Assessment of the Biofield Energy Healing-Based Proprietary Test Formulation on Modulation of Immune Response in Human Monocytic Cell Line (THP-1)
Mahendra Kumar Trivedi1, Alice Branton1, Dahryn Trivedi1 and Snehasis Jana2*
1Trivedi Global, Inc., Henderson, USA
2Trivedi Science Research Laboratory Pvt. Ltd., India
Submission: April 21, 2021; Published: May 18, 2021
*Corresponding author: Snehasis Jana, Trivedi Science Research Laboratory Pvt. Ltd., Thane (W), Maharashtra, India
How to cite this article: Mahendra Kumar Trivedi, Alice Branton, Dahryn Trivedi and Snehasis Jana. Assessment of the Biofield Energy Healing-Based Proprietary Test Formulation on Modulation of Immune Response in Human Monocytic Cell Line (THP-1). Int J Cell Sci & Mol Biol. 2023; 7(1): 555702. DOI: 10.19080/IJCSMB.2023.07.555702
Abstract
The present study was aimed to evaluate the effect of the Trivedi Effect®- Biofield Energy Treated/Blessed Test formulation/Item (TI) composed of minerals (magnesium, zinc, copper, calcium, selenium, and iron), vitamins (ascorbic acid, pyridoxine HCl, alpha tocopherol, cyanocobalamin, and cholecalciferol), Panax ginseng extract, CBD isolates, and β-carotene on cytokine (IL-6 and TNF-α) levels in Human monocytic cell line (THP-1) cell line in RPMI. Each constituents of test formulation were divided into two parts; one section was defined as the untreated test formulation (UT), while the other portion of the test formulation received Biofield Energy Healing/Blessing Treatment (BT) by a renowned Biofield Energy Healer, Mr. Mahendra Kumar Trivedi. The test items were treated with Biofield Energy Healing Treatment/Blessing and divided as Biofield Energy Treated (BT) and untreated (UT) test items. MTT data showed that the test formulation in various concentrations was found as safe and nontoxic in the tested concentrations with viability range 77% to 134%. IL-6 level was significantly inhibited by 48.7% and 21.1% at 5 and 10μg/mL, respectively in the BT-RPMI+UT-TI group, while 11.8%, 20.8%, and 44% inhibition of IL-6 was reported at 0.01, 5, and 10μg/mL respectively, in the UT-RPMI+BT-TI group, and 422.9%, 84.2%, and 14.7% inhibition of IL-6 at 0.01, 0.1, and 10μg/mL, respectively in the BT-RPMI+BT-TI group as compared with the untreated test formulation and RPMI group. Similarly, TNF-α inhibition by 26.1%, 29.2%, and 155.2% in the UT-RPMI+BT-TI group at 0.01, 0.1, and 5μg/mL respectively, as compared with the untreated group. However, BT-RPMI+UT-TI group showed a significant inhibition of TNF-α by 50% and 14.9% at 0.01 and 10μg/mL, respectively, while BT-RPMI+BT-TI group showed a significant inhibition of TNF-α by 46.4%, 17.5%, 21.2%, and 228.4% at 0.01, 0.1, 1, and 10μg/mL, respectively as compared with the untreated test formulation and RPMI group. Overall, the in vitro cell line data suggested significance inhibition of pro-inflammatory cytokines (TNF-α and IL-6) at all the testes concentration of Biofield Energy Treated test formulation. Overall, the experimental results showed the significant improved immune profile by regulation of cytokines profile, which can be used against immunity disorders, mental illness, stress, lethargic conditions, alertness, migraine, minimizing toxic effects of free radicals, and many other immune related disorders.
