Effectiveness of Mhealth to Increase Cervical Cancer Screening: Systematic Review of Interventions
Jacques L Tamuzi1*, Ley M Muyaya1, Jonathan L Tshimwanga2 and Linda Zeng2
1Community Health Division, Stellenbosch University, South Africa
2Family Medicine Division, Stellenbosch University, South Africa
Submission: March 03, 2017; Published: October 27, 2017
*Corresponding author: Jacques L Tamuzi, Community Health Division, Faculty of Medicine and Health Sciences, Stellenbosch University, Matieland, South Africa, Email: drjacques.tamuzi@gmail.com
How to cite this article: Jacques L Tamuzi, Ley M Muyaya, Jonathan L Tshimwanga, Linda Zeng. AEffectiveness of Mhealth to Increase Cervical Cancer Screening: Systematic Review of Interventions. Int J Pul & Res Sci. 2017; 2(3): 555586. DOI:10.19080/IJOPRS.2017.02.555586
Abstract
Background: Estimated one million-plus women worldwide are currently living with cervical cancer. Many of them have not any access to health services for prevention, curative treatment or palliative care. Cervical cancer is a consequence of a long-term infection with human papillomavirus (HPV), and the majority of cervical cancer cases (>80%) are currently found in low- and middle-income countries. In fact, an increasing body of literature indicates that HIV-positive women have an increased risk of developing cervical cancer in comparison with their HIV-negative counterparts. Cervical cancer is most notable in the lower-resource countries of sub-Saharan Africa as the result of the highest incidence of HIV-infected women.
Pilot mHealth projects have shown that, particularly in developing countries, mobiles phones improve communication and information-delivery and information-retrieval processes over vast distances between healthcare service providers and patients. Mobiles phones provide remote access to healthcare facilities, facilitate trainings for, and consultations among health workers, and allow for remote monitoring and surveillance to improve public health programs awareness.
mHealth interventions can potentially influence health-related behavior (and, in turn, health outcomes) via effecting changes in mediators of behavior change such as knowledge, attitudes, community peer norms, beliefs and self-efficacy. SMS can be customized to fit the needs of specific individuals by delivering tailored messages that are more likely to catch the individual’s attention and be perceived as personally relevant and interesting.
This systematic review will investigate whether mHealth interventions could improve cancer screening uptake in risk women.
Objective: To assess the effectiveness of different mHeath (SMS, calls, letters and emails reminders) interventions to improving cervical cancer screening in risk women.
Search methods: We searched for studies in MEDLINE, Scorpus, PsychINFO, Cochrane Central Register of Controlled Trials (CENTRAL), CINAHL, World Health Organization Global Health Library regional index, Mobile Active http:// www.mobileactive.org, Web of Science and Grey literature. In addition, hand-searching was performed for the original published version of this review, but not for this update. Issues of the following journals will be hand-searched: AIDS, AIDS Care, Health Education Journal, Health Psychology and Journal of the American Medical Association
Selection criteria: We included the following studies design: randomized control trials, quasi-experimental studies and non-randomized control trials assessing different mHealth interventions in improving cervical cancer screening outcomes.
Data collection and analysis: Two reviewers independently (JT and LM) identified and critically appraised all included studies. Study design, characteristics of study populations, interventions, controls and study results were extracted by two review authors. In addition, the risk of bias of included studies was assessed independently by two reviewers. We interpreted the results from meta-analysis. We reported the odds ratio with 95% confidence intervals for the different outcomes.
Main results: We found 4731 studies in different electronic databases, 3004 studies were included after removing duplicated studies. Among them, 79 studies were fully assessed and then, 51 were excluded and 28 studies were assessed for eligibility criteria. 11 studies were excluded with reasons and 17 studies were included in meta-analysis. The overall results revealed that call reminders increased 44% of cervical cancer screening compared to the standard care, with p-value of0.01. 8 studies were included in this meta-analysis and the total number of participants was 29477. Call reminders improved 89% of cervical cancer screening adherence, with highly statistical results (Test for overall effect: Z = 5.23, P < 0.00001). 3 studies and 1340 participants were included. Lastly, letter reminders improved 20 % of cervical cancer screening compared to the standard care. 8 studies and 345835 participants were found in the overall results. Therefore, this result was not statistically significant (P=0.15).
The effect of call reminders on cervical cancer screening and its adherence was high; therefore the impact of letter reminders on cervical cancer was moderate.
Authors’ conclusion: This systematic review supports the use of call reminders in improving cervical cancer screening and adherence to testing. The main outcomes were graded as high level of evidence. Then, call reminders could be suggested to be encompassed in different national policy in screening cervical cancer in risk populations. The lack of sufficient evidence on the subject limits the reliability of the current cervical cancer screening guidelines for high risk women is the leading cause of diagnosing cervical cancer in the last stage. Further studies in this field will provide the sole for preventing cervical cancer. However, this review could orientate public health policy makers.
Background
Description of the condition
An estimated one million-plus woman worldwide is currently living with cervical cancer [1]. Many of them have not any access to health services for prevention, curative treatment or palliative care [1]. Cervical cancer is a consequence of a long-term infection with human papillomavirus (HPV), and the majority of cervical cancer cases (>80%) are currently found in low- and middleincome countries [1].
