Imaging Findings in Pulmonary Fat Embolism: Literature Review and Practical Aspects
Felipe Velasquez-Botero1*, Radhika Bassi2, Flor Andrea Alcocer-Rondon3, Jessica Mariela Amaya-Alvarez4, Coralvia Yaroslangna Villanueva-Perez5, Aditi Gupta6, Ishwar Basavan6, Mariangel Teresa Gonzalez-Hernandez7, Marlon Reyes8, Laura Sofia Triviño9, Tesfahunegn Hailemariam10, Jhon Navarro-Gonzalez11 and Adriana Carolina Toro-Velandia9
1CES University, Colombia. Larkin Community Hospital, USA
2Ross University School of Medicine, Barbados
3Universidad de Oriente, Venezuela
4Universidad Salvadorena Alberto Masferrer, El Salvador. Larkin Community Hospital, USA
5Universidad Nacional Experimental Francisco de Miranda, Venezuela
6Jawaharlal Nehru Medical College, India
7Universidad de Carabobo, Venezuela
8American University of Antigua College of Medicine, Antigua and Barbuda
9Juan N Corpas University, Colombia. Larkin Community Hospital, USA
10Addis Ababa University College of Health Sciences. Center for Innovative Drug Development and Therapeutic Trials for Africa (CDT-AFRICA), Ethiopia
11Universidad Del Zulia, Venezuela
Submission: December 22, 2022; Published: January 04, 2023
*Corresponding author: Felipe Velasquez-Botero, MD. CES University, Larkin Community Hospital, Miami, Florida, USA
How to cite this article: Felipe V-B, Radhika B, Flor Andrea A-R, Jessica M A-A, Coralvia Yaroslangna V-P. Imaging Findings in Pulmonary Fat Embolism: Literature Review and Practical Aspects. Int J Pul & Res Sci. 2023; 6(3): 555686. DOI: 10.19080/IJOPRS.2023.06.555686
Abstract
Fat embolism syndrome is an uncommon but potentially fatal condition associated with trauma or long bone surgery, which presents predominantly with pulmonary symptoms. While medical advances have resulted in a reduction in mortality rates, the accurate diagnosis of the condition remains challenging due to its ability to mimic other causes of respiratory distress. Since the symptoms, laboratory tests, and imaging studies are often nonspecific, the identification of fat embolism must be based on a combination of these elements. The use of pulmonary imaging techniques, particularly chest computed tomography, is crucial to the assessment of this condition. When hypoxia occurs after surgery or trauma, the presence of diffuse and well-defined ground glass opacities or centrilobular nodules on CT are highly suggestive of fat embolism. As this disorder is mainly managed via supportive measures, prevention and early identification are essential to improving patient outcomes. This review describes the main clinical and imaging aspects of pulmonary fat embolism.
Keywords: Fat embolism; Pulmonary fat embolism; High resolution computed tomography; Chest x-ray; Thoracic CT scan
Abbreviations: FE: Fat Embolism; FES: Fat Embolism Syndrome; ARDS: Adult Respiratory Distress Syndrome; ESR: Erythrocyte Sedimentation Rate; ABG: Arterial Blood Gas; EKG: Electrocardiogram; CT: Computed Tomography; BAL: Broncho Alveolar Lavage; TEE: Trans-Esophageal Echocardiography; MRI: Magnetic Resonance Imaging; DWI: Diffusion-Weighted Image; HRCT: High-Resolution Computed Tomography
Introduction
Fat embolism (FE) is defined as the presence of fat globules in the peripheral or pulmonary vasculature, which can trigger the development of a multi-organic insult, also known as fat embolism syndrome (FES) [1]. This condition usually occurs when tissues and intramedullary fat are manipulated, particularly after trauma or orthopedic procedures involving long bone or pelvic fractures. However, it has been described that, in rare cases, this condition can also occur after certain procedures and in non-trauma patients (5% of all cases of FES). The pathophysiology of FE is not entirely understood, but it is assumed to be primarily caused by a vascular-obstruction-induced inflammatory response [2]. Studies have shown that fat globules in the pulmonary circulation may be present in up to 82% of patients after blunt trauma [3]. However, the actual prevalence of FES is much lower, ranging between 0.05-3% (based on trauma severity, affected bone, associated comorbidities, and age) [2]. The incidence is more significant in femoral fractures (4.8%-7.5%) and after intramedullary nail fixation (11%).