Keywords: Biofield treatment; IL-6; TNF-α; The Trivedi Effect®; TMP-1
Introduction
Immune system plays a vital role in regulation of health of humans and animals. Immunity and immune functions can be varied by various factors including diets known as immune modulation [1]. To study the immunity profile and to study the effect of any test compound, cell line activity using estimation of cytokines would be the meaningful approach because it minimizes the effects of culture period and genetic variation [2]. In addition, it can also provide mechanistic approach and detailed information of any test compound or plant based novel formulations [3]. In vitro-ex vivo systems are the initial step forward to mimic the in vivo situation and to study the immunity parameters; THP-1 (human leukemia monocytic cell line) has been extensively used to evaluate the monocyte/macrophage functions, signaling pathway, mechanism, and drug transport. Cytokines are the small, secreted proteins derived from immune cells, which play a major role in the differentiation, maturation, and activation of different immune function. IL-6 and TNF-α was used as the major cytokines estimation in lipopolysaccharide (LPS) induced THP-1 cell line. Anti-inflammatory cytokines are involved in most of the immune-related disorders and defined as the standard parameter for evaluation the effect test compound. Cytokines are divided based on the specific local microenvironment i.e., proinflammatory or anti-inflammatory effects, or both [4,5]. They are responsible to create the bridge between innate and adaptive immune systems [6,7]. The present study focused on the detection of pro-inflammatory cytokines as a major target in THP-1 cell line. Thus, a novel test formulation was designed with the combination of vital minerals (Ca, Zn, Mg, Se, Fe, Cu), vitamins (B12, E, D3, C, B6), and some biological active plant-based extracts such as β-carotene, Ginseng, and cannabidiol isolate (CBD). Vitamins are the major source that builds immunity using different pathways to boost energy level. Vitamin C, D are the major immunity-based vitamins along with minerals used in the test formulations. All the minerals and vitamins used in the test formulation have significant functional role to provide vital physiological role [8-10]. Besides, cannabidiol itself has wide range of pharmacological profile and was reported to role in different disorders [11,12], while ginseng extract is regarded as the one of the best immune boosters for overall immunity [13]. Thus, using this novel test formulation, present study was designed to study the effect of test formulation on the level of cytokines in presence of LPS. The test formulation was treated with Biofield Energy Treatment (a Complementary and Alternative Medicine, CAM) by a renowned Biofield Energy Healer, on THP-1 cell line. Biofield Energy Healing Treatment has been considered as a Complementary and Alternative Medicine (CAM) treatment approach, which is also recommended by National Center for Complementary/Alternative Medicine (NCCAM) due to its significant effects against various disorders [14-16], when used along with the conventional treatment approach [17]. There are some other CAM therapies that have been accepted by the National Centre of Complementary and Integrative Health (NCCIH) along with Biofield Energy Healing, such as deep breathing, Tai Chi, yoga, therapeutic touch, Reiki, chiropractic/osteopathic manipulation, relaxation techniques, pranic healing, meditation, homeopathy, Ayurvedic medicine, movement therapy, mindfulness, traditional Chinese herbs, and medicines in biological systems, etc. [18,19]. The impact of the Trivedi Effect®-Consciousness Energy Healing/Blessing Treatment is similarly found to be useful due to its beneficial impact on various living and non-living things. Its effect has been reported by various scientific studies in different disciplines such as materials science [20,21], agriculture science [22], antiaging [23], gut health [24], nutraceuticals [25], pharmaceuticals [26], and overall human health and wellness. In this study, the authors sought to study the impact of the Biofield Energy Treatment (the Trivedi Effect®) on the given novel test formulation on the level of cytokines (IL-6 and TNF-α) levels in THP-1 cell line.
Material and Methods
Chemicals and reagents
Pyridoxine hydrochloride (vitamin B6), calcitriol, zinc chloride, magnesium (II) gluconate, and β-carotene (retinol, provit A) were purchased from TCI, Japan. Copper chloride, cyanocobalamin (vitamin B12), calcium chloride, vitamin E (Alpha-Tocopherol), cholecalciferol (vitamin D3), iron (II) sulfate, and sodium carboxymethyl cellulose (Na-CMC) were procured from Sigma- Aldrich, USA. Ascorbic acid (vitamin C) and sodium selenate were obtained from Alfa Aesar, India. Cannabidiol isolate and Panax ginseng extract were obtained from Panacea Phytoextracts, India and Standard Hemp Company, USA, respectively. Imipramine Hydrochloride was purchased from Sigma, USA. Human monocytic cell line (THP-1) cell line was procured from NCCS, Pune.