Nowadays, Cervical cancer constitutes a major health problem worldwide [2]. Recent studies have demonstrated cervical cancer is the leading cause of female cancer mortality and second most common cancer in women worldwide [3] and It is responsible for 528,000 new cases of cancer and causes 270,000 deaths each year (WHO 2012) [4]. Several demographic, economical and behavioral risk factors have been studied in relation to cervical cancer [5]. Most of them may influence the risk of cancer through their effects on the risk of HIV and HPV infection Ali-Risasi [5]. Different studies have shown that HIV infection has been associated with an increased risk of cervical cancer Kumakech [6]. Epidemiological studies have clearly established human papillomavirus (HPV) infection as the main cause of cervical cancer. In most studies, HPV16 and HPV18 are the predominant genotypes: they cause about 70 % of precancerous lesions and cervical cancer [7,5]. In Sub- Saharan Africa however, other oncogenic genotypes have been reported 5,8,9,10]. In fact, an increasing body of literature indicates that HIV-positive women have an increased risk of developing cervical cancer in comparison with their HIV-negative counterparts [11,12]. Sub-Saharan has the highest incidence of HIV-infected women, and then cervical cancer is most notable in the lower-resource countries of sub-Saharan Africa [4]. In sub- Saharan Africa, 34.8 new cases of cervical cancer are diagnosed per 100 000 women annually, and 22.5 per 100 000 women die from the disease [4]. Compared to North America where there are 6.6 new cases of cervical cancer diagnosed per 100 000 women annually, and 2.5 per 100 000 women die, Sub-Saharan Africa has 34.8 and 22.5 per 100 000 respectively [4].With increasing attention to cervical cancer prevention in developing countries [13], several pilot screening programs have been initiated throughout sub-Saharan Africa Rosser [14]. The World Health Organization (WHO) recommends a more aggressive cervical cancer screening [15].
In fact, among all malignant tumours, cervical cancer is the one that is most easily preventable by screening Arbyn M [16]. The detection of cytological abnormalities by microscopic examination of “Pap smears”, and the subsequent treatment of women with high-grade cytological abnormalities, avoids development of cancer [16,17]. With increasing attention to cervical cancer prevention in developing countries, several pilot screening programs have been initiated throughout sub-Saharan Africa [14]. Therefore, some challenges are associated with screening, ranging from low levels of cervical cancer screening due to poor access to organized screening, a lack of or low information on cervical cancer screening, stigma, women’s perception of low threat of disease and overburdened health care facilities which lack equipment and are understaffed [18,19].
Description of the intervention
Mobile telecommunication technologies into the health arena is also known as mobile health, mHealth or eHealth [20]. Mobile phone technology is increasingly viewed as a promising communication channel that offers the potential to improve health care delivery and promote behavior change among vulnerable populations [20].
Pilot mHealth projects have shown that, particularly in developing countries, mobile phones improve communication and information-delivery and information-retrieval processes over vast distances between healthcare service providers and patients [21,22]. Mobiles provide remote access to healthcare facilities, facilitate trainings for, and consultations among, health workers, and allow for remote monitoring and surveillance to improve public health programs. This phenomenon has the potential to lead to an overall increase in the efficiency and effectiveness of under-resourced health infrastructures, ultimately translating into benefits for patients [22].
SMS-based interventions enable patients and providers to ‘‘interact’’ via two-way communication. To date, this feature has been implemented in various ways. For example, most studies have used systems to automate the message delivery process for providers, ranging from fully automated clinical appointment reminders [23] to staff developing and delivering the messages themselves. SMS interventions also have enabled patients to communicate with providers to confirm thier adherence to any health interventions or outcomes [24,25]. Other studies have mixed SMS, call, email and letter reminders to improve health related outcomes. In fact, letter reminders could be used in network inaccessible areas or cellphone deprived women.
The use of mHealth to improve health related outcomes is receiving more attention in public health as emerging evidence suggests reminder messages, call, email and letter can improve several health outcomes.
How the intervention might work
Individual and cultural factors, such as stigma, isolation, symptoms of illness, and psychological distress [26-28] may contribute then to non-adherence of cervical cancer screening.
mHealth interventions can potentially influence healthrelated behavior (and, in turn, health outcomes) via effecting changes in mediators of behavior change such as knowledge, attitudes, community peer norms, beliefs and self-efficacy [29]. SMS can be customized to fit the needs of specific individuals by delivering tailored messages that are more likely to catch the individual’s attention and be perceived as personally relevant and interesting [30]. Then, mHealth plays an active role in one’s health and medical care leads to better healthcare quality, better clinical health outcomes, and likely lower healthcare costs [31]. Interventions aimed at increasing patient involvement have shown beneficial effects on satisfaction and functional status [32,25], quality of life [33], perceived control over cervical cancer.
Why it is important to do this review
Studies have shown that well-organized cytological screening at the population level, every three to five years, and the incidence of cervical cancer can be reduced up to 80% [34,16]. Furthermore, the vaccination against the most common oncogenic human papillomavirus (HPV) types, HPV-16 and HPV- 18, could prevent development of up to 70% of cervical cancers worldwide [35]. Therefore, this vaccine is quite inaccessible in developing countries; by the way, the Pap smear reminds the cornerstone of cervical cancer screening in developing countries. Then, improving cervical screening through different behavioral intervention is the only way that could decrease drastically the morbidity and mortality of cervical cancer.
Eight studies exploring reasons women did not utilize cervical cancer screening were included. Women in Sub-Saharan Africa reported similar barriers despite cultural and language diversity in the region [36]. Women reported fear of screening procedure and negative outcome, low level of awareness of services, embarrassment and possible violation of privacy, lack of spousal support, societal stigmatization, cost of accessing services and health service factors like proximity to facility, facility navigation, waiting time and health care personnel attitude [36].
This systematic review will investigate whether mHealth interventions could improve cancer screening uptake in risk women.
Objectives
To assess the effectiveness of different mHeath (SMS, calls, letters and emails reminders) interventions to improving cervical cancer screening in risk women.