In most cases, FE occurs in males between the ages of 20 and 40 and within 48 hours after a lower extremity fracture (i.e., delayed intra-medullary nailing). Clinical manifestations include a classic triad characterized by pulmonary distress, neurologic symptoms, and petechial rash. However, it is important to note that the triad is present in less than 30-50% of cases, and many patients might show a broad spectrum of clinical findings [1,2]. Most patients present with respiratory symptoms (up to 95%). However, due to the lack of precise diagnostic laboratory tests, these are difficult to differentiate from other more frequent etiologies of respiratory distress after trauma or surgery (i.e., pneumonia, atelectasis, aspiration pneumonitis). Therefore, an accurate diagnosis of this condition can be significantly challenging and must be based on a combination of signs/symptoms, laboratory results, and imaging findings.
Pulmonary imaging studies are of vital importance in the assessment of this disease since they can provide crucial information for an early diagnosis and prompt treatment. Chest imaging (particularly thoracic computed tomography) is the standard imaging modality for assessing pulmonary conditions, playing a paramount role in diagnosing FES and alternative differentials. This article aims to provide a comprehensive overview of pulmonary fat embolism syndrome, focusing on the role of imaging studies and their relevance for promptly recognizing this rare but potentially fatal condition.
Etiology and Pathogenesis
There are two possible origins of fat embolism syndrome: traumatic and non-traumatic. Non-traumatic FES has been described during the presence of acute illness (i.e., pancreatitis, sickle cell disease, osteomyelitis, diabetes mellitus, fatty liver disease) or after iatrogenic intervention (i.e., transplant, cesarean section, liposuction, bone marrow harvest) [4]. However, FES is most commonly associated with significant traumatic injuries, particularly long bone, and pelvic fractures. In 95% of cases, long bone fractures and orthopedic surgery disrupt intramedullary fat, but only a minority of patients develop clinical symptoms. It is important to note that the term fat embolism refers to the presence of fat droplets in the circulatory system, which can be detected in the blood and urine of almost all patients with fractures. On the other hand, FES is a clinical diagnosis requiring the presence of major and minor criteria. Several risk factors are related to the development of fat embolism syndrome, including young age, closed fractures, multiple fractures, and prolonged conservative management of long bone fractures [5].
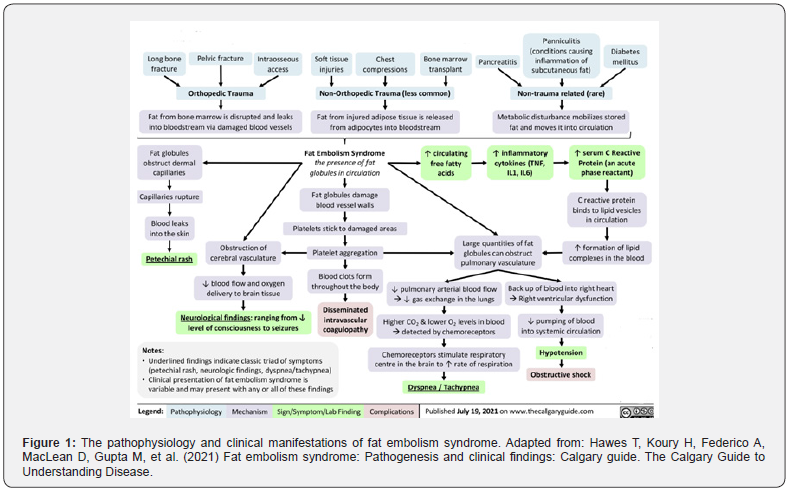
Several attempts have been made to comprehend the pathological process of FES, particularly why some individuals undergo severe respiratory distress and others do not. Although the physiopathology still needs to be fully understood, it can be rationalized by two main hypotheses: mechanical and biochemical [6]. The mechanical theory, postulated by Gassling et al. [7] describes that large fat droplets are released from the tissues into the venous system after a specific vascular insult (trauma, surgery), leading to vascular obstruction and subsequent organ damage and inflammation [7]. On the other hand, the biochemical theory provides an alternate explanation for fat embolization. This hypothesis proposes that the inflammatory response to trauma causes the release of free fatty acids from the bone marrow into the venous system [6]. A flow chart illustrating the pathophysiology and clinical manifestations of FES is presented in (Figure 1).
