Pulmonary Angioplasty in Chronic Thromboembolic Pulmonary Hypertension. Are Intravascular Ultrasound and Stents Helpful?
Alvaro Calabuig1, Enric Domingo1,2*, Manuel Lopez Messeguer3, Juan Carlos Grignola4, Antonio Roman3, Bruno Garcia1 and Carles Bravo3
1Cardiology, Hospital Universitari Vall d Hebron, Spain
2Department of Physiology, Universitat Autonoma de Barcelona, Spain
3Neumology, Hospital Universitari Vall d Hebron, Spain
4Pathophysiology Department, Universidad dela Republica, Uruguay
Submission: July 15, 2022; Published: July 22, 2022
*Corresponding author: Enric Domingo, Cardiology, Hospital Universitari Vall d Hebron, Barcelona, Spain Address: Unitat de Hemodinamica, Hospital Universitari Vall d Hebron, P. Vall d Hebron 119, Barcelona, 08035, Spain
How to cite this article: Alvaro C, Enric D, Manuel L M, Juan Carlos G, Antonio R, et al. Pulmonary Angioplasty in Chronic Thromboembolic Pulmonary Hypertension. Are Intravascular Ultrasound and Stents Helpful?. Int J Pul & Res Sci. 2022; 6(1): 555677. DOI: 10.19080/IJOPRS.2022.06.555677
Abstract
Pulmonary arterial angioplasty (PA) in chronic thromboembolic pulmonary hypertension (CTEPH) is an option for patients rejected for surgical pulmonary endarterectomy. Preliminary results seem encouraging, although pulmonary injure rates are reported. 11 patients (6 woman) with CTEPH, mean age 64 ± 16 underwent PA. Four patients were in functional class 3, mean TAPSE was 15 ± 4mm. Before the procedure, mean pulmonary pressure (mPAP) was 49 ± 13mmHg, all had normal left ventricular ejection fraction and were under a chronic anticoagulant therapy. 10 out of 11 patients were done under intravascular ultrasound (IVUS) guidance in order to precisely estimate the vessel size. 20 stents were delivered to correct severe initial recoil or arterial occlusive dissection after initial balloon dilatation. There were no procedural complications (no regional pulmonary edema, no pulmonary vessel rupture). On follow-up (16.5 ± 6,4 months), we observed improvement in functional class in 9 out of the 11 patients. No hospital admission and no dead were seen on follow-up. Pulmonary angioplasty under IVUS guidance seems to improve the procedure safety in our series, probably avoiding vessel rupture due to a more precise vessel sizing. Stent implantation seems to be safe and has no restenosis.
Keywords: Pulmonary hypertension; Pulmonary angioplasty; Chronic thromboembolic pulmonary hypertension; Intravascular ultrasound; Stents
Abbreviations: PA: Pulmonary Arterial Angioplasty; CTEPH: Chronic Thromboembolic Pulmonary Hypertension; mPAP: Mean Pulmonary Pressure; IVUS: Intravascular Ultrasound; PEA: Pulmonary Endarterectomy; BPA: Balloon Pulmonary Angioplasty; ESC/ERS: European Society of Cardiology/European Respiratory Society; OCT: Optical Coherence Tomography; PH: Pulmonary Hypertension
Introduction
Chronic thromboembolic pulmonary hypertension (CTEPH) is characterized by precapillary pulmonary hypertension (PH) hemodynamically defined as mean pulmonary arterial pressure (mPAP) ≥20mmHg and a normal pulmonary artery wedge pressure ≤15mmHg during resting right heart catheterization , with thromboembolic evidence showing mismatched perfusion defects in pulmonary arteries on computed tomographic pulmonary angiography, lung perfusion scintigraphy or pulmonary angiography despite effective anticoagulation therapy for at least three months. If left untreated, CTEPH will rapidly progress and seriously threaten people’s lives [1], and CTEPH patients with mPAP >30mmHg have a relatively poor prognosis [2], with a 5-year survival rate of 30% for those with mPAP >40mmHg and 10% for those with mPAP >50mmHg [3,4].
