Outcome of Patients with Incidental Pulmonary Nodules in Veterans Population
Aria Hong2, Sanford B Church2*, Catherine S Sassoon1,2 and Bahman Saatian1,2
1Department of Medicine, Veterans Affairs Long Beach Healthcare System, USA
2Department of Medicine, University of California, USA
Submission: January 20, 2023; Published: February 07, 2023
*Corresponding author: Sanford Church, Department of Medicine, Division of Pulmonary and Critical Medicine, VA Long Beach Healthcare System, 5901 East 7th Street, Long Beach, CA 90822, USA
How to cite this article: Aria Hong, Sanford B Church, Catherine S Sassoon and Bahman Saatian. Outcome of Patients with Incidental Pulmonary Nodules in Veterans Population. Int J Pul & Res Sci. 2023; 6(3): 555687. DOI: 10.19080/IJOPRS.2023.06.555687
Abstract
Background: Lung cancer is the leading cause of cancer death. Chest computed tomography (CT) has resulted in increased detection of incidental pulmonary nodules requiring close monitoring. Fleischner Society guidelines have been applied in the management of those pulmonary nodules. However, it is unclear whether the guidelines are sufficient in the management of veterans with high lung cancer risks.
Objectives: To assess whether shorter interval of monitoring is required in veterans with incidental detection of pulmonary nodules. We hypothesize that shorter interval of monitoring is needed to detect progression of lung nodules in high-risk veterans than recommended by the Fleischner Society.
Method: In this retrospective study, all Chest CTs labeled Code 1-Tumor between 2012 and 2014 at single Veterans Affairs Medical Center were reviewed. A team of Pulmonary and Radiology physicians made decisions for a diagnostic procedure and/or follow up using the Fleischner Society guidelines. Clinical data were collected including reasons for imaging, tobacco use, premorbid conditions, type, and number of nodules, change in size, and duration of follow up.
Results: All nodules less than 8 mm were benign. At initial detection, the average size of malignant nodules was significantly greater than that of the benign nodules. The rate of lung cancer in veterans with incidental lung nodules was higher (7.7%) than in general population (5.5 %) with average follow up of 5.6 months.
Conclusion: The rate of malignancy of incidentally detected pulmonary nodules in veterans is relatively high, and the Fleischner Society guidelines are sufficient to monitoring those nodules.
Keywords: Lung cancer; Pulmonary nodule; Part-solid nodule; Ground glass nodule; Chest computed tomography
Abbreviations: CT: Computed Tomography; CXR: Compared to Chest x-ray; VA: Veterans Affairs; COPD: Chronic Obstructive Pulmonary Disease; PET: Positron Emission Tomography
Introduction
Lung cancer is the leading cause of cancer death in men and women [1]. Lung cancer causes more deaths every year in men than the next four most common cancers combined, including prostate, colorectum, pancreas, and liver cancer. Veterans have nearly double the rate of lung cancer compared to the general population [2]. The 5-year survival rate for all stages of lung cancer combined is only 19 % [3]. Lung cancers have poor prognosis because they are usually detected at an advanced stage since signs and symptoms do not develop until the tumor is large. With the increased use of low dose chest computed tomography (CT) for lung cancer screening in high-risk patients compared to chest x-ray (CXR), there has been a significant increase in incidental lung nodules detected [4]. One study showed that more than 4.8 million Americans underwent at least one CT scan, and 1.57 million nodules were identified in 2010 [5].
The Fleischner Society has published guidelines for the management of incidentally detected lung nodules in 2005 [6], subsequently updated for solid, subsolid, and multiple nodules in 2013 [7], and most recently, in 2017 for changes in nodule size and length of follow up [8]. These recommendations are the most frequently cited for management of small pulmonary nodules detected by Chest CT scan. Based on the size of the nodule and the relevant risk factors of the individual, Fleischner society makes recommendations for serial monitoring of pulmonary nodules with repeat Chest CT for up to 2 years for solid pulmonary nodules and 5 years for subsolid nodules [8].
However, the Fleischner Society made the recommendations based on studies performed on non-veterans population. Given the higher prevalence of smoking in US veterans compared to non-veterans leading to increased risk of lung cancer [9], the Fleischner Society recommendations may not be applicable to detect progression of lung nodules to malignancy in the veterans population. Shorter monitoring interval of incidental pulmonary nodules may be needed to detect lung cancer at an early stage when there is potential for cure. The purpose of this study is to assess the outcomes of incidental pulmonary nodules in a single Veterans Affairs (VA) Medical Center based on the Fleischner Society guidelines prior to the initiation of lung cancer screening at our institution.
