Med 48(2): 214-220.
- Schrevens L, Lorent N, Dooms C, Vansteenkiste J (2004) The Role of PET Scan in Diagnosis, Staging, and Management of Non-Small Cell Lung Cancer. Oncologist 9(6): 633-643.
- Andrew W, Henderson WW (2022) Solitary Pulmonary Nodule. StatPearls.
- Detterbeck FC, Mazzone PJ, Naidich DP, Bach PB (2013) Screening for lung cancer: diagnosis and management of lung cancer, (3rd edn): American College of Chest Physicians evidence-based clinica lpractice guidelines. Chest 143(5 Suppl): e78S-92S.
- Kikano GE, Picard FA, Robert S (2015) Evaluation of the Solitary Pulmonary Nodule. American family physician 92(12): 1084-1091.
- Patel VK, Naik SK, Naidich DP, Travis WD, Weingarten JA, et al. (2013) A practical algorithmic approach to the diagnosis and management of solitary pulmonary nodules: part 2: pretestprobability and algorithm. Chest 143(3): 840-846.
- (2011) Cancer du poumon, Bilan initial, collection Recommandations et référentiels. Boulogne-Billancourt: INCa.
- Naik SK, Naidich DP, Travis WD, Weingarten JA, Lazzaro R, et al. (2013) A practical algorithmic approach to the diagnosis and management of solitary pulmonary nodules: part 1: radiologic characteristics and imaging modalities. Chest 143(3): 825-839.
- Cronin P, Dwamena BA, Kelly AM, Carlos RC (2008) Solitary pulmonary nodules: meta-analytic comparison of cross-sectional imaging modalities for diagnosis of malignancy. Radiology 246(3): 772-782.
- Travis WD, Brambilla E, Riely GJ (2013) New pathologic classification of lung cancer: relevance for clinical practice and clinical trials. J Clin Oncol 31: 992-1001.
- Lederlin M, Revel MP, Khalil A, Ferretti G, Milleron B, et al. (2013) Management strategy of pulmonary nodule in 2013. Diagn Interv Imaging 94: 1081-1094.
- Hammer MM, Palazzo LL, Kong CY, Hunsaker AR (2019) Cancer Risk in Subsolid Nodules in the National Lung Screening Trial. Radiology 293(2): 441-448.
- Albert RH, Russell JJ (2009) Evaluation of the solitary pulmonary nodule. Am Fam Physician 80(8): 827-831.
- Nair A, Devaraj A, Callister MEJ, Davod RB (2018) The Fleischner Society 2017 and British Thoracic Society 2015 guidelines for managing pulmonary nodules: keep calm and carry on. Thorax 73: 806-812.
- Wyker A, Henderson WW (2022) Solitary Pulmonary Nodule. In: StatPearls [Internet].
- Winokur RS, Pua BB, Sullivan BW, Madoff DC (2013) Percutaneous lung biopsy: technique, efficacy, and complications. Semin Intervent Radiol 30(2): 121-127.






