Methods
Criteria for considering studies for this review
Types of studies
- Randomized control trials
- Quasi-experimental studies
- Non randomized control trials
4.3. Types of participants
Women at risk of developing cervical cancer
Types of interventions
SMS reminders
- Call reminders
- E-mail reminders
- Letter reminders
Types of outcome measures
Primary outcomes
- Pap smear uptake
- Adherence to test pap smea
Secondary outcomes
Proportion of abnormal pap smear
Search methods for identification of studies
(Cellular phone) OR (telephone) OR (mobile phone) OR (text messag*) OR (testing) OR (short messag*) OR (cell phones) OR (SMS) OR (short message service) OR (text) OR (mobile health) OR (telemedicine) OR (health) OR (health communication) OR (health education) OR (behavior) OR (ehealth)
(Uterine Cervical Neoplasm) OR (Cervical Neoplasms) OR (Cervical Neoplasm) OR (Cervix Neoplasms) OR (Cervix Neoplasm) OR (Cancer of the Uterine Cervix) OR (Cancer of the Cervix) OR (Cervical Cancer) OR (Uterine Cervical Cancer) OR (Cancer of Cervix) OR (Cervix Cancer)
(Test, Papanicolaou) OR (Pap Test) OR (Test, Pap) OR (Pap Smear) OR (Smear, Pap) OR (Papanicolaou Smear)
(Randomized controlled trial) OR (controlled clinical trial) OR (randomized controlled trials) OR (random allocation) OR (double-blind method) OR (single-blind method) OR (clinical trial) OR (trial) OR (clinical trials) OR (clinical trial) OR (singl* OR doubl*) OR (trebl* OR tripl*) AND (mask* OR blind*) OR (placebos) OR (placebo*) OR (random*).
Electronic searches
We searched for studies in:
MEDLINE
- Scorpus
- PsychINFO
- Cochrane Central Register of Controlled Trials (CENTRAL)
- CINAHL
- World Health Organization Global Health Library regional index
- Mobile Active http:// www.mobileactive.org
- Web of Science
- Grey literature
Searching other resources
Hand-searching was performed for the original published version of this review, but not for this update. Issues of the following journals was hand-searched: AIDS, AIDS Care, Health Education Journal, Health Psychology and Journal of the American Medical Association.
Data Collection and Analysis
Selection of studies
Inclusion criteria was applied to all titles and, where available, abstracts identified from the literature search by two review authors. Potentially relevant references was then retrieve for further screening by one review author and check by a second. Any disagreement was resolved through discussion with recourse to a third review author when necessary.
Data extraction and management
The following data were extracted:
- Author and year of publication
- Country, town, Setting
- study design
- Total number of intervention groups
- Unit of data analysis
- Sample size calculation
- Duration of follow-up
- total number enrolled
- Eligible participants
- Age
- Ethnicity
- Intervention details: type of intervention, description of intervention, frequency and duration of intervention
- comparator group(s)
- Outcomes measures
Assessment of risk of bias in included studies
Risk of bias assessed in included studies using the Cochrane Collaboration’s Risk of Bias tool. The tool includes the following domains: random sequence generation; allocation concealment; blinding of participants and personnel; blinding of outcome assessment; incomplete outcome data; selective reporting; and other sources of bias. Any disagreement will be resolved by consensus, by consulting a third author.
Measures of treatment effect
We used only dichotomous outcomes we used the odds ratio and its 95% CI was calculated.
Unit of analysis issues
The unit of analysis was individuals. After adjustment for possible confounding, data derived from cluster-randomized controlled trials produced same results. We included clusterrandomized trials in the meta-analysis along with individuallyrandomized trials. We adjusted for design effect using an ‘approximation method’.
Dealing with missing data
We did not experience any missing data in this systematic review
Assessment of heterogeneity
Heterogeneity between trials was assessed by visual inspection of forest plots, by estimation of the percentage of I2 between trials which could be ascribed to sampling variation, by a formal statistical test of the significance of the heterogeneity and, if possible, by sub-group analyses. If we find substantial heterogeneity, the possible reasons for this was investigated and reported.
Assessment of reporting biases
Funnel plots corresponding to meta-analysis of the primary outcome was examined if we have 10 or more studies. We then assessed the potential for small study effects. If there is evidence of small-study effects, publication bias was considered as only one of a number of possible explanations. If these plots suggested that treatment effects may not be sampled from a symmetric distribution, as assumed by the random effects model, sensitivity analyses was carried out using fixed effects models.
Data synthesis
Data synthesis was based on the heterogeneity of the studies. When heterogeneity was not too large, we performed a metaanalysis. In the presence of homogeneity, we used a fixed-effect model for the meta-analysis. In the case of moderate or high heterogeneity, we used a random-effects model to produce the overall results.
Results
Results of the search

Included studies
Seventeen studies were included in this systematic review (see annex tables: Characteristics of included studies). Twelve RCTs [2,37-47], two cluster randomized control trials [48,49], two quasi-randomized control trial [50,51] and one non randomized control trial [52].