Clinical Manifestations and Diagnosis
Fat embolism syndrome is characterized by pulmonary insufficiency, neurologic symptoms, and hematologic disturbance [8]. It is reported that FES often presents insidiously 24 to 72 hours after an injury, with a median presentation time of 48.5 hours following a long bone fracture [9]. The most common clinical finding is hypoxia (90% of cases), followed by changes in mental status and petechiae. However, the classically described symptomatic triad is not seen in all patients, producing a clinical spectrum that can vary from an asymptomatic process to respiratory failure. Also, many other signs and symptoms may arise depending on the organ affected (i.e., fever, renal/retinal changes, jaundice) [10].
Among the most significant characteristics of FES is its potential to cause severe respiratory effects, such as adult respiratory distress syndrome (ARDS) and respiratory failure (seen in up to 10% of cases). Neurologic symptoms are present in 60% of patients and include irritability, confusion, convulsions, delirium, and coma [11]. Petechiae typically appear on the trunk, face, axillary folds, and conjunctiva (present in up to 50% of patients). It should be noted that the later clinical findings constitute the major diagnostic criteria for FES, while symptoms such as tachycardia or fever are considered minor diagnostic criteria [12]. The diagnostic criteria for FES are presented in (Table 1).
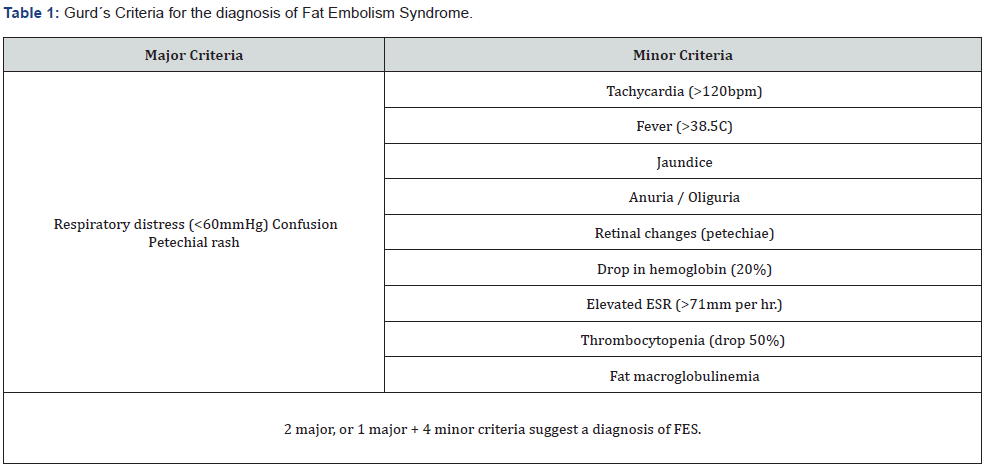
*Adapted from: (2019) JAAOS - Journal of the American Academy of Orthopaedic Surgeons 27(8): e346-e355.
Considering its broad range of clinical manifestations and the absence of a gold standard exam, fat embolism can be challenging to diagnose. The approach to these patients should therefore include laboratory tests and imaging studies. Routine blood analyses will show a rise in erythrocyte sedimentation rate (ESR) and a fall in hemoglobin, hematocrit, and platelets. Moreover, an arterial blood gas (ABG) analysis might reveal hypoxia, hypocarbia, and respiratory alkalosis. It has been demonstrated that hypoxemic patients are at significantly higher risk of developing FES and its related complications [13]. Electrocardiogram (EKG) findings are usually non-specific and might include tachycardia, ST-segment alterations, T wave changes, and right axis deviation. In the context of neurological impairment, brain imaging has proven beneficial in certain situations. Brain CT may be normal or exhibit minimal brain edema or diffuse white matter petechial hemorrhages.
