Effects of Selenium Nanoparticles on Bleomycin-Induced Pulmonary Fibrosis in Rats
Rana Shahabi1, Ali Anissian2, Seyed Ali Javadmoosavi3, Zahra Behroozi4, Leila Aryan5 and Farinaz Nasirinezhad6*
1Department of Physiology, Iran University of Medical Sciences, Iran
2Veterinary Pathology Department, Islamic Azad University, Iran
3Air Pollution Center of Iran University of Medical Sciences, Iran
4Physiology Research Center, Kerman University of Medical Sciences, Iran
5Department of Physiology, Iran University of Medical Sciences, Iran
6Physiology Research Center and Department of Physiology, Iran University of Medical Sciences, Iran
Submission: February 24, 2022; Published: April 29, 2022
*Corresponding author: Farinaz Nasirinezhad, Physiology Research Center and Department of Physiology, Iran University of Medical Sciences, Tehran, Iran
How to cite this article: Rana S, Ali A, Seyed Ali J, Zahra B, Leila A, et al . Effects of Selenium Nanoparticles on Bleomycin-Induced Pulmonary Fibrosis in Rats. Int J Pul & Res Sci. 2022; 5(5): 555675. DOI: 10.19080/IJOPRS.2022.05.555675
Abstract
Background: Idiopathic pulmonary fibrosis (IPF) is one of the interstitial lung diseases. To date, most drug trials for the treatment of IPF have yielded disappointing results. Recently, selenium nano-particles (SeNP) have received attention for potential use in treatments, due in part to their established abilities to exert size-dependent anti-oxidant/-inflammatory effects.
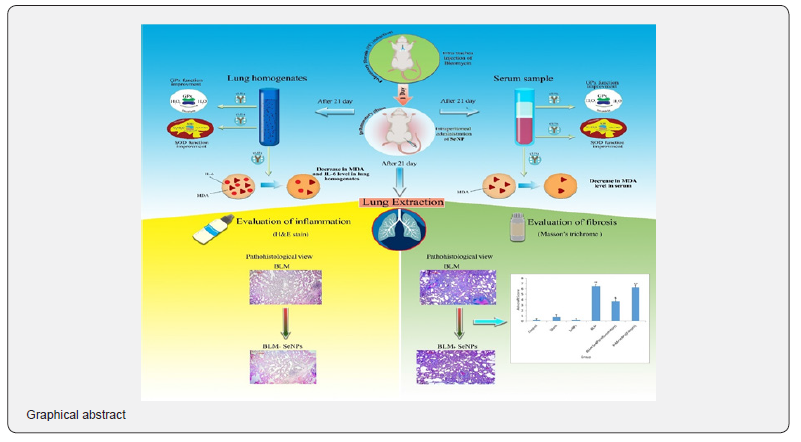
Materials and Methods: the rats were treated with SeNPs by intraperitoneal injection (0.5mg SeNP/kg) for five consecutive days in the inflammatory phase (a day after injection of bleomycin) and fibrotic phase (a week after injection of bleomycin).
Results: The results showed that administration of SeNP during the inflammatory phase improved the activity of enzymes antioxidant (i.e., glutathione peroxidase and superoxide dismutase) and also led to significant decreases in interleukin (IL)-6 and malondialdehyde (MDA) levels in lung homogenates. Histopathology, measures of lung index and body weight, and evaluations of lungs using Ashcroft criteria supported the mitigative effects found above. Notably, treatment with the SeNP during the fibrotic phase imparted no ameliorative effects. Lastly, the SeNP themselves seemed to impart no overt toxicities in naïve rat hosts.
Conclusion: Thus, the findings suggest that SeNP, in part by improving the antioxidant enzyme activities, might impart a therapeutic effect against PF induced by bleomycin in rats. This potentially might mean that SeNP could represent a new therapeutic for treatment of this disease, at least in its earliest phases. (Graphical abstract)
Keywords: Pulmonary fibrosis; Selenium nanoparticles; Bleomycin; Antioxidant enzymes; Interleukin- 6
Abbreviations: IPF: Idiopathic Pulmonary Fibrosis; SOD: Superoxide Dismutase; CAT: Catalase GPx: Glutathione Peroxidase; BLM: Bleomycin; PF: Pulmonary Fibrosis; IL: Interleukin; MDA: Malondialdehyde; Se: Selenium; TrxR: Thioredoxin Reductase; IP: Intraperitoneal; DLS: Dynamic Light Scattering; TEM: Transmission Electron Micrograph; ANOVA: Analysis Of Variance; Lavage BAL: Bronchoalveolar
Introduction
Idiopathic pulmonary fibrosis (IPF) is an interstitial lung disease that is a chronic, advanced fibrosis of unknown cause [1]. The prognosis is poor and average survival time after diagnosis is 5 years [2]. All organs, including the lungs, possess a wide range of antioxidant enzymes to defend against oxidative stress. Among the various enzymes involved in this defense are superoxide dismutase (SOD), catalase (CAT), and glutathione peroxidase (GPx) [3]. A decrease in overall antioxidant defenses/status in the lung leads to increased oxidative stress, inflammation, and ultimately, activation of fibroblasts. Such deposition of extracellular matrix elements into the lung parenchyma results in the destruction of alveolar structures and, even loss of lung function [4]. Hence, it is believed that oxidative stress and oxidation/anti-oxidation imbalances are major mechanisms involved in the pathogenesis and progression of IPF [5].