Pulmonary endarterectomy (PEA) remains the potentially curative treatment of choice with an in-hospital mortality rate of 2.2% for all eligible CTEPH patients at experienced centers and should be considered whenever possible [5]. Currently, inoperable candidates, either due to comorbidities or to distal arterial disease, have more options to receive medical and/or interventional therapies at specialized centers. Balloon pulmonary angioplasty (BPA) is a catheter-based interventional technique which uses appropriately sized balloons to dilate and open up stenotic or occluded pulmonary vessels under angiography guidance. Accumulating evidence suggests that it can strikingly improve hemodynamics, cardiac function and exercise tolerance with an acceptable incidence of complications, and the long-term prognosis is encouragingly comparable to PEA surgery [6-10].
After more than 30 years of development and refinements, BPA is emerging as a promising alternative treatment option for CTEPH patients. However, there are still many issues worth exploring for BPA, such as the selection of patients, the establishment of a standardized operation process and the reduction of complications. After years of unremitting efforts, the 2015 European Society of Cardiology/European Respiratory Society (ESC/ERS) guidelines recommended that BPA only be performed in experienced CTEPH expert center [11]. Intravascular imaging techniques such as optical coherence tomography (OCT) [12,13] and intravascular ultrasound (IVUS) [14] offer endovascular information of the vessel wall and lumen that may potentially be useful for a better vessel selection and for a more adequate technique during BPA in order to increase its effectivity and decrease its complications.
OCT produces optically scattered images showing high-resolution internal tissue microstructure. OCT-guided BPA allows us to precisely assess lesion type and vessel diameter and facilitate the selection of appropriate balloon size and length and can also be utilized to evaluate the effectiveness of BPA; however, it may result in potential volume overload and right heart failure [15-18]. IVUS is an endovascular imaging technique that uses a miniaturized ultrasound probe to generate sound waves and produce real-time intravascular images, and due to relatively lower resolution mainly applied to determine vessel diameter so as to select the appropriate balloon. In addition, virtual histology IVUS is capable of identifying vascular lesions vulnerable to balloon compression and helps us decide which organized thrombus can be treated to avoid potential pulmonary artery rupture [17].
The world largest multicenter registry from Japan described pulmonary injury (17.8%) and hemoptysis (14.0%) as the most frequent complications, pulmonary artery perforation, dissection and rupture accounted for 2.9%, 0.4%, and 0.1%, respectively [6]. The recent French BPA experience revealed that lung injury, hemoptysis, pulmonary artery perforation and dissection occurred in 9.1%, 7.1%, 2.8, and 1.9%, respectively, among 1006 BPA sessions, and the incidence of lung injury decreased over time from initial 13.3% to recent 5.9%, clearly indicating that the occurrence of BPA complications depends on center experience [7]. Pulmonary artery stenting may be an option in proximal CTEPH when elastic recoil phenomenon makes balloon angioplasty of a large vessel ineffective [19]. It has been suggested that IVUS-guided pulmonary angioplasty in chronic thromboembolic pulmonary hypertension may increase safety of the procedure [20].