Materials and Methods
In view of the reported low adherence to the Fleischner Society guidelines among radiologists and pulmonologists [10,11], the VA Long Beach Medical Center has an existing protocol in which all nodules or masses detected on Chest imaging are labeled Code-1 Tumor. Subsequently, a multidisciplinary team of Radiologist and Pulmonologist reviews the imaging and risk factors as described below. When the nodule is detected on a Chest radiograph and patient has high-risk factors, a thin-cut Chest CT is obtained. This retrospective study evaluated Chest CTs labeled Code 1-Tumor from 2012 to 2014. Using the Fleischner Society guidelines of 2005 [6] and 2013 [7], the team jointly arrived at a consensus decision for a diagnostic procedure and/or follow up. Inclusion criteria were patients over 35 years old with nodule size of >2mm to 30mm.
Patients were excluded if they had a known metastatic malignancy or active lung cancer. Demographic information including age, gender, smoking history, presence of chronic obstructive pulmonary disease (COPD), exposure to asbestos, family history of lung cancer, and personal distant history of malignancy or radiation to chest was collected. Non melanoma skin cancers were excluded. Clinical data were collected including reason for imaging, type of nodule (ground glass, solid, or part-solid), location and size of nodule (≥2-4mm, >4-6mm, >6-8mm, >8-30mm), change in size of the lung nodule, number of nodules, and duration of follow up. In addition, information on nodule management was recorded, including biopsy, resection, radiation therapy, and/or chemotherapy. The nodule was determined malignant if it was pathologically proven by biopsy or resection.
Statistical Analysis
Quantitative variables were expressed as means ± standard deviation. ANOVA and Chi-Square tests were used to determine statistical significance when performing comparisons (SPSS Software version 18). A P value of <0.05 was deemed statistically significant.
Result
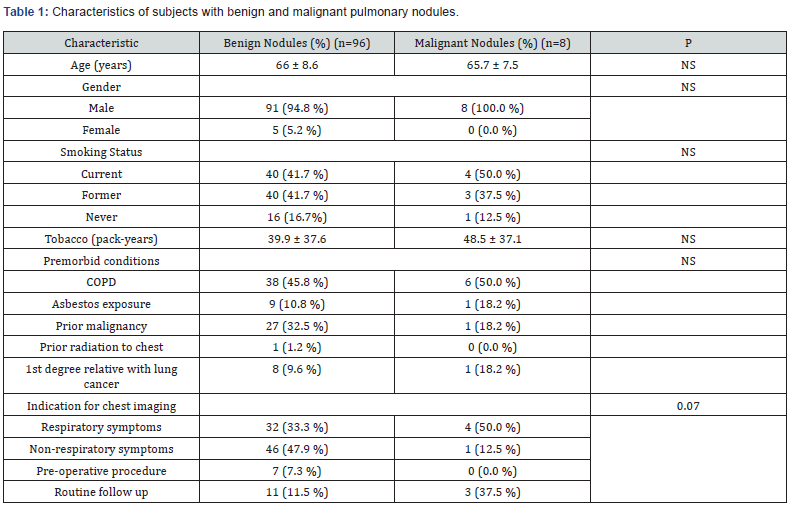
Values are mean ± SD for Age and Tobacco use. NS = not significant. P<0.05 is statistically significant. Number in parenthesis is percent of total number of subjects in each category, except for premorbid conditions, the denominator is the total of premorbid conditions as a subject may have none, one, or more premorbid conditions.
One hundred four subjects were included in the study. Subjects’ characteristics are outlined in (Table 1). The average age of our subjects was 66 (range 47 to 89) years, with the majority were males (99/104, 95%). Of the 104 patients, 96 patients (92.3%) had benign nodules, while 8 (7.7%) had a malignant lung nodule. Smoking status was similar in patients with benign compared with malignant nodules (41.7% vs. 50% current smokers, 41.7% vs. 37.5% former smokers, and 16.7% vs. 12.5% never smokers, respectively). The number of pack years of tobacco use was similar for those with benign (39.9 pack-years) and malignant (48.5 pack-years) nodules. There was no significant difference in premorbid conditions (risk factors) for benign compared with malignant nodules: COPD, asbestos exposure, prior distant history of malignancy, prior radiation to the chest, or first degree relative with lung cancer.
The prior malignancies were all extrapulmonary, except one patient had a history of lung cancer that was treated with surgical resection and chemotherapy 10 years prior to a new malignant lung nodule detection. Only one patient with a benign nodule had prior radiation to chest, while none in patients with malignant nodule. The most common indications for chest imaging that led to the detection of pulmonary nodules tended to be significantly different for malignant compared with benign nodules (P = 0.07, Table 1)). For benign nodules, nodule was most commonly detected after imaging was obtained for non-respiratory symptoms (47.9%), while for malignant nodules, the most common reason for obtaining imaging was for respiratory symptoms (50.0%).