Bleomycin (BLM), an anti-cancer drug, causes lung damage and induces pulmonary fibrosis (PF). Therefore, BLM is used to create an animal model of PF [6,7]. BLM induces an oxidative stress in the lung that is characterized by low endogenous GPx, SOD, and CAT antioxidant enzymes activities [8]. In addition to those changes, BLM induces formation of interleukin (IL)-6, a cytokine that mediates inflammatory responses in lung fibrosis [9]. The induced lung injury is biphasic, i.e., there is an initial inflammatory phase characterized by leukocytic infiltration to the lungs and damage to alveolar epithelial cells over the first week, that is then followed by a fibrotic phase characterized by fibroblast proliferation and extracellular matrix deposition over the second week [10]. In recent years, studies have been performed to mitigate the adverse effects of BLM-induced pulmonary fibrosis [11] in the hopes that development of such drugs might in turn be used in the treatment of IPF. To date, such studies and novel drugs have not been very promising [12].
Selenium (Se) is an essential element due to its action in the active site of antioxidant enzymes such as GPx and thioredoxin reductase (TrxR), as well as having a role in immune responses and inflammation in humans [13]. By itself, Se has antioxidant and anti-inflammatory effects [14,15], and its presence insures malondialdehyde (MDA) levels to remain normal [16,17]. Nevertheless, there is a narrow margin between effective and toxic doses for selenium, regardless of its chemical form [18]. Elemental selenium nanoparticles (SeNP), because of low toxicity and high biological activities, have received great attention as a potential therapeutic agent [19]. In part, due to findings SeNPs (5-200nm) have shown size-dependent effects in scavenging free radicals in vitro [20]. SeNPs have also been revealed to have anti-inflammatory/-oxidant effects in vivo [21-24]. The present study was carried out to evaluate possible anti-inflammatory/-oxidant activities of SeNP against BLM-induced pulmonary fibrosis in male rats. Potential effects of the particles were evaluated in both the inflammatory and fibrotic phases of the disease, as it is completely unknown if there is any preferential period in which the SeNP might be most beneficial.
Materials and methods
Animals
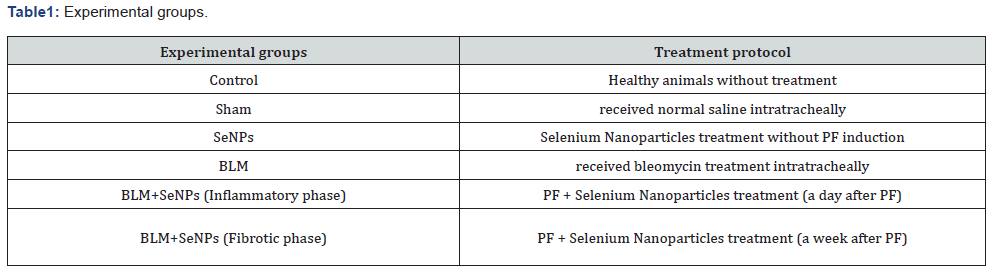
Male Wistar rats 200-250g weight (eight to ten weeks of age) were purchased from the Laboratory Animal Breeding Center of Iran University of Medical Sciences for the study. All rats were housed under controlled conditions (temperature 22 ± 2°C, and 50% relative humidity) with a 12-hr light/dark cycles. All rats had ad libitum access to filtered water and standard rodent chow. For the experiment, after a 2-wk acclimatization period, rats were randomly allocated into six experimental groups (10/group) (Table 1). All experimental protocols were approved by the Iran University of Medical Sciences Ethics Committee. The study was performed according to the animal ethics committee guidelines of the Principles of Laboratory Animals Care"(NIH Publication No. 85-23, revised 2011).
Pulmonary fibrosis (PF) induction
Bleomycin-induced PF was induced in rats using the protocols of Zaafan et al. [25]. In brief, rats were anesthetized via intraperitoneal (IP) injection of a mixture of ketamine (100mg/kg) and xylazine (10mg/kg). Thereafter, the trachea was cannulated. For induction of PF, the rats received a single dose of 4mg BLM/kg (Bleomycin, Cell Pharm Gmbh, Hannover, Germany) in 50µl saline. Sham animals received saline without BLM. The cannula was then removed and the skin was sutured.
Characteristics of selenium nanoparticles (SeNP)
The hydrodynamic size of the nanoparticles was measured by dynamic light scattering (DLS) (Malvern, Worcestershire, UK) and zeta potential was measured by Zeta Sizer (Malvern, Worcestershire, UK) according to the manufacturer’s instructions.