Case Series
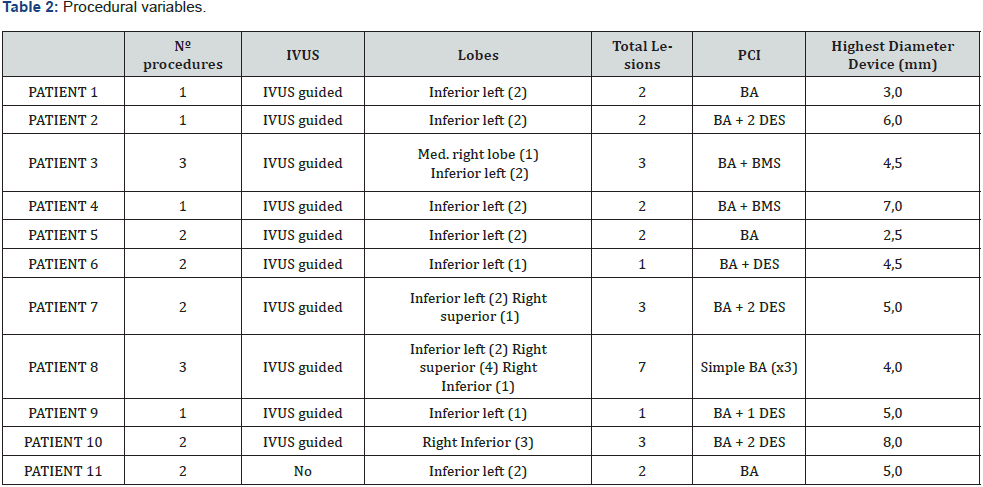
We here in describe a case series of IVUS-guided BPA in CTEPH where the IVUS information was useful for the effectiveness and safety of the procedure. Patients submitted to pulmonary angioplasty were either patients with distal disease not candidates for surgery or patients rejected for surgery due to comorbidities despite being angiographically good surgical candidates. No peri nor post procedural complications were observed (no regional pulmonary edema, no pulmonary vessel rupture). All patients were dismissed the day after the procedure. Right heart catheterization was performed through the femoral vein in 7 and through the brachial vein in 13 procedures. 11 patients were submitted to 20 procedures and a total of 28 lesions were treated. IVUS guided PA (Boston Scientific, Opti Cross 40MHz) was performed in 10 out of 11 patients and in 17 out of 20 procedures. Stents were delivered in 10 out of 28 lesions, due to severe immediate elastic recoil or due to angiographic occlusive dissection after initial balloon dilatation.
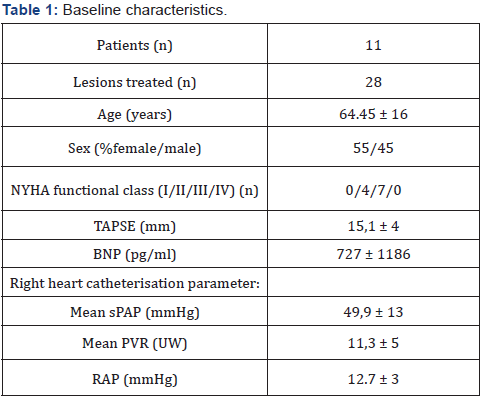
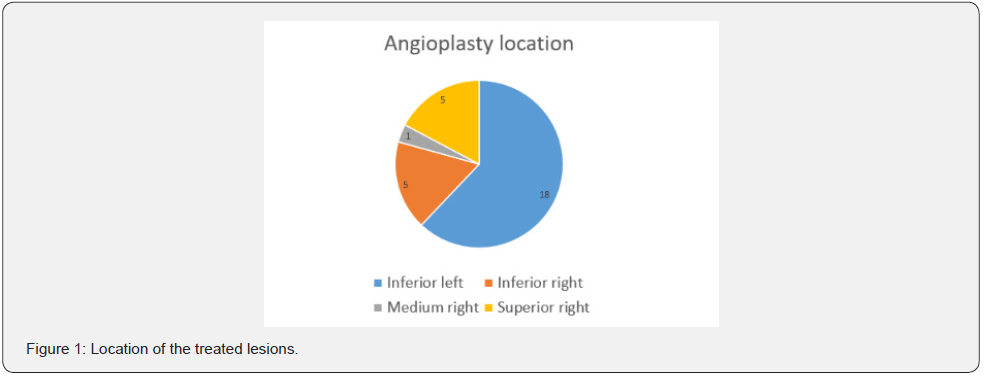
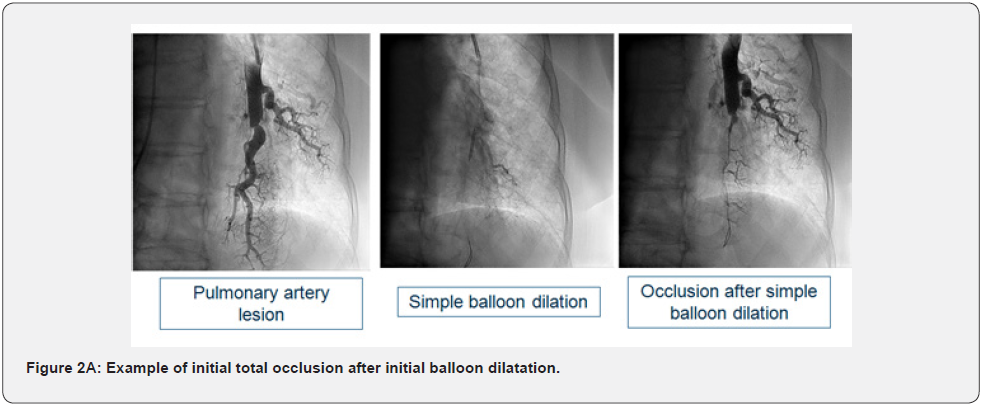
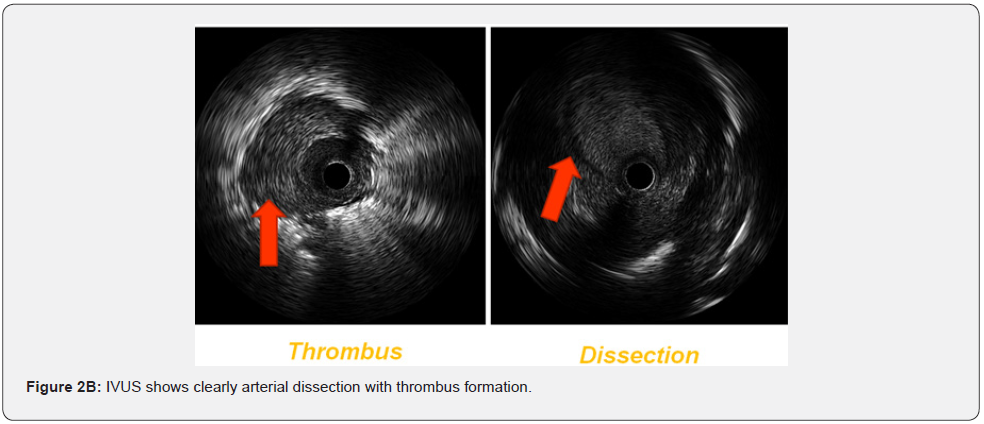
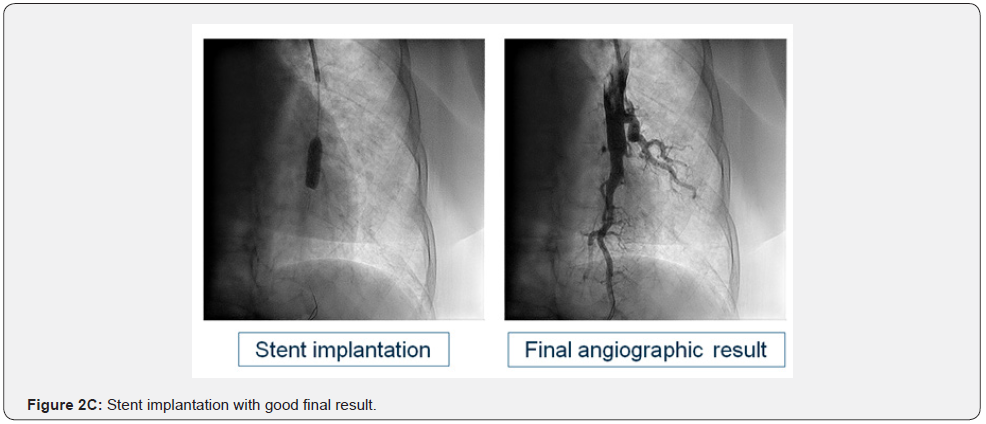
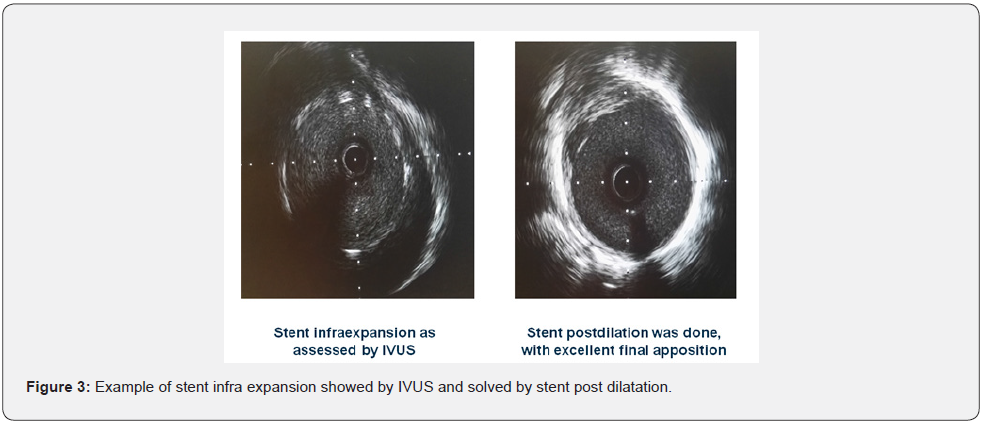