According to several studies, magnetic resonance imaging (MR) has the highest sensitivity for diagnosing cerebral FE [14].
Therefore, it should be performed in every patient presenting with acute deterioration in mental status after orthopedic surgery or trauma in the presence of a normal CT scan. T2-weighted brain MRI images commonly reveal multiple small, scattered, non-confluent, hyperintense lesions involving grey and white matter at an early stage of the disease; revealing even small cerebral infarctions not detected by other imaging methods [11]. It has been suggested that the number and size of the lesions correlate directly with the degree of neurological disability. An MRI may detect these lesions as early as four hours after the onset of symptoms [15]. Diffusion-weighted images (DWI) show tiny, point-like hyperintense foci in a dark background, where they follow a watershed distribution (resulting in an appearance of a “starfield”). Interestingly, these lesions can be detected on DWI as early as one hour after the onset of FES symptoms.
Pulmonary imaging studies for FES include chest plain films and thoracic/lung CT scans. Radiological abnormalities develop gradually and commonly exhibit diffuse “fluffy” bilateral infiltrates (snowstorm appearance), predominantly in the lung bases and the periphery [16]. The use of thoracic and lung CT scans may be particularly helpful in patients with symptoms compatible with FES and relatively normal chest X-rays (i.e., small lung infarcts/lesions). Other diagnostic modalities might include bronchoalveolar lavage (BAL) and transesophageal echocardiography (TEE) [12]. However, the diagnostic performance of these tools has yet to be validated since the presence of fat globules within pulmonary macrophages, or systemic circulation is non-specific and can be present in other clinical settings (i.e., multi-organ failure, sepsis, nutrition) [17].