Excluded studies
Ten studies were excluded from the review among which [53- 62] (see annex tables: Characteristics of excluded studies)
Risk of bias in included studies
Allocation (selection bias)
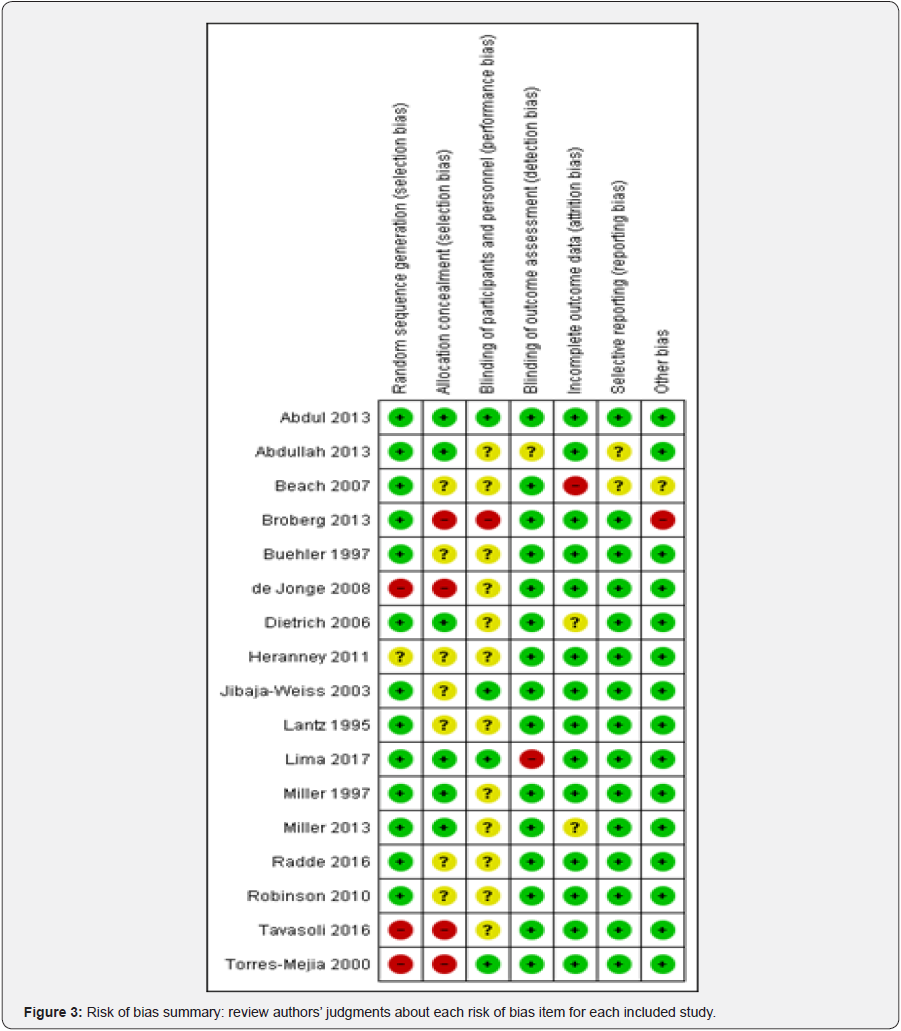
Allocation concealment was minimized in Abdul [37]; Abdullah F [48]; Dietrich [40]; Lima [51]; Miller [44]; Miller [2]; Radde [45]. In Beach [49]; Buehler [39]; Heranney [41]; Jibaja- Weiss [42]; Lantz [43]; Robinson [46], selection bias was unclear, therefore high in Broberg [38]; de Jonge [50]; Tavasoli [52]; Torres-Mejia [47].
Blinding (performance bias and detection bias)
Bias assessment stool revealed that performance bias was reduced in Abdul [37]; Abdullah [48]; Lima [51]; Jibaja-Weiss [42]; Torres-Mejia [47]. unclear Beach [49]; Buehler [39]; de Jonge [50]; Dietrich [40]; Heranney [41]; Lantz [43]; Miller [44]; Miller [2]; Radde [45]; Robinson [46]; Tavasoli [52] and high Broberg [38].
Incomplete outcome data (attrition bias)
We found that incomplete outcome data(attrition bias) Abdul [37]; Abdullah [48]; Broberg [38]; Buehler [39]; de Jonge [50]; Heranney [41]; Jibaja-Weiss [42]; Lantz [43]; Lima [51]; Miller [44]; Radde [45]; Robinson [46]; Tavasoli [52]; Torres-Mejia [47] were low risk of bias, Dietrich [40]; Miller [2] were unclear and Beach [49] was high.
Selective reporting (reporting bias)
Critical appraisal revealed that Abdul [37]; Broberg [38]; Buehler [39]; de Jonge [50]; Dietrich [40]; Heranney [41]; Jibaja- Weiss [42]; Lima [51]; Radde [45]; Tavasoli [52] were low risk of bias. Therefore Lantz [43]; Miller [44]; Miller [2]; Robinson [46]; Torres-Mejia [47] were unclear and Abdullah [48]; Beach [49] were high risk of bias
Other potential sources of bias
We judged as low risk of bias Abdul [37]; Abdullah [48]; Buehler [39]; de Jonge [50]; Dietrich [40]; Jibaja-Weiss [42]; Lantz [43]; Lima [51]; Miller [44]; Miller [2]; Radde [45]; Robinson [46]; Tavasoli [52]; Torres-Mejia [47] as unclear Beach [49]and Broberg [38]; Heranney [41] were judged as high risk of bias (Figure 2 & 3).
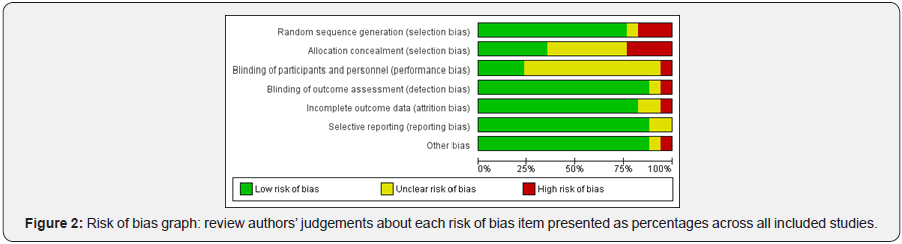
Summary of Main Results
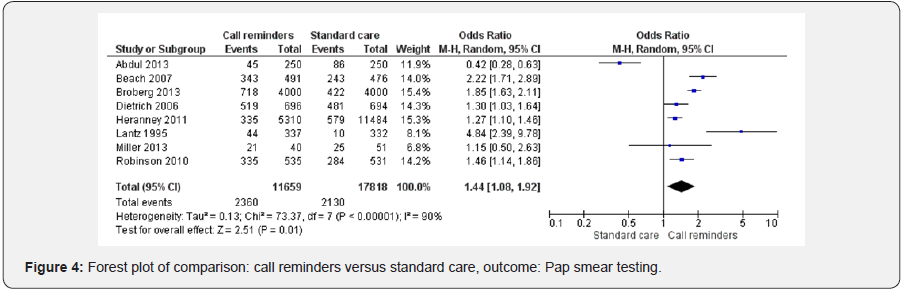
Call reminders and cervical cancer screening
Height studies [2,37,38,40,41,43,46,49] were included in the forest plot analyzing the effect of call reminders on cervical cancer screening in risk women. Call reminders were statistically significant in increasing cervical cancer screening compared to the standard care (OR 1.44 95% CI 1.08, 1.92, 29477 participants, 8 studies, Heterogeneity: Tau² = 0.13; Chi² = 73.37, df = 7 (P < 0.00001); I² = 90%, random effects). Test for overall effect: Z = 2.51 (P = 0.01) (Figure 4).