Basal clinical and hemodynamic data are listed in (Table 1). Procedural variables are listed in (Table 2). Location of dilated lesions is showed in (Figure 1). No immediate or mid-term complications were observed (mean follow up 16.5 ± 6.4 months). Baseline functional class was II in 35% and III in 65% of patients. 9 patients improved at least 1 functional class (6 improved 1 and 3 patients 2) (p<0.01) and 2 remained stable. Only a modest improvement was observed in mean PAP (49.9mmHg vs. 42.8mmHg), and BNP (727 vs. 222pg/dl). No angiographic stent restenosis was seen in angiographic control. No clinical or angiographic complications related to stent delivery were detected on the control angiography at 3-6 months follow-up. Examples of the peri procedural usefulness of IVUS (depicting the mechanisms of arterial occlusion after initial balloon dilatation) are depicted in (Figure 2A, 2B and 2C). Example of stent under expansion not evident in the arterial angiography and showed by the endovascular IVUS image is depicted in (Figure 3).
Conclusion
IVUS allows a better vessel sizing of pulmonary artery and thus a more accurate selection of balloon and stent diameters. IVUS is better than angiography alone for detecting balloon post dilatation recoil and stent under expansion. IVUS guided PA may also detect potential complications, such as arterial dissection, differentiating true vs false lumen, thrombus formation, and may increase the safety of the procedure. No angiographic stent restenosis was seen in angiographic control.