Administration of selenium nanoparticles (SeNP)
SeNPs (size ~23nm) were purchased from the Iranian Nonmaterials Pioneers Company; NANOSANY (Mashhad, Iran). The purchased SeNPs were examined by transmission electron micrograph (TEM) to confirm integrity (Figure 1). One day after the treatment with BLM [or saline] (i.e., inflammatory phase), rats received an IP dose of 0.5g SeNP/kg; this was repeated on each of four successive days. On day 7 after treatment with BLM [or saline] (i.e., fibrotic phase) parallel sets of rats received an IP dose of 0.5g SeNP/kg; this was repeated on each of four successive days [22]. This particular dose of SeNP was selected based upon the study by Peng et al. [20].

Sample collection and analytical procedures
On day 21 after induction of PF (or dosing with saline), rats were anesthetized with ketamine/xylazine and blood samples were collected from the abdominal aorta. After euthanization (exsanguination), the lungs were removed, weighed, and washed with ice-cold saline. From this, the lung index was determined [ratio of net lung weight (g): body weight (g)]. The right lung was then immersed in 10% buffered formaldehyde for histopathologic analysis. The left lung was placed at -80°C for later measures of SOD and GPx activity, as well as of MDA and IL-6 levels. Each blood sample was centrifuged (10,000 RPM, 5min, 4°C), and the serum was isolated and stored at -80°C, until later analysis of SOD, GPx and MDA.
Body weight and lung index
All rats were weighed in the course of the experiment every 7 days. The changes in body weight were measured. Also, to assess lung index, lung tissue was weighed. Lung index was determined by the ratio of net lung weight (g) to the body weight (g) for each rat.
Histopathology
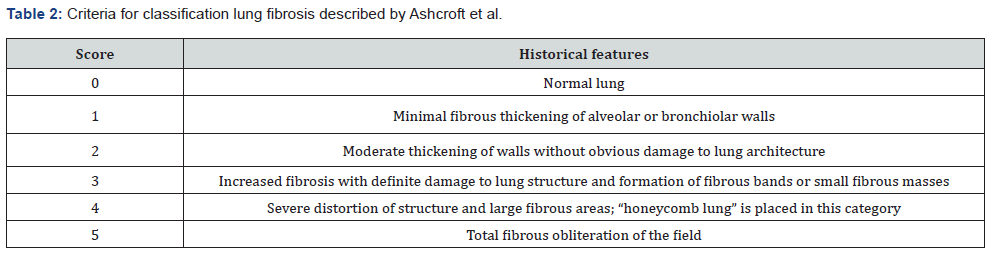
The right lung was fixed in buffered formaldehyde for one week at room temperature. After this, the tissue was dried using graded ethanol, fixed in paraffin, and then sectioned with 4μm thickness. The sections were then stained with hematoxylin and eosin (H&E) to permit evaluation of the severity of interstitial fibrosis; Masson's trichrome stain was also used to analyze collagen content. All slides were examined by a certified histologist (blinded manner) using a labomed lx400 light microscope (Fremont, California, USA) at 400 magnifications. The degrees of fibrosis and inflammation in lung parenchyma were evaluated through the semi-quantitative classification system Ashcroft et al. [26] (Table 2). A total of 10 fields/slide (and 10 slides/rat) was examined. The mean score of all fields in each section was considered as the fibrosis score.
Lung homogenate preparation
A small portion of the left lung (lower lobe) was isolated and placed in phosphate-buffered saline (PBS, pH 7.4 to 100mg tissue/ml). The samples were then homogenized using a WiseTis HG-15D Homogenizer (Witeg Labortechnik GmbH, Wertheim, Germany), centrifuged (4000-6000 RPM, 4°C) for ≈20min, and each supernatant was then recovered for use in the assaying of SOD and GPx activities, as well as of MDA and IL-6 levels.
SOD, GPx and MDA
SOD, GPx, enzyme activities and MDA levels were analyzed in lung homogenates and serum using commercial ELISA kits (ZellBio, GmbH, Veltlinerweg, Germany), according to manufacturer protocols. Homogenate IL-6 level was measured via an ELISA kit (Biorbyt, Cambridge, UK). The sensitivity of the kits was 1 U SOD/ml, 10U GPx/ml, 0.1µM MDA, and 15pg IL-6/ml. All samples were analyzed in triplicate. All ELISAs were evaluated using a Synergy HT Multi-microplate reader (BioiTek, Winooski, VT).
Statistical Analysis
All data are presented as mean ± SEM. To compare differences in body weight between groups, a two-way analysis of variance (ANOVA) was performed using student's t-test. For evaluation of outcomes based on histology, as well as lung indices, a one-way ANOVA was used. In all cases, a p-value <0.05 was considered significant. All analyses were performed using SPSS v.21.0 (Sacramento, CA).