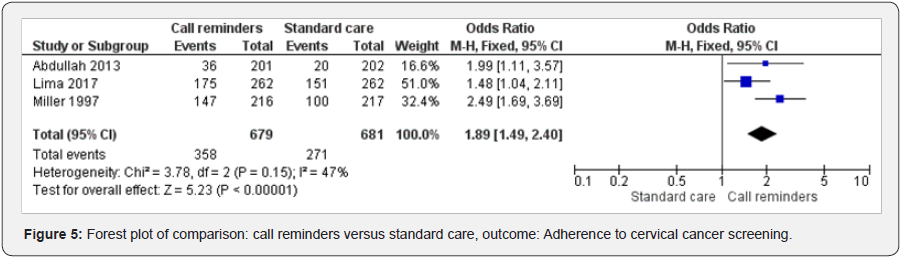
Call reminders and adherence to cervical cancer screening
Three studies [48,51,44] were included in examining the effect of call reminders on cervical cancer screening adherence. Call reminders versus standard care has shown statistically significant results (OR 1.89 95% CI 1.49, 2.40, 1360 participants, 3 studies). Heterogeneity: Chi² = 3.78, df = 2 (P = 0.15); I² = 47%, fixed effects). Test for overall effect: Z = 5.23 (P < 0.00001) (Figure 5).
Letter reminders and cervical cancer screening
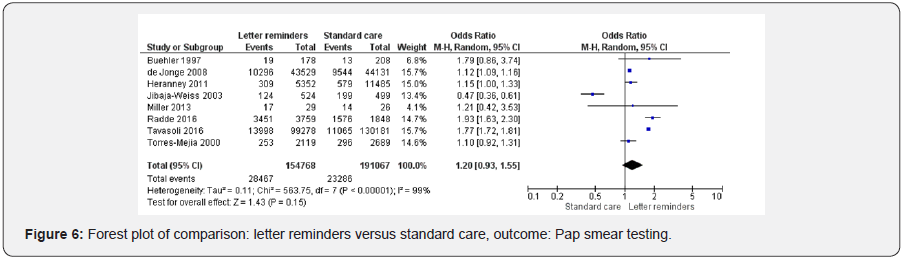
Height studies were included in letters reminders versus standard care [39,41,42,2,45,52,47,50]. Letter reminders did not improve cervical cancer screening (OR 1.20 95% CI 0.93, 1.55, 345835 participants, 8 studies, Heterogeneity: Tau² = 0.11; Chi² = 563.75, df = 7 (P < 0.00001); I² = 99%, random effects). Test for overall effect: Z = 1.43 (P = 0.15) (Figure 6).
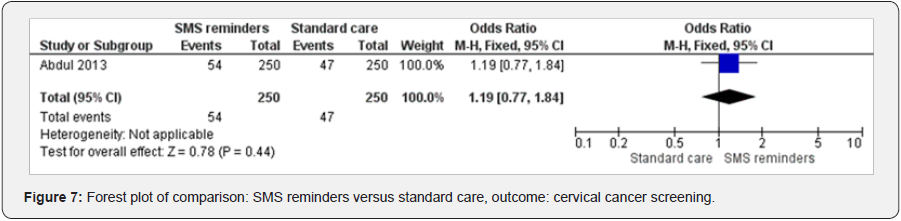
SMS reminders and cervical cancer screening
One study analyzed the effect of SMS reminders on cervical cancer [37]. SMS reminders increased cervical cancer screening (OR 1.19 95%CI 0.77 to 1.84, 500 participants, 1 study, test for heterogeneity not applicable, fixed effects) (Figure 7).
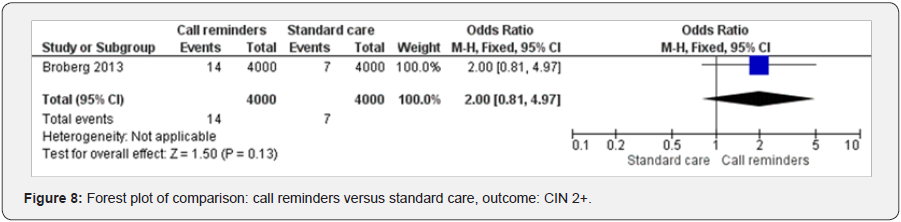
Call reminders and CN 2+
One study examined the effect of call reminders on diagnosing CN 2+ [38]. The result has shown the call reminders improved CN 2+ diagnostic (OR 2.00 95% CI 0.81 to 4.97, 8000 participants, 1 study, test for heterogeneity not applicable, fixed effects) (Figure 8).
Discussion
The overall completeness and applicability of evidence could be judged high when we considered the impact of call reminders on cervical cancer screening and adherence to screening. This illustrated the strength of this review. Then, this study could influence public health policy in screening cervical cancer in risk population. This evidence is strengthened by a recent review that has shown automated telephone communication systems interventions can modify patients’ health behaviors, improve clinical outcomes and increase healthcare uptake with positive effects in multiple health areas among which immunization, screening, appointment attendance, and adherence to medications or tests [63].