References
- Madani M, Ogo T, Simonneau G (2017) The changing landscape of chronic thromboembolic pulmonary hypertension management. Eur Respir Rev 26(146): 170105.
- Lewczuk J, Piszko P, Jagas J, Porada A, Wojciak S, et al. (2001) Prognostic factors in medically treated patients with chronic pulmonary embolism. Chest 119(3): 818-823.
- Riedel M, Stanek V, Widimsky J, Prerovsky I (1982) Longterm follow-up of patients with pulmonary thromboembolism. Late prognosis and evolution of hemodynamic and respiratory data. Chest 81(2): 151-158.
- Fedullo P, Kerr KM, Kim NH, Auger WR (2011) Chronic thromboembolic pulmonary hypertension. Am J Respir Crit Care Med 183(12): 1605-1613.
- Madani MM, Auger WR, Pretorius V, Sakakibara N, Kerr KM, et al. (2012) Pulmonary endarterectomy: recent changes in a single institution's experience of more than 2,700 patients. Ann Thorac Surg 94(1): 97-103.
- Ogawa A, Satoh T, Fukuda T, Sugimura K, Fukumoto Y, et al. (2017) Balloon Pulmonary Angioplasty for Chronic Thromboembolic Pulmonary Hypertension: Results of a Multicenter Registry. Circ Cardiovasc Qual Outcomes 10(11): e004029.
- Brenot P, Jais X, Taniguchi Y, Garcia Alonso C, Gerardin B, et al. (2019) French experience of balloon pulmonary angioplasty for chronic thromboembolic pulmonary hypertension. Eur Respir J 53(5): 1802095.