Result
Hydrodynamic size and surface charge of selenium nanoparticles
The results of DLS showed that the hydrodynamic size was 25 ±2nm and the results of Zeta potential indicated that the surface charge was -29mV.
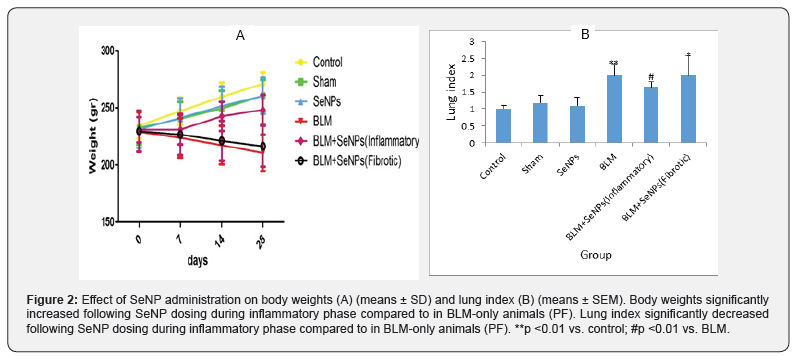
Body weights and lung Index
Body weights of BLM rats were significantly decreased compared with control rats at 21 days’ post-treatment (p <0.001). Dosing with SeNP during the inflammatory phase inhibited the loss of weight (p <0.001). In contrast, administration of SeNP initiated during the fibrotic phase did not affect body weights (p = 0.11) (Figure 2A). As with body weights, the lung index was significantly changed on Day 21 (df: 5; F = 120.13; p <0.001) (Figure 2B). In BLM-only-treated rats, there was a significant increase in lung index compared to in control rats (p <0.001). Dosing with SeNP during the inflammatory phase led to a significant decrease in index compared with values for the BLM-only counterparts (p <0.001). In contrast, administration of SeNP initiated during the fibrotic phase did not affect lung index compared to the BLM-only values (p = 0.41). There were no significant differences between score values among the control, sham, and SeNP-only rats.
Histopathology
Histological changes in lung tissues stained with H&E and Masson’s trichrome were seen 21 days after BLM injection (Figure 3A, 3B). Control animals showed normal histological structure including thin interalveolar septa, clear alveoli, and few inflammatory cells in the pulmonary mesenchyme. In BLM rats, there was a marked change, including peribronchiolar thickening of the interalveolar septa, collapse of alveolar spaces, inflammatory cell infiltration, proliferation of fibroblasts, and evidence of extracellular matrix deposition. There was also increased interstitial collagen deposition. In rats dosed with SeNP during the inflammatory phase, there were attenuated thickening of the interalveolar septa, inflammatory cell infiltration, collagen deposition, and damaged structures relative to what was noted in the BLM-only rats.
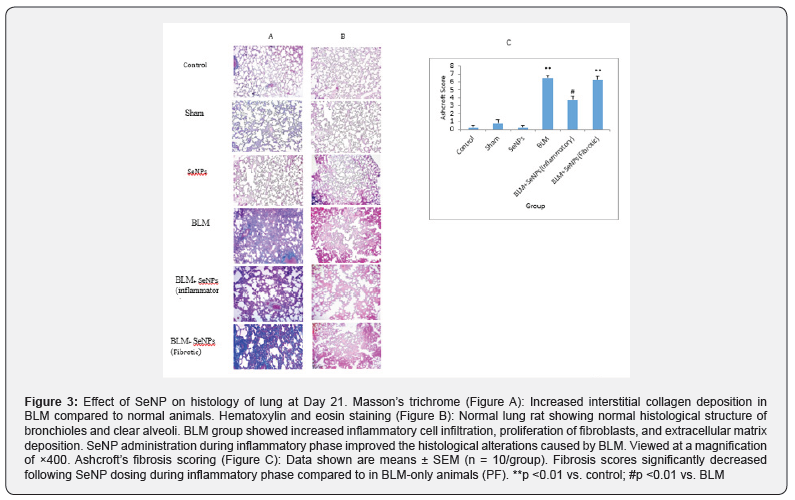
Administration of SeNP during the fibrotic phase did not give rise to pathologic changes similar to what occurred with dosing during the inflammatory phase. Ashcroft’s fibrosis scoring showed the differences between the groups on day 21 after BLM injection (p <0.001) (Figure 3C). Administration of BLM resulted in significant increase in scores for fibrosis (p <0.001). Administration of SeNP during the inflammatory phase led to a significantly lowered score compared to BLM-only rats (p <0.001). Administration of SeNP during the fibrotic phase had no effects on the BLM-induced fibrosis (p = 0.99). In no cases were there significant differences between score values among the control, sham, and SeNP-only rats.