In view of the above, the proper diagnosis of FES must rely on a correct assessment and interpretation of patient clinical findings, laboratory results, and imaging studies.
Pulmonary Imaging Studies
Chest X-Ray
The use of imaging studies is crucial in diagnosing pulmonary FE. Commonly, the initial radiographic tool in the context of respiratory distress includes a chest x-ray, which principal function is to exclude other conditions that clinically mimic fatty pulmonary embolism. In most cases, chest x-ray findings are normal during the early stages of the disease [18]. However, as pulmonary FE progresses, both lungs can be observed with an increased pulmonary broncho vascular marking, flake-like pulmonary shadow, patchy or diffuse infiltrates, and interstitial and nodular opacities (Figure 2 & 3) [19]. However, these findings are non-specific and may be indistinguishable from other conditions such as pulmonary edema, aspiration, or infection. Therefore, the efficacy of chest radiographs in the initial diagnosis of FES is limited, but it can serve as a modality for monitoring disease progression [13].
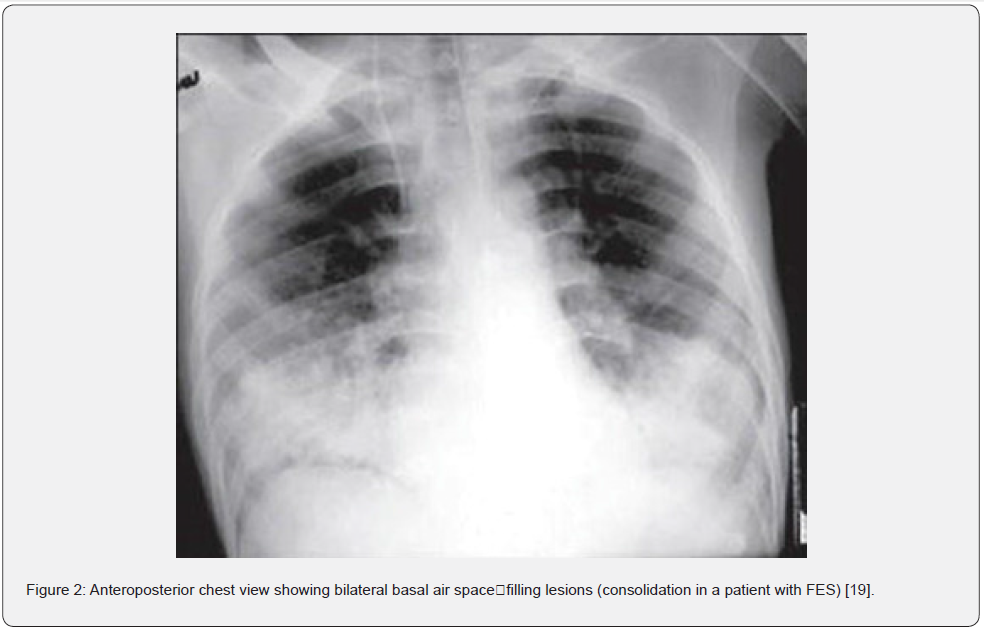
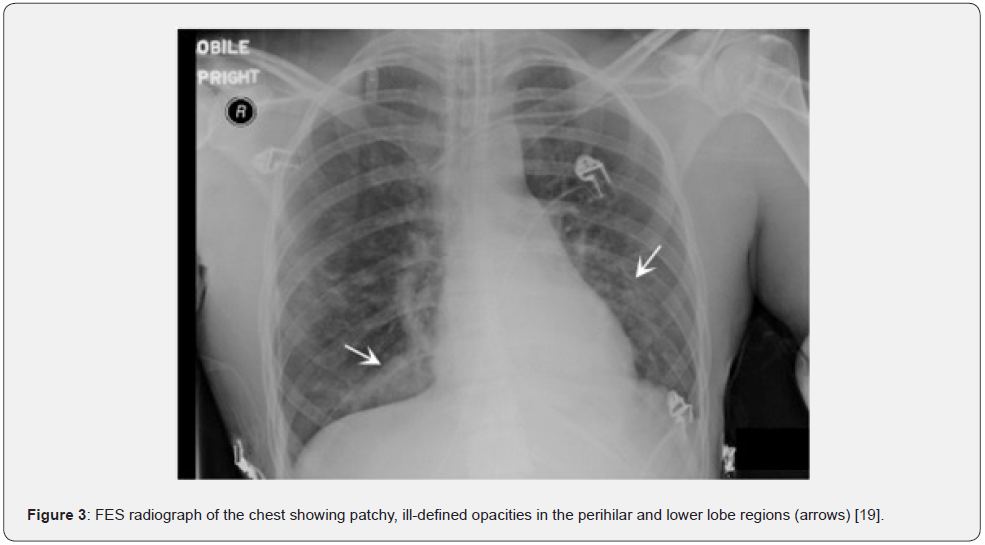
In later stages, x-ray findings may be similar to those associated with acute respiratory distress syndrome, including regional oligemia and dilatation of pulmonary vessels (Westermark sign), enlarged right descending pulmonary artery (Palla sign), and extended curvilinear densities reaching the pleural surface (Fleischner lines) [20]. Most of these imaging findings become evident around 24-48 hours after the injury and tend to disappear gradually over time (< 30 days) [21]. Notably, the sensitivity and specificity of chest x-rays in detecting pulmonary embolism are relatively low. Hence, this imaging modality should only be used to rule out potential differential diagnoses.