Letter reminders have shown to improve cervical cancer screening outcomes; therefore the results were not statistically significant compared to recent studies conducted in this field [45,52]. The quality of evidence was moderate when analyzing the effect of letter reminders on cervical cancer screening in risk population. Letter reminders could still constitute an option in improving cervical cancer screening. However, these strategies would be challenging to implement in the context of a jurisdictionally centralized screening program [52].
We found only one RCT that investigated the effect of SMS on cervical cancer screening. The result was not significant. In addition, the quality of evidence was moderate. Further studies should be conducted in this field even if several reviews have shown positive effect of short messaging on health outcomes. Only one RCT was found in analyzing the impact of mHealth on the diagnosis of cervical intraepithelial neoplasia grade 2. The quality of evidence was moderate; the overall result was not significant. Therefore, further studies are needful in this field.
Telephone interventions is a resource associated with the nursing practice, which can produce significant changes in the health outcomes, highlighting the importance of technical and clinical knowledge for the interventions by the professional [51]. Furthermore, the use of technology for healthcare development requires trained professionals to promote the convergence between human development and technological knowledge, aiming at the desired goals [51].
The lack of high-quality evidence on the prevention of cervical cancer for high risk women, which is important for implementing efficient screening and treatment strategies, results then in the absence of a clearly defined health program in low and middle income countries [13]. This is responsible for the low screening uptake and high mortality rates [13].
As said above, several knowledge gaps might inhibit women from undergoing cervical cancer screening. This review could be useful in overcoming certain gaps, and then cervical cancer screening could be ameliorated.
Authors’ conclusion
Nowadays, the risk of developing cervical precancerous and cancerous lesions is high; therefore close monitoring and specific schedule for follow constitute a big challenge. This review supports the use of call reminders in improving cervical cancer screening and adherence to testing. The level of evidence is high.Then, call reminders could be suggested to be incorporated in different national policy in screening cervical cancer in risk populations. The lack of sufficient evidence on the subject limits the reliability of the current cervical cancer screening guidelines for high risk women is the leading cause of diagnosing cervical cancer in the last stage. Further studies in this field will provide more solid foundations for preventing cervical cancer. However, this review could orientate public health policy makers.
Acknowledgment
We are grateful for all the review team in their different contributions.
References
- http://www.who.int/reproductivehealth/topics/cancers/fightcervical- cancer/en/
- Miller SM, Hui SK, Wen KY, Scarpato J, Zhu F, et al. (2013) Tailored telephone counseling to improve adherence to follow-up regimens after an abnormal pap smear among minority, underserved women. Patient education and counseling 93(3): 488-495.
- Wenzel L, Osann K, Hsieh S, Tucker JA, Monk BJ, et al. (2015) Psychosocial telephone counseling for survivors of cervical cancer: results of a randomized biobehavioral trial. Journal of clinical oncology: official journal of the American Society of Clinical Oncology 33(10): 1171-1179.
- http://www.who.int/mediacentre/factsheets/fs297/en/
- Ali-Risasi C, Verdonck K, Padalko E, VandenBroeck D, Praet M, et al. (2015) Prevalence and risk factors for cancer of the uterine cervix among women living in Kinshasa, the Democratic Republic of the Congo: a cross-sectional study [Computer program].Infect age cancer 10: 20.
- Kumakech E, Andersson S, Wabinga H, Berggren V (2015) Integration of HIV and cervical cancer screening perceptions and preferences of communities in Uganda. BMC women’s health 15: 23.
- Bouvard V, Baan R, Straif K, Grosse Y, Secretan B, et al. (2009) A review of human carcinogens--Part B: iological agents. Lancet Oncol 10(4): 321-322.
- Denny L, Adewole I, Anorlu R, Dreyer G, Moodley M, et al. (2014) Human papillomavirus prevalence and type distribution in invasive cervical cancer in sub-Saharan Africa. International journal of cancer. Journal international du cancer 134(6): 1389-1398.
- Bruni L, Diaz M, Castellsague X, Ferrer E, Bosch FX, et al. (2010) Cervical human papillomavirus prevalence in 5 continents: meta-analysis of 1 million women with normal cytological findings. J Infect Dis 202(12): 1789-1799.
- Maranga IO, Hampson L, Oliver AW, Gamal A, Gichangi P, Opiyo A, et al.(2013)Analysis of factors contributing to the low survival of cervical cancer patients undergoing radiotherapy in Kenya. PloS one 8(10): e78411.
- Massad LS, Einstein MH, Huh WK, Katki HA, Kinney WK, Schiffman M, et al. (2013) Updated consensus guidelines for the management of abnormal cervical cancer screening tests and cancer precursors. Obstetrics and gynecology 122(4): 393.
- Denslow SA, Rositch AF, Firnhaber C, Ting J, Smith JS, et al. (2014) Incidence and progression of cervical lesions in women with HIV: a systematic global review. International journal of STD & AIDS 25(3): 163-77.
- Viviano M, DeBeaudrap P, Tebeu PM, Fouogue JT, Vassilakos P, Petignat P,et al. (2107) A review of screening strategies for cervical cancer in human immunodeficiency virus-positive women in sub-Saharan Africa. International journal of women’s health 9: 69-79.
- Rosser JI, Njoroge B, Huchko MJ (2015) Knowledge about cervical cancer screening and perception of risk among women attending outpatient clinics in rural Kenya. International journal of gynaecology and obstetrics: the official organ of the International Federation of Gynaecology and Obstetrics 128(3): 211-5.