Characteristics of the pulmonary nodules are listed in (Table 2). Of the 104 nodules detected, 88 (84.6%) were solid, 12(11.5%) were ground glass, and 4(3.8%) were part solid. Eight of the 88 solid nodules (9.1%), and none of the ground glass or part-solid nodules were malignant. Lung nodules ranged from 3 to 28mm in longest diameter. The distribution of nodule size was significantly different (P=0.001). All nodules of 8 mm or less (n=67) were benign. Of the 37 nodules greater than 8mm, 8 were malignant (21.6%) and 29 were benign (78.4%). The average size of malignant nodules (17.4mm ± 5.8mm (± SD)) was significantly greater than that of the benign nodules (8.4mm ± 5.4mm) (P<0.001). The total number of nodules detected varied. For the 96 patients with benign nodules, most patients (n=34 (35.4%)) had 1 nodule, followed by those with greater than 4 nodules (n=29 (30.2%)).
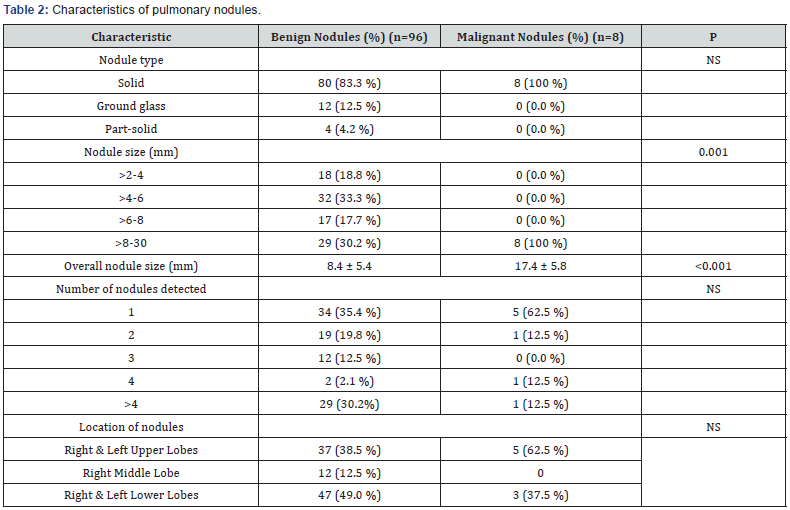
Values are mean ± SD for overall nodule size. NS = not significant. P<0.05 is statistically significant. Number in parenthesis is percent of total number of subjects in each category.
For patients with malignant nodules, 5 of 8 patients (62.5%) had solitary nodules, 1 patient had 2 nodules, 1 patient had 4 nodules, and 1 patient had more than 4 nodules. The distribution of benign or malignant nodules in the different lobes was not significantly different, although most common location of malignant nodules was in the upper lobes (62.5 %) than in the lower lobes (37.5%), and none was found in the right middle lobe (Table 2). Benign nodules were found mostly in the lower lobes (49%) than in the upper lobes (38.5%), or in the right middle lobe (12.5%). (Table 3) shows the average duration of follow up of various nodule types, and changes in size. The time to follow up tended to be significantly different with the shortest interval for solid nodules of 16.6 months; and an average of 5.6 (± 7.2 (± SD)) months for the 8 malignant solid nodules due to the prompt workup of suspicious nodules [6].
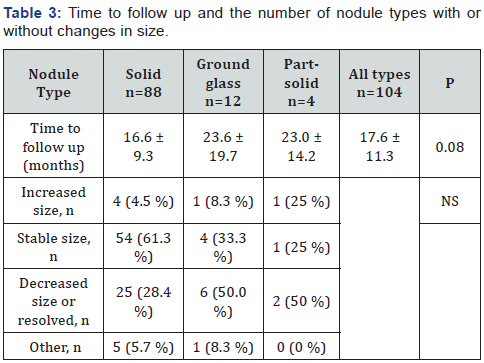
Values are mean ± SD. NS = not significant. P<0.05 is statistically significant. Number in parenthesis is percent of total nodule type in each category. Comparisons are made among solid, ground glass, and part-solid nodule types. Time to follow up follows Fleischner Society recommendations from the initial nodule detection to confirmed diagnosis, or when follow up was deemed no longer necessary. Other: change in size does not apply, since immediate workup was initiated, and nodule was not monitored over time.