Glutathione peroxidase (GPx) activity
Analysis revealed changes in GPx activity in the serum on day 21 after BLM injection (p <0.001), with values being significantly decreased compared to control (Figure 4A). Injection of SeNP during the inflammatory phase caused a significant increase in serum GPx compared to that of the BLM-only rats (p = 0.02), with values reaching those of control rats (p = 0.30). GPx in lung homogenates (p <0.001) were significantly decreased as a result of BLM (Figure 4B). Injection of SeNP during inflammatory phase caused a significant increase in lung homogenate GPx compared to that seen in the BLM rats (p = 0.008), with levels reaching those in control rats (p = 0.30). In contrast, administration of SeNP during the fibrotic phase did not results in any changes of GPx in serum or lung homogenates compared to BLM-only rats (p = 0.33 and p = 0.93, respectively). In no cases were there significant differences between values among the control, sham, and SeNP-only rats.

Superoxide dismutase (SOD) activity
Treatment with BLM led to a significant decrease in serum SOD on day 21 (p <0.001) (Figure 5A). Administration of SeNP during the inflammatory phase caused significant increase in serum SOD compared to that of BLM-only rats (p = 0.035). Similarly, BLM administration caused significant decrease in SOD in lung homogenates (p<0.001) (Figure 5B). Administration of SeNP during the inflammatory phase caused a significant increase in lung homogenate SOD compared to that of BLM-only rats (p = 0.017), with levels reaching those seen in control rats (p = 0.55). No changes were seen in SOD in the serum or lung homogenates ((p = 0.66 and p = 0.38, respectively, relative to in BLM-only rats) in rats that received the SeNP during the fibrotic phase. In no cases were there significant differences between values among the control, sham, and SeNP-only rats.
Malondialdehyde (MDA) levels
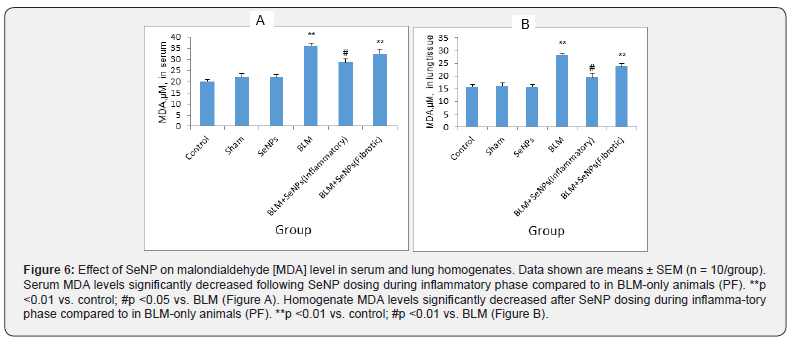
On day 21 after BLM administration, rats presented enhanced serum MDA levels (index of lipid peroxidation) (p <0.001) (Figure 6A). Treatment with SeNP during the inflammatory phase resulted in significant decrease in serum MDA (p = 0.021). It was also seen that BLM alone led to a significant increase in MDA level in lung homogenates (p <0.001) compared with the controls (Figure 6B). Compared to the BLM-only rats, SeNP treatment led to significant decrease in these levels in the homogenates (p <0.001) to the point that they were on par with control group levels (p = 0.26). There were no significant differences in MDA levels in serum and homogenates between the BLM and fibrotic groups (p = 0.60 and p = 0.12 respectively). In no cases were there significant differences between values among the control, sham, and SeNP-only rats.
Interleukin (IL)-6 levels
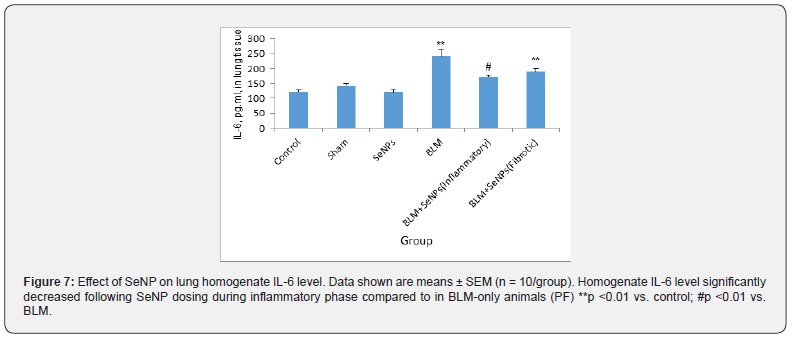
Lung homogenate IL-6 levels were significantly increased in BLM rats after 21 days (p <0.001) (Figure 7). Administration of SeNP during the inflammatory phase caused a significant decrease in homogenate IL-6 levels compared to those in the BLM-only rats (p = 0.006), with levels reaching those seen in control rats (p = 0.10). In contrast, administration of SeNP during the fibrotic phase did not lead to any change in lung homogenate IL-6 levels relative to those in the BLM-only rats (p = 0.072). In no cases were there significant differences between values among the control, sham, and SeNP-only rats.