- Sankaranarayanan R, Budukh AM, Rajkumar R (2001) Effective screening programmes for cervical cancer in low- and middle-income developing countries. Bulletin of the World Health Organization 79(10): 954-62.
- Arbyn M, Ronco G, Anttila A, Meijer CJ, Poljak M, Ogilvie G, et al. (2012) Evidence regarding human papillomavirus testing in secondary prevention of cervical cancer. Vaccine 31(52): 6266.
- Miller B, Morris M, Rutledge F, Mitchell MF, Atkinson EN, Burke TW, et al. (1993) Aborted exenterative procedures in recurrent cervical cancer. Gynecologic oncology 50(1): 94-99.
- Makin-Byrd K, Azar ST (2011) Beliefs and attributions of partner violence perpetrators: the physical and psychological violence of adolescent males. Violence and victims 26(2): 177-190.
- Kivuti-Bitok LW, Pokhariyal GP, Abdul R, McDonnell G (2013) An exploration of opportunities and challenges facing cervical cancer managers in Kenya. BMC research notes 6: 136.
- Gurman TA, Rubin SE, Roess AA (2012) Effectiveness of mHealth behavior change communication interventions in developing countries: a systematic review of the literature. Journal of health communication 17Suppl 1: 82-104.
- Tamrat T, Kachnowski S (2012) Special delivery: an analysis of mHealth in maternal and newborn health programs and their outcomes around the world. Maternal and child health journal 16(5): 1092-101.
- Chib A, Wilkin H, Hoefman B (2013) Vulnerabilities in mHealth implementation: a Ugandan HIV/AIDS SMS campaign. Global health promotion 20(1 Suppl): 26-32.
- Downer SR, Meara JG, Da Costa AC, Sethuraman K (2006) SMS text messaging improves outpatient attendance. Australian health review: a publication of the Australian Hospital Association 30(3): 389-396.
- Hardy S, White J, Deane K, Gray R(2011) Educating healthcare professionals to act on the physical health needs of people with serious mental illness: a systematic search for evidence. Journal of psychiatric and mental health nursing 18(8): 721-727.
- Coomes CM, Lewis MA, Uhrig JD, Furberg RD, Harris JL, Bann CM,et al. (2012) Beyond reminders: a conceptual framework for using short message service to promote prevention and improve healthcare quality and clinical outcomes for people living with HIV. AIDS care 24(3): 348-357.
- Gonzalez AB, Salas D, Umpierrez GE (2011) Special considerations on the management of Latino patients with type 2 diabetes mellitus. Current medical research and opinion 27(5): 969-979.
- Zelaya CE, Sivaram S, Johnson SC, Srikrishnan AK, Suniti S, Celentano DD,et al. (2012) Measurement of self, experienced, and perceived HIV/ AIDS stigma using parallel scales in Chennai, India. AIDS care 24(7): 846-855.
- Cook SA, Salmon P, Dunn G, Holcombe C, Cornford P, Fisher P,et al. (2015) The association of metacognitive beliefs with emotional distress after diagnosis of cancer. Health psychology : official journal of the Division of Health Psychology, American Psychological Association 34(3): 207-215.
- Shepherd HL, Butow PN, Tattersall MH(2011)Factors which motivate cancer doctors to involve their patients in reaching treatment decisions. Patient education and counseling 84(2): 229-235.
- Kreuter MW, Oswald DL, Bull FC, Clark EM (2000) Are tailored health education materials always more effective than non-tailored materials? Health education research 15(3): 305-315.
- Hibbard JH, Stockard J, Mahoney ER, Tusler M (2004) Development of the Patient Activation Measure (PAM): conceptualizing and measuring activation in patients and consumers. Health services research 39(4 Pt 1): 1005-1026.
- Green JR (1988) Reports on health facility development and education. Australia. The Journal of health administration education 6(4 Pt 1): 857-61.
- Waqner CW (2001) Paired for life? Controlling allergic rhinitis can improve pediatric asthma. Advance for nurse practitioners 9(4): 72, 77-78.
- Franceschi S (2005) The IARC commitment to cancer prevention: the example of papillomavirus and cervical cancer. Recent results in cancer research 166: 277-297.
- Harper DM (2004) Why am I scared of HPV? CA: a cancer journal for clinicians 54(5): 245-247.
- Lim JN, Ojo AA (2017) Barriers to utilisation of cervical cancer screening in Sub Sahara Africa: a systematic review. European journal of cancer care 26(1).
- Abdul Rashid RM, Mohamed M, Hamid ZA, Dahlui M et al. (2013) Is the phone call the most effective method for recall in cervical cancer screening?--results from a randomised control trial. Asian Pacific journal of cancer prevention: APJCP 14(10): 5901-5904.
- Broberg G, Jonasson JM, Ellis J, Gyrd-Hansen D, Anjemark B, Glantz A, et al. (2013) Increasing participation in cervical cancer screening: telephone contact with long-term non-attendees in Sweden. Results from RACOMIP, a randomized controlled trial. International journal of cancer 133(1): 164-171.
- Buehler SK, Parsons WL (1997) Effectiveness of a call/recall system in improving compliance with cervical cancer screening: a randomized controlled trial. CMAJ 157(5): 521-526.
- Dietrich AJ, Tobin JN, Cassells A, Robinson CM, Greene MA, Sox CH, et al. (2006) Telephone care management to improve cancer screening among low-income women: a randomized, controlled trial. Annals of internal medicine 144(8): 563-571.
- Heranney D, Fender M, Velten M, Baldauf JJ (2011) A prospective randomized study of two reminding strategies: telephone versus mail in the screening of cervical cancer in women who did not initially respond. Actacytologica 55(4): 334-340.
- Jibaja-Weiss ML, Volk RJ, Kingery P, Smith QW, Holcomb JD, et al. (2003) Tailored messages for breast and cervical cancer screening of low-income and minority women using medical records data. Patient education and counseling 50(2): 123-132.