Thoracic CT scan
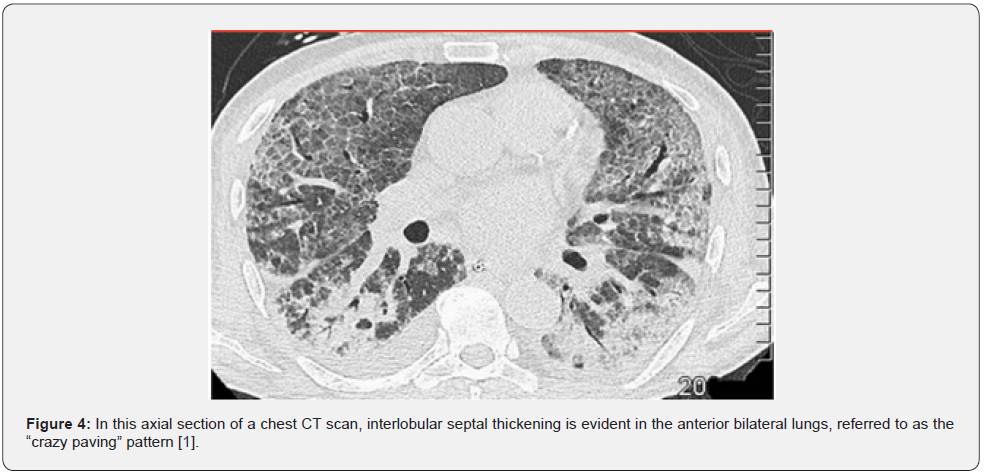
In suspected cases of FES, a chest CT scan, particularly a high-resolution CT scan (HRCT), is recommended for assessing the lung parenchyma. It may be helpful not only to establish a diagnosis but also to identify alternate causes of respiratory distress [22]. HRCT thoracic imaging is more effective than a chest x-ray in diagnosing fat embolism syndrome. The most common findings include diffuse patchy ground-glass opacities and consolidations with ‘crazy-paving’ pattern depicting interlobular septal thickening (Figure 4) [1,23]. Consolidation of the airspace has also been reported, evidently in more severe FES cases, indicating extensive pulmonary bleeding and edema. A direct correlation has been observed between the extent of airspace opacities and the severity of pulmonary symptoms, which may reflect the degree of acute lung injury. On HRCT, patients with mild pulmonary FES typically demonstrate a patchy ground-glass pattern, while patients with severe cases will exhibit consolidations (hemorrhage, edema).
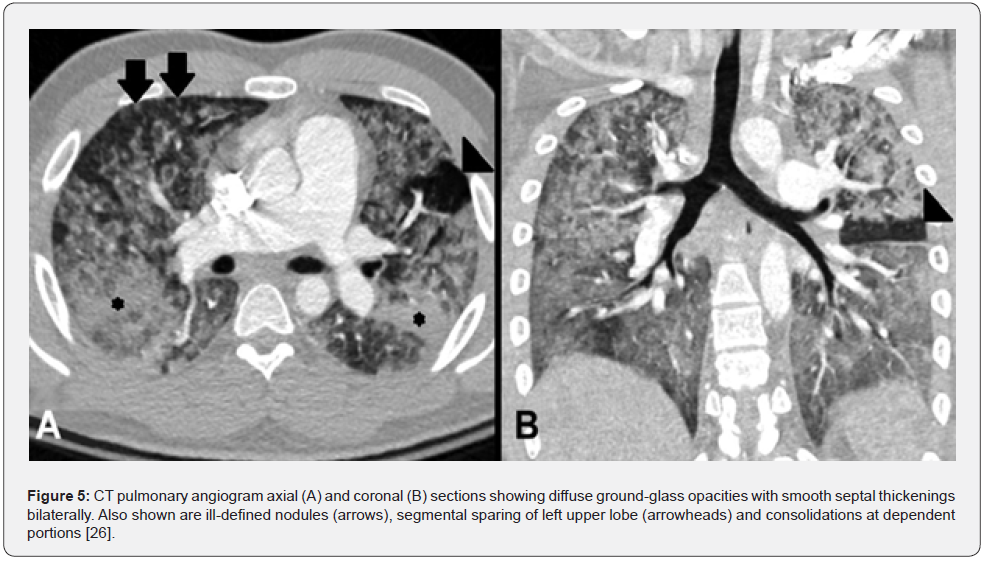
There is a correlation between the evolution of imaging findings and the progression of respiratory symptoms, with symptoms and the extent of the disease peaking approximately 48 hours after post-traumatic lipid release [24]. Less commonly seen in CT, but much more specific, is the presence of a fat embolus within the pulmonary vasculature. This can be observed as small areas of fat attenuation within the pulmonary artery lumen (Figure 5). Other imaging findings include the presence of small (< 5-10mm) and ill-defined nodules, typically described in a predominantly peripheral and upper lobe distribution, which represent inflamed intrapulmonary lymph nodes. It is still unclear when the pulmonary FE findings on CT will disappear, but the time period can be estimated between two weeks and one month. In mild cases, CT abnormalities may resolve within 7 to 15 days, while in more complicated cases, they may take up to 2 months [4,25,26].