Discussion
The current study evaluated the effects of administration of SeNP on oxidant/antioxidant status and potential improvements against BLM-induced pulmonary fibrosis (PF) in rats. The results showed that administration of SeNP during the inflammatory phase increased GPx and SOD enzymes activities, and decreased levels of MDA and inflammatory IL-6. The data also showed SeNP application at that point mitigated BLM-related effects on body weight and lung indices, as well as on histologic markers of damage. Treatment with SeNP starting in the fibrotic phase did not have any beneficial effects. The main part of the pathogenesis of pulmonary fibrosis is an inflammatory response. That is the initial response, as a result, the employment of both residents and circulating lymphocytes, neutrophils, and macrophages.
These Inflammatory cells can produce large quantities of reactive oxygen species (ROS) and inflammatory cytokines like IL-6, which stimulate proliferation, migration, secretory activities, and production of collagen by fibroblasts which eventually contributes to pulmonary fibrosis [9,27,28]. It is well-known that BLM causes oxidative damage and increases ROS production by inflammatory cells in the lungs [29]. In addition to oxidative stress, intratracheal injection of BLM leads to increased expression of IL-6 and this cytokine could exacerbate the lung injury [30]. SeNPs have lower toxicity and high biological activities than selenium itself (or various forms, soluble/insoluble selenium) [19]. Several studies have shown that SeNPs have anti-inflammatory and antioxidant activities in vivo and in vitro [21,31,32] which this confirms our results in the antioxidant and anti-inflammatory effects of SeNP in inflammatory phase.
In the present study, BLM led to increased levels of MDA (marker for lipid peroxidation) in the blood and lung tissues, inflammatory IL-6 in lung tissues, and a simultaneous reduction amount and activity of antioxidant GPX and SOD in sera and lung tissues. These findings were similar to those in previous reports [25]. Furthermore, the study here found that treatment with SeNP during the inflammatory phase significantly reduced MDA levels in serum and lung tissues, IL-6 in lung tissues, and simultaneously increased levels of GPx and SOD in serum and lung tissues. These data are consistent with results of Ali E et al. who showed that SeNP had a potential to significantly increase GPx and SOD and improve the markers of oxidative damage during lung cancer [23]. Here, SeNP treatment during the inflammatory phase seemed to mitigate negative effects of BLM on SOD, GPx and MDA; such outcomes might be related to antioxidant effects from the SeNP [33,34].
Such antioxidant proprieties of SeNP have previously been demonstrated through decreases in MDA levels and increasing the activity of GPx and SOD in the livers of mice treated with cyclophosphamide [35]. It has been reported that BLM administration in rat leads to an immediate increase in the production of IL-6 [9]. In accordance with these findings, in the current study, it was seen that levels of this inflammatory cytokine were significantly increased in BLM groups. Application of the SeNP during the inflammatory phase causes significant decreases in lung levels of IL-6. Such results were in agreement with those of Zaid et al. who showed SeNP administration led to decreased levels of brain tissue IL-6 that had been altered in association with a neurotoxicity induced by acrylamide [24].
It has been shown that the prescription of SeNP is capable to ameliorate the activity of antioxidant enzymes such as GPx, CAT, and SOD and decrease the level of MDA [36]. Turgut et al. witnessed a significant improvement in lung fibrosis score, body weight, lung Index, activity of antioxidant enzymes, MDA, I-6 levels by intratracheal BLM application into rat lung by the treatment of naringin, an antioxidant and anti-inflammatory agent, given 5min after administration of BLM for 14 consecutive days [6]. In addition, Akgedik et al. reported that lung fibrosis score, hydroxyproline content, accumulation of inflammatory cells in bronchoalveolar lavage (BAL) fluid, MDA and total antioxidant capacity in the lung tissue and serum were improved following oral administration of Resveratrol as an antioxidant and anti-inflammatory compound compared to the BLM group [37].
In another study, Kilic et al. [38] reported administration of apocynin as an ROS production inhibitor before (12hr before) and after (14 days after) BLM injection. Apocynin caused a significant increase in GPx activity and significantly decreases in lung levels of MDA, IL-6, and IL-8, and reversed/mitigated histological damage (all compared to lungs of rats that received only the BLM treatments) [38]. The authors in the above studies suggested that their test agents might have protective and therapeutic effects against BLM-induced lung fibrosis. In contrast, the present data showed that SeNP only was effective during the inflammatory phase (i.e., could improve [reduce] pulmonary fibrosis scores, cause increase in GPx and SOD activity, decrease MDA and IL-6 levels) but did not show any therapeutic effects during the fibrotic phase. In another study, Hassanin et al. evaluated the effect of antioxidant SeNP on thyroid damage by oxidative stress. They showed that the prescription of SeNP for 5 consecutive days was able to improve the activity of antioxidant enzymes in the thyroid gland.