- Kataoka M, Inami T, Hayashida K, Shimura N, Ishiguro H, et al. (2012) Percutaneous transluminal pulmonary angioplasty for the treatment of chronic thromboembolic pulmonary hypertension. Circ Cardiovasc Interv 5(6): 756-762.
- Mizoguchi H, Ogawa A, Munemasa M, Mikouchi H, Ito H, et al. (2012) Refined balloon pulmonary angioplasty for inoperable patients with chronic thromboembolic pulmonary hypertension. Circ Cardiovasc Interv 5(6): 748-755.
- Sugimura K, Fukumoto Y, Satoh K, Nochioka K, Miura Y, et al. (2012) Percutaneous transluminal pulmonary angioplasty markedly improves pulmonary hemodynamics and long-term prognosis in patients with chronic thromboembolic pulmonary hypertension. Circ J 76(2): 485-488.
- Galie N, Humbert M, Vachiery JL, Gibbs S, Lang I, et al. (2015) 2015 ESC/ERS Guidelines for the diagnosis and treatment of pulmonary hypertension: The Joint Task Force for the Diagnosis and Treatment of Pulmonary Hypertension of the European Society of Cardiology (ESC) and the European Respiratory Society (ERS): Endorsed by: Association for European Paediatric and Congenital Cardiology (AEPC), International Society for Heart and Lung Transplantation (ISHLT). Eur Respir J 46(4): 903-975.
- E Domingo, J Grignola, R Aguilar, M Montero, C Arredondo, et al. (2013) In vivo assessment of pulmonary arterial wall fibrosis by intravascular optical coherence tomography in pulmonary arterial hypertension: a new prognostic marker of adverse clinical follow-up. Open Respir Med J 7: 26-32.
- E Domingo, R Aguilar, M Lopez-Meseguer, G Teixido, M Vazquez, et al. (2009) New concepts in the invasive and non invasive evaluation of remodelling of the right ventricle and pulmonary vasculature in pulmonary arterial hypertension. Open Respir Med Journal 3: 31-37.
- J Rodes-Cabau, E Domingo, A Roman, J Majo, B Lara, et al. (2003) Intravascular ultrasound of the elastic pulmonary arteries: a new approach for the evaluation of primary pulmonary hypertension. Heart 89(3): 311-315.
- Raber L, Ueki Y, Lang IM (2019) Balloon pulmonary angioplasty for the treatment of chronic thromboembolic pulmonary hypertension. EuroIntervention 15(9): e814-e815.
- Araszkiewicz A, Jankiewicz S, Janus M, Lanocha M, Mularek-Kubzdela T, et al. (2017) Optical coherence tomography reveals the mechanisms of balloon pulmonary angioplasty in inoperable chronicthromboembolic pulmonary hypertension. Cardiol J 24(3): 334-335.
- Kopec G, Waligora M, Stępniewski J, Zmudka K, Podolec P, et al. (2015) In vivo characterization of changes in composition of organized thrombus in patient with chronic thromboembolic pulmonary hypertension treated with balloon pulmonary angioplasty. Int J Cardiol 186: 279-281.
- Roik M, Wretowski D, Labyk A, Kostrubiec M, Rowinski O, et al. (2014) Optical coherence tomography of inoperable chronic thromboembolic pulmonary hypertension treated with refined balloon pulmonary angioplasty. Pol Arch Med Wewn 124(12): 742-743.
- Szymon D, Radoslaw P, Marta B, Arkadiusz P, Lukasz K, et al. (2020) Balloon Pulmonary Angioplasty with Stent Implantation as a Treatment of Proximal Chronic Thromboembolic Pulmonary Hypertension. Diagnostics 10(6): 363.
- Alvaro CG, Bernat SC, Gerard MA, Antonio RB, M Lopez-Messeguer, et al. (2018) Abstract 16076: Initial Experience on Ivus-Guided Pulmonary Artery Angioplasty and Stent Implantation in Patients with Chronic Thromboembolic Pulmonary Hypertension. Circulation 138: A16076.






