- Lantz PM, Stencil D, Lippert MT, Beversdorf S, Jaros L, Remington PL, et al. (1995) Breast and cervical cancer screening in a low-income managed care sample: the efficacy of physician letters and phone calls. American journal of public health 85(6): 834-836.
- Miller SM, Siejak KK, Schroeder CM, Lerman C, Hernandez E, Helm CW. Enhancing adherence following abnormal Pap smears among lowincome minority women: a preventive telephone counseling strategy. Journal of the National Cancer Institute 1997;89(10):703-8.
- Radde K, Gottschalk A, Bussas U, Schulein S, Schriefer D, Seifert U, et al. (2016) Invitation to cervical cancer screening does increase participation in Germany: Results from the MARZY study. International journal of cancer 139(5): 1018-1030.
- Robinson CM, Beach ML, Greene MA, Cassells A, Tobin JN, Dietrich AJ, et al. (2010) Staffing time required to increase cancer-screening rates through telephone support. The Journal of ambulatory care management 33(2): 143-154.
- Torres-Mejia G, Salmeron-Castro J, Tellez-Rojo MM, Lazcano-Ponce EC, Juarez-Marquez SA, Torres-Torija I, et al .(2000) Call and recall for cervical cancer screening in a developing country: a randomised field trial. International journal of cancer 87(6): 869-873.Top of Form
- Abdullah F, Su TT (2013) Applying the Transtheoretical Model to evaluate the effect of a call-recall program in enhancing Pap smear practice: a cluster randomized trial. Preventive medicine 57: S83-6.
- Beach ML, Flood AB, Robinson CM, Cassells AN, Tobin JN, Greene MA, et al. (2017) Can language-concordant prevention care managers improve cancer screening rates? Cancer epidemiology, biomarkers & prevention: a publication of the American Association for Cancer Research, cosponsored by the American Society of Preventive Oncology 16(10): 2058-2064.
- de Jonge E, Cloes E, Op de Beeck L, Adriaens B, Lousbergh D, Orye GG, et al. (2008) A quasi-randomized trial on the effectiveness of an invitation letter to improve participation in a setting of opportunistic screening for cervical cancer. European journal of cancer prevention: the official journal of the European Cancer Prevention Organisation (ECP) 17(3): 238-242.
- Lima TM, Nicolau AI, Carvalho FH, Vasconcelos CT, Aquino PS, et al. (2017) Telephone interventions for adherence to colpocytological examination. Rev Lat Am Enfermagem 25: e2844.
- Tavasoli SM, Pefoyo AJ, Hader J, Lee A, Kupets R, et al. (2016) Impact of invitation and reminder letters on cervical cancer screening participation rates in an organized screening program. Preventive medicine 88: 230-236.
- Bergmeir C, Garcia Silvente M, Benitez JM (2012) Segmentation of cervical cell nuclei in high-resolution microscopic images: A new algorithm and a web-based software framework. Computer methods and programs in biomedicine 107(3): 497-512.
- Catarino R, Vassilakos P, Scaringella S, Undurraga-Malinverno M, Meyer-Hamme U, et al. (2015) Smartphone Use for Cervical Cancer Screening in Low-Resource Countries: A Pilot Study Conducted in Madagascar. PloS one 10(7): e0134309.
- Del Mistro A, Frayle H, Ferro A, Fantin G, Altobelli E, et al. (2017) Efficacy of self-sampling in promoting participation to cervical cancer screening also in subsequent round. Preventive medicine reports 166- 168.
- Eichhorn JH, Brauns TA, Gelfand JA, Crothers BA, Wilbur DC, et al. (2005) A novel automated screening and interpretation process for cervical cytology using the internet transmission of low-resolution images: a feasibility study. Cancer 105(4): 199-206.
- Giorgi Rossi P, Fortunato C, Barbarino P, Boveri S, Caroli S, et al. (2015) Self-sampling to increase participation in cervical cancer screening: an RCT comparing home mailing, distribution in pharmacies, and recall letter. British journal of cancer 112(4): 667-675.
- Kobetz E, Seay J, Amofah A, Pierre L, Bispo JB, et al. (2017) Mailed HPV self-sampling for cervical cancer screening among underserved minority women: study protocol for a randomized controlled trial. Trials 18(1): 19.
- Quinley KE, Gormley RH, Ratcliffe SJ, Shih T, Szep Z, et al. (2011) Use of mobile telemedicine for cervical cancer screening. Journal of telemedicine and telecare 17(4): 203-209.
- Ricard-Gauthier D, Wisniak A, Catarino R, van Rossum AF, Meyer- Hamme U, et al. (2015) Use of Smartphones as Adjuvant Tools for Cervical Cancer Screening in Low-Resource Settings. Journal of lower genital tract disease 19(4): 295-300.
- Sherman ME, Dasgupta A, Schiffman M, Nayar R, Solomon D, et al. (2007) The Bethesda Interobserver Reproducibility Study (BIRST): a web-based assessment of the Bethesda 2001 System for classifying cervical cytology. Cancer 111(1): 15-25.
- Yabroff KR, Zapka J, Klabunde CN, Yuan G, Buckman DW, et al. (2011) Systems strategies to support cancer screening in U.S. primary care practice. Cancer epidemiology, biomarkers & prevention: a publication of the American Association for Cancer Research, cosponsored by the American Society of Preventive Oncology 20(12): 2471-2479.Top of Form
- Posadzki P, Mastellos N, Ryan R, Gunn LH, Felix LM, et al. (2016) Automated telephone communication systems for preventive healthcare and management of long-term conditions. The Cochrane database of systematic reviews 12: CD009921.






