Furthermore, the prescription of SeNP alone in the nano-selenium-treated group had no toxic effect in compared to control group [22]. Mohammed et al. [34] reported that selenium nanoparticles in two different sizes, i.e., 3-5nm and 10-20nm, exerted antioxidant effects and could decrease the MDA level and tumor necrosis factor (TNF)-α [34]. The mechanism through which SeNP could limit fibrosis seems probably to be related to its capability to diminish oxidative damage and IL-6 in the lung structures in the inflammatory phase of the disease process. However, with advancement of the disease and onset of fibrotic processes (i.e., proliferation of fibroblasts. collagen deposition), treatment with SeNP was not effective. It appeared that anti-fibrotic agents would be needed for treatment at this phase of the disease. The present study was the first in vivo study to investigate potential effects of SeNP on the restoration of the activity of antioxidant enzymes and levels of anti-inflammatory factor following induction of PF.
Conclusion
The results of the current study have shown that SeNP due to anti-inflammatory and antioxidant properties could significantly suppress BLM-induced pulmonary fibrosis (PF) in rats when administered during the inflammatory phase of PF. These changes were accompanied by significant reductions in lung lipid peroxidation levels and increases in the activities of antioxidant enzymes. On the other hand, when provided during the fibrotic phase of PF, the SeNP could not have any ameliorative effects. Thus, it seems these compounds are not a viable option for the treatment of PF in its advanced stages. Still, based on the findings, SeNP may be a promising new therapeutic for potential use in early stages of PF.
References
- Shahabi R, Anissian A, Javadmoosavi SA, Farinaz N (2021) Protective and anti-inflammatory effect of selenium Nano-particles against bleomycin-induced pulmonary injury in male rats. Drug Chem Toxicol 44(1): 92-100.
- Olson AL, Jeffrey JS, Dennis CL, Jill MN, Carla GW, et al. (2007) Mortality from pulmonary fibrosis increased in the United States from 1992 to 2003. Am J Respir Crit Care Med 176(3): 277-284.
- Cheresh P, Seok-Jo K, Sandhya T, David WK (2013) Oxidative stress and pulmonary fibrosis. Biochim Biophys Acta 1832(7): 1028-1040.
- Todd NW, IG Luzina, SP Atamas (2012) Molecular and cellular mechanisms of pulmonary fibrosis. Fibrogenesis Tissue Repair 5(1): 11.
- Matsuzawa Y, Tatsuo K, Ryosei K, Sho H, Tamako I, et al. (2015) Change in serum marker of oxidative stress in the progression of idiopathic pulmonary fibrosis. Pulm pharmacol Ther 32: 1-6.
- Turgut NH, Haki K, Sahende E, Koksal D, Huseyin G, et al. (2016) The Protective effect of naringin against bleomycin-induced pulmonary fibrosis in Wistar rats. Pulm Med 2016: 7601393.
- Moore BB, CM Hogaboam, (2008) Murine models of pulmonary fibrosis. Am J Physiol Lung Cell Mol Physiol 294(2): L152-L160.
- Crestani BS, Marchand-Adam, S Schneider (2005) Traitements medicamenteux dela fibrose pulmonaire idiopathique. Revue de Pneumologie Clinique 61(3): 221-231.
- Saito F, Sadatomo T, Ken-Ichiro I, Keisuke M, Yasushi N, et al. (2008) Role of interleukin-6 in bleomycin-induced lung inflammatory changes in mice. Am J Respir Cell Mol Biol 38(5): 566-571.
- Reinert T, Clarissa SRB, Frederico APN, Adriana ASS (2013) Bleomycin-induced lung injury. Journal of Cancer Research 2013: 9.
- Tashiro M, Koichi I, Daisuke Y, Shigeki N, Shintaro K, et al. (2008) Lung fibrosis 10 years after cessation of bleomycin therapy. Tohoku J Exp Med 216(1): 77-80.
- Talmadge EK, Albera C, Williamson ZB, Ulrich C, Phil H, et al. (2009) Effect of interferon gamma-1b on survival in patients with idiopathic pulmonary fibrosis (INSPIRE): a multicentre, randomised, placebo-controlled trial. Lancet 374(9685): 222-228.
- Rayman MP (2000) The importance of selenium to human health. Lancet 356(9225): 233-241.
- Pedrero Z, Y Madrid (2009) Novel approaches for selenium speciation in foodstuffs and biological specimens: a review. Anal Chim Acta 634(2): 135-152.
- Kahya MC, M Naziroglu, B Çig (2015) Melatonin and selenium reduce plasma cytokine and brain oxidative stress levels in diabetic rats. Brain inj 29(12): 1490-1496.
- Boostani A, Sadegh AA, Naser MS, C Mohamad, Kashan N (2015) The effects of organic, inorganic, and nano-selenium on blood attributes in broiler chickens exposed to oxidative stress. Acta Scientiae Veterinariae 43.
- Placha I, J Takacova, M Ryzner, K Cobanova, A Laukova, et al. (2014) Effect of thyme essential oil and selenium on intestine integrity and antioxidant status of broilers. Br Poult Sci 55(1): 105-114.
- Zhang JS, XY Gao, LD Zhang, YP Bao (2001) Biological effects of a nano red elemental selenium. Biofactors 15(1): 27-38.