Most solid nodules remained stable (61.3%), 4.5% increased in size, and 28.4% decreased in size or resolved. Assessment on change in size was impossible in 5.7% of nodules (4 solid malignant, 1 benign nodule) due to workup soon after detection. Ground glass and part-solid nodules had a longer average time to follow up of 23.6 and 23.0 months, respectively. The ground glass and part-solid nodules decreased in size or resolved more frequently than the solid nodules (50% for both ground glass and part-solid nodules). Only one ground glass nodule and one part-solid nodule increased in size, but both were benign. The remaining of the nodule types were stable in size. Regarding nodule evaluation and patients’ outcome, 14 of 96 patients (14.6%) with benign nodules and all the 8 patients with malignant nodules had a positive positron emission tomography (PET) scan.
7 out of 8 patients with a malignant nodule had avid fluorodeoxyglucose uptake greater than background activity. Only 3 of 96 patients (3.1%) with benign nodules, while 7 of 8 patients (87.5%) with malignant nodules had a lung biopsy. One patient with malignant nodule had lung resection which confirmed malignancy. None of the patients with benign nodules had surgery for evaluation of lung nodule. For the 8 patients with malignant nodules, the most common type of cancer detected was adenocarcinoma (n=4), followed by small cell carcinoma (n=2), and squamous cell carcinoma (n=1) (Table 4). One patient in the “other” category found to have a metastatic lesion from primary gastric adenocarcinoma that was not discovered until workup for the nodule was initiated. Of the 4 patients with adenocarcinoma, 3 patients underwent lobectomy; one patient was not a candidate for surgical resection and had stereotactic body radiation therapy.
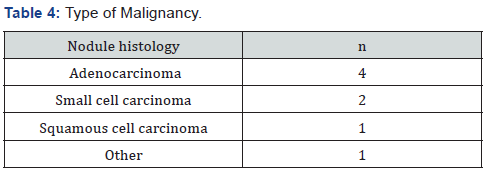
One patient with adenocarcinoma developed metastasis to the spine and received chemotherapy and radiation therapy. Both patients with small cell carcinoma were nonresectable; and were treated with chemo- and radiation therapy. One patient had wedge resection and was found at follow up to have squamous cell carcinoma, T3N0M0 (Stage IIb) and was treated with chemo- and radiation therapy. Of the 8 patients with malignant nodules, 6 patients had died upon chart review, and lung cancer was the cause of death in 4 of these patients or a mortality rate of 50% in 2 years.
Discussion
The primary findings in this study were the high rate of lung cancer incidence, the significantly large size of malignant compared with benign nodules at the initial detection of lung nodules on Chest CT. Applying the alert method of Code-1 Tumor, team approach, and adherence to the Fleischner Society guidelines, we have been able to obtain an expeditious lung cancer diagnosis in our veterans presented with incidental lung nodules. There are more than 1 million incidental lung nodules detected every year in the United States. A recent study reported a 5.2% lung cancer rate for nodules 4 to 30mm in size within 2 years of initial lung nodule detection [5]. In this study, we found a high rate of lung cancer in veterans with incidental lung nodules (7.7% over an average follow up period of 5.6 months). Our follow up followed the 2013 Fleischner Society guidelines (published online in 2012) at 3 months for nodule size greater than 8mm unless the team recommended aggressive work up with immediate biopsy or surgical resection at initial detection.
Another study which included the Pan-Canadian Early Detection of Lung Cancer Study (PanCan) database, a cohort of 2537 patients undergoing lung cancer screening, and the British Columbia Cancer Agency (BCCA) database, a cohort of 1090 patients undergoing lung cancer screening, demonstrated a 5.5% malignancy rate over a median follow up period of 3.1 years and a malignancy rate of 3.7% over a median follow up of 8.6 years, respectively [12]. The low rate of lung cancer over relatively prolonged period in both the Pan Can and BCCA cohorts was likely due to the lung screening nature of the study. This emphasizes the importance of lung cancer screening in veterans population. Like those studies, we found a high probability of lung cancer with nodule size of greater than 8mm. Due to the small sample size, we were unable to detect statistically significant differences in risk factors for malignancy, including smoking status, COPD, asbestos exposure, prior malignancy, and first degree relative with lung cancer. As in prior studies, malignant nodules were more likely located in the upper lobe. None of the ground glass or part solid nodules were malignant. The chart review was done 3-5 years after initial nodule detection and none of these ground glass or part solid nodules were diagnosed as malignant by that time.