Thoracic CT Differential Diagnosis of FES
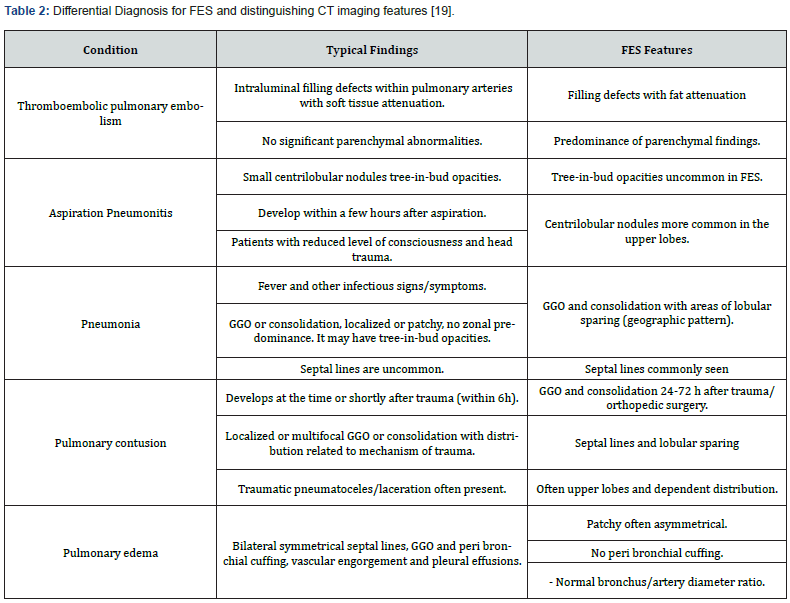
Considering that pulmonary FE is not as common and can resemble many other pulmonary and/or systemic inflammatory conditions, it is important to consider the differential diagnoses before making the diagnosis of fat embolism [16]. On CT, the differential diagnosis of parenchymal findings of FES may include pulmonary contusion, edema, thromboembolic pulmonary embolism, aspiration, and pneumonia. (Table 2) summarizes the typical imaging features of each diagnosis.
Abbreviations: FES: Fat Embolism Syndrome; GGO: Ground-Glass Opacities
Conclusion
Pulmonary fat embolism is a respiratory disorder that occurs mainly after trauma or orthopedic procedures. Despite being a rare condition, it represents significant morbidity since it is associated with respiratory failure, neurocognitive deficit, and death. Furthermore, accurate diagnosis can be challenging and/or delayed because FES often has numerous clinical manifestations. For this reason, it can be confounded with other more common pulmonary and/or systemic inflammatory conditions that occur after an acute organic insult, such as trauma or surgery. In addition, there are no specific laboratory tests to achieve a precise diagnosis, which complicates the process of identifying the disease in its early stages.
As a result, the assessment of FES depends on a combination of clinical features, laboratory tests, and ruling out alternative differentials. In this context, imaging studies are advantageous when evaluating these patients, particularly chest CT. For example, findings such as diffuse, ill-defined ground glass opacities or centrilobular nodules on chest CT suggest pulmonary FE in a surgical or post-traumatic setting. Even though these types of findings cannot always be detected, images can assist in identifying differential diagnoses. Thus, radiological studies are an integral part of the diagnostic work-up of patients with suspected FES. Consequently, clinicians should understand how to utilize these tools effectively to enhance the early diagnosis and successful treatment of FES.
References
- Rothberg DL, Makarewich CA (2019) Fat Embolism and Fat Embolism Syndrome. J Am Acad Orthop Surg 27(8): e346-e355.
- Stein PD, Yaekoub AY, Matta F, Kleerekoper M (2008) Fat embolism syndrome. Am J Med Sci 336(6): 472-477.
- Eriksson EA, Pellegrini DC, Vanderkolk WE, Minshall CT, Fakhry SM, et al. (2011) Incidence of pulmonary fat embolism at autopsy: an undiagnosed epidemic. J Trauma 71(2): 312-315.