- Al-Quraishy S, MA Dkhil, AEA Moneim (2015) Anti-hyperglycemic activity of selenium nanoparticles in streptozotocin-induced diabetic rats. Int J Nanomedicine 10: 6741-6756.
- Peng D, Jinsong Z, Qingliang L, Ethan WT (2007) Size effect of elemental selenium nanoparticles (Nano-Se) at supranutritional levels on selenium accumulation and glutathione S-transferase activity. J Inorg Biochem 101(10): 1457-1463.
- Zhang BJ, Li XY, (2010) Protective effects of selenium nanoparticles on oxidative stress and antioxidant enzymes activities induced by microcystins in the liver of mice. Acta Hydrobiologica Sinica 34(3).
- Hassanin KMA, SHA El-Kawi, KS Hashem (2013) The prospective protective effect of selenium nanoparticles against chromium-induced oxidative and cellular damage in rat thyroid. Int J Nanomedicine 8: 1713-1720.
- Ali E, S El-Sonbaty, F Salem (2013) Evaluation of selenium nanoparticles as a potential chemo preventive agent against lung carcinoma. Int J Pharm Biol Sci 2(4): 38-46.
- Zaid OARA, SM El- Sonbaty, WE El-AM Barakat (2017) Ameliorative effect of selenium nanoparticles and ferulic acid on acrylamide-induced neurotoxicity in rats. Annals of Medical and Biomedical Sciences 3(2): 35-45.
- Zaafan MA, Hala FZ, AI El-Brairy, Sanaa AK (2016) Pyrrolidinedithiocarbamate attenuates bleomycin-induced pulmonary fibrosis in rats: Modulation of oxidative stress, fibrosis, and inflammatory parameters. Exp Lung Res 42(8-10): 408-416.
- Ashcroft T, JM Simpson, V Timbrell (1988) Simple method of estimating severity of pulmonary fibrosis on a numerical scale. J Clin Pathol 41(4): 467-470.
- Shahabi R, Dehghani M, Javadmoosavi SA, Bahareh S, Omid P, et al. (2022) The effect of nanoparticles on pulmonary fibrosis: a systematic review and Meta-analysis of preclinical studies. Arch Environ Occup Health Pp: 1-11.
- Le T-TT, H Karmouty-Quintana, Ernestina M, Thanh-Truc TL, Tingting W, et al. (2014) Blockade of IL-6 trans signaling attenuates pulmonary fibrosis. J Immunol 193(7): 3755-3768.
- Inghilleri S, Patrizia M, Tiberio O, Sergio B, Carla F (2006) In situ assessment of oxidant and nitrogenic stress in bleomycin pulmonary fibrosis. Histochem Cell Biol 125(6): 661-669.
- Chaudhary NI, A Schnapp, JE Park (2006) Pharmacologic differentiation of inflammation and fibrosis in the rat bleomycin model. Am J Respir Crit Care Med 173(7): 769-776.
- El-Ghazaly MA, N Fadel, E Rashed, A El-Batal, SA Kenawy (2017) Anti-inflammatory effect of selenium nanoparticles on the inflammation induced in irradiated rats. Can J Physiol Pharmacol 95(2): 101-110.
- Wang J, Yifeng Z, Yahong Y, Tianli Y (2014) Immunomodulatory of selenium nano-particles decorated by sulfated Ganoderma lucidum polysaccharides. Food Chem Toxicol 68: 83-189.
- Dehordi SK, A Mohebbi, K Shahanipour (2015) Effects of the selenium nanoparticles on the biomarkers of oxidative status in the Wistar rats spleen following the experimental cadmium toxicity.
- Mohammed E, G Safwat (2013) Assessment of the ameliorative role of selenium nanoparticles on the oxidative stress of acetaminophen in some tissues of male albino rats. Beni-Suef University Journal of Basic and Applied Sciences 2(2): 80-85.
- Bhattacharjee A, Abhishek B, Prosenjit G, Jaydip B, Sudin B (2014) Protective effect of Selenium nanoparticle against cyclophosphamide induced hepatotoxicity and genotoxicity in Swiss albino mice. J Biomater Appl 29(2): 303-317.
- Dkhil MA, Rafat Z, S Al-Quraishy, AEA Moneim (2016) Selenium Nanoparticles Attenuate Oxidative Stress and Testicular Damage in Streptozotocin-Induced Diabetic Rats. Molecules 21(11): 1517.
- Akgedik R, Sukran A, Harun K, Sema U, Bulent B, et al. (2012) Effect of resveratrol on treatment of bleomycin-induced pulmonary fibrosis in rats. Inflammation 35(5): 1732-1741.
- Kilic T, Hakan P, Elif T, Sedat Y, Alaadin P, et al. (2015) Protective and therapeutic effect of apocynin on bleomycin-induced lung fibrosis in rats. Inflammation 38(3): 1166-1180.






