Given the higher rate of lung cancer in the veterans compared to the general population, we wanted to evaluate whether the Fleischner Society guidelines are sufficient for follow up of incidental lung nodules in our veterans. No nodules of 8 mm or less were found to be malignant. In fact, the smallest malignant nodule was 13mm at initial detection. In view of the size, a follow up at and every 3 months with aggressive diagnostic work up was performed unless contraindications exist. Given our findings, the Fleischner Society guidelines are adequate for monitoring of incidental pulmonary nodules in the veterans population. In view of the high incidence of lung cancer [13] and the high mortality rate within less than 5 years in our study population, lung cancer screening in veterans with proper follow up is of paramount importance in veterans. Discouragingly, recent report in lung cancer screening demonstrated less than optimal adherence on subsequent follow up of Chest CTs, 82% and 65% at first and second follow up imaging, respectively [14].
Only a small minority of patients with benign nodules required further evaluation with PET scan or biopsy. Most lung cancers diagnosed were adenocarcinoma. The other lung cancers, including squamous cell and small cell, were advanced by the time of diagnosis, and required multimodality treatment. This study is limited as it is a single center, retrospective study with a small sample size. Due to the retrospective nature of the study, some variables that were utilized for risk-stratifying patients were not recorded, such as spiculation of nodules or exposure history such as to radon. Furthermore, definitive documentation of certain history such as asbestos exposure or radiation to the chest were not always available that limits the accuracy of the information. In conclusion, our study demonstrates that veterans had a relatively high rate of lung cancer of incidental pulmonary nodules, all of which were greater than 8 mm, and were diagnosed soon after presentation or after a relatively short interval of follow up. Based on our small study, the Fleischner society guidelines are sufficient to monitor incidentally detected lung nodules in the veterans population.
Acknowledgement
We thank Mr. Mehrtash Hashemzadeh for assistance with statistical analysis.
References
- Siegel RL, Miller KD, Jemal A (2020) Cancer statistics, 2020. CA Cancer J Clin 70(1): 7-30.
- Harris RE, Hebert JR, Wynder EL (1989) Cancer risk in male veterans utilizing the veterans administration medical system. Cancer 64(5): 1160-1168.
- (2020) Cancer Facts & Figures 2020. Atlanta: American Cancer Society.
- National Lung Screening Trial Research Team, Aberle DR, Adams AM, Berg CD, Black WC, et al. (2011) Reduced Lung-Cancer Mortality with Low-Dose Computed Tomographic Screening. N Engl J Med 365(5): 395-409.
- Gould MK, Tang T, Liu IA, Lee J, Zheng C, et al. (2015) Recent trends in the identification of incidental pulmonary nodules. Am J Respir Crit Care Med 192(10): 1208-1214.
- MacMahon H, Austin JH, Gamsu G, Herold CJ, Jett JR, et al. (2005) Guidelines for management of small pulmonary nodules detected on CT scans: a statement from the Fleischner Society. Radiology 237(2): 395-400.
- Naidich DP, Bankier AA, MacMahon H, Schaefer-Prokop CM, Pistolesi M, et al. (2013) Recommendations for the management of subsolid pulmonary nodules detected at CT: a statement from the Fleischner Society. Radiology 266(1): 304-317.
- MacMahon H, Naidich DP, Goo JM, Lee KS, Leung ANC, et al. (2017) Guidelines for management of incidental pulmonary nodules detected on CT images: from the Fleischner Society 2017. Radiology 284(1): 228-243.
- Brown DW (2010) Smoking prevalence among US veterans. J Gen Intern Med 25(2): 147-149.
- Eisenberg RL, Bankier AA, Boiselle PM (2010) Compliance with Fleischner Society Guidelines for management of small lung nodules: a survey of 834 radiologists. Radiology 255(1): 218-224.
- Bueno J, Landeras L, Chung JH (2018) Updated Fleischner Society Guidelines for Managing Incidental Pulmonary Nodules: Common Questions and Challenging Scenarios. Radiographics 38(5): 1337-1350.
- McWilliams A, Tammemagi MC, Mayo JR, Roberts H, Liu G, et al. (2013) Probability of Cancer in Pulmonary Nodules Detected on First Screening CT. N Engl J Med 369(10): 910-919.
- Kinsinger LS, Anderson C, Kim J, Larson M, Chan SH, et al. (2017) Implementation of lung cancer screening in the veterans health population. JAMA Intern Med 177(3): 399-406.
- Tanner NT, Brasher PB, Wojciechowski B, Ward R, Slatore C, (2020) et al. Screening Adherence in the Veterans Administration Lung Cancer Screening Demonstration Project. Chest 158(4): 1742-1752.






