- Kwiatt ME, Seamon MJ (2013) Fat embolism syndrome. Int J Crit Illn Inj Sci 3(1): 64-68.
- Singh S, Goyal R, Baghel PK, Sharma V (2018) Fat embolism syndrome: A comprehensive review and update. J Orthop Allied Sci 6(2): 56-63.
- Akhtar S (2009) Fat embolism. Anesthesiol Clin 27(3): 533-50.
- Chen HI (2009) From neurogenic pulmonary edema to fat embolism syndrome: a brief review of experimental and clinical investigations of acute lung injury and acute respiratory distress syndrome. Chin J Physiol 52(5 Suppl): 339-344.
- Mellor A, Soni N (2001) Fat embolism. Anaesthesia 56: 145-154.
- Ufuk F, Kaya F, Sagtas E, Kupeli A (2020) Non-thrombotic pulmonary embolism in emergency CT. Emerg Radiol 27(3): 343-350.
- George J, George R, Dixit R, Gupta RC, Gupta N (2013) Fat embolism syndrome. Lung India 30(1): 47-53.
- Maitre S (2006) Causes, clinical manifestations, and treatment of fat embolism. Virtual Mentor 8(9): 590-592.
- Shaikh N, Parchani A, Bhat V, Kattren MA (2008) Fat embolism syndrome: clinical and imaging considerations: case report and review of literature. Indian J Crit Care Med 12(1): 32-36.
- Taviloglu K, Yanar H (2007) Fat embolism syndrome. Surg Today 37(1): 5-8.
- Richards RR (1997) Fat embolism syndrome. Can J Surg 40(5): 334-339.
- Arakawa H, Kurihara Y, Nakajima Y (2000) Pulmonary fat embolism syndrome: CT findings in six patients. J Comput Assist Tomogr 24(1): 24-29.
- Matusov Y, Tapson VF (2021) Radiologic mimics of pulmonary embolism. Postgrad Med 133(sup1): 64-70.
- Muenzel D, Fingerle AA, Zahel T, Andreas S, Alain V, et al. (2017) CT Angiography: Post-processed Contrast Enhancement for Improved Detection of Pulmonary Embolism. Acad Radiol 24(2): 131-136.
- Ong SCL, Balasingam V (2017) Characteristic imaging findings in pulmonary fat embolism syndrome (FES). BMJ Case Rep 2017: bcr2017223007.
- Newbigin K, Souza CA, Torres C, Edson M, Ashish G, et al. (2016) Fat embolism syndrome: State-of-the-art review focused on pulmonary imaging findings. Respir Med 113: 93-100.
- Torres PPTES, Mançano AD, Zanetti G, et al. (2020) Multimodal indirect imaging signs of pulmonary embolism. Br J Radiol 93(1108): 20190635.
- Donnamaria V, Palla A, Petruzzelli S, Carrozzi L, Pugliesi O, et al. (1993) Early and late follow-up of pulmonary embolism. Respiration 60(1): 15-20.
- Malagari K, Economopoulos N, Stoupis C, Zoe D, Spyros P, et al. (2003) High-resolution CT findings in mild pulmonary fat embolism. Chest 123(4): 1196-1201.
- McCabe BE, Veselis CA, Goykhman I, Hochhold J, Eisenberg D, et al. (2019) Beyond Pulmonary Embolism; Nonthrombotic Pulmonary Embolism as Diagnostic Challenges. Curr Probl Diagn Radiol 48(4): 387-392.
- Rossi SE, Goodman PC, Franquet T (2000) Nonthrombotic pulmonary emboli. AJR Am J Roentgenol 174(6): 1499-1508.
- Agbelele P, Van Maris F, Sanguina M, Zerkly B, Djebara AE, et al. (2020) Use of chest CT-scan images to differentiate between SARS-CoV-2 infection and fat embolism: A clinical case. Radiol Case Rep 15(10): 2047-2050.
- Nucifora G, Hysko F, Vit A, Vasciaveo A (2007) Pulmonary fat embolism: common and unusual computed tomography findings. J Comput Assist Tomogr 31(5): 806-807.






