Cell culture
THP-1 was used as test system in the present study. The cell line was maintained in RPMI growth medium for routine culture supplemented with 10% FBS. Growth conditions were maintained as 37°C, 5%CO2, and 95% humidity and subculture by trypsinization followed by splitting the cell suspension into fresh flasks and supplementing with fresh cell growth medium. Three days before the start of the experiment (i.e., day -3), the growth medium of near-confluent cells was replaced with fresh phenolfree RPMI, supplemented with 10% charcoal dextran stripped FBS (CD-FBS) and 1% penicillin-streptomycin [27].
Experimental design
The experimental groups consisted of cells in baseline control, vehicle control groups (0.05% DMSO with Biofield Energy Treated/Blessed and untreated RPMI-1640), positive control group (calcitriol) and five different experimental test groups. The experimental groups included the combination of the Biofield Energy Treated/Blessed and untreated test formulation/Medium (RPMI-1640). It consisted of five major treatment groups on specified cells with Untreated-RPMI + Untreated-Test item (UTTI), UT-RPMI + Biofield Energy Treated/Blessed test item (BT-TI), BT-RPMI + UT-TI, and BT-RPMI + BT-TI.
Consciousness energy healing strategies
The novel test formulation was consisted of zinc chloride, iron (II) sulfate, copper chloride, vitamin B6, vitamin B12, vitamin D3, sodium selenate, calcium chloride, ascorbic acid, vitamin E, beta carotene, Panax ginseng extract, cannabidiol and magnesium (II) gluconate. Each ingredient of the novel test formulation was divided into two parts. The test formulation was divided into two parts, one part of the test compound was not received any sort of treatment and were defined as the untreated or control sample. The second part of the test formulation was treated with the Trivedi Effect®-Energy of Consciousness Healing Treatment/ Blessing (Biofield Energy Treatment) by a renowned Biofield Energy Healer, Mr. Mahendra Kumar Trivedi under laboratory conditions for ~3 minutes in the research laboratory of Dabur Research Foundation, New Delhi, India. The energy transmission was done without touching the samples. After that, the Biofield Energy Treated samples was kept in the similar sealed condition and used as per the study plan. In the same manner, the control test formulation group was subjected to “sham” healer for ~3 minutes Energy Treatment, under the same laboratory conditions. The “sham” healer does not have any knowledge about the Biofield Energy Treatment. The Biofield Energy Treated/Blessed test medium was also taken back to experimental room for further culture methods.
Determination of non-cytotoxic concentration
The single cell suspension of THP-1 cells was prepared in RPMI with 10% FBS. The cells were counted on a hemocytometer, while the cells were seeded with specific cell density (30,000 cells/ well/180μL in RPMI-1640 + 10% FBS in 96-well plates). The cells were incubated in a CO2 incubator for 24 hours. After 24 hours, medium was removed, and following treatments were given in medium along with the 10% FBS in various experimental groups. After incubation for 24 hours, the effect of the test formulation on cell viability was assessed by 3-(4, 5-dimethylthiazol-2-yl)- 2,5-diphenyl tetrazolium bromide (MTT) assay. A 20μL of 5mg/ mL of MTT was added to all the wells and incubated at 37°C for 3 hours. The cells were centrifuged to obtain the pellet. The supernatant was removed and 150μL of DMSO was added to all wells to dissolve formazan crystals. Further, all the wells were reported using optical density (OD) values at 540nm using Biotek Reader. The effect of the test formulation on viability of cells was determined using equation 1.
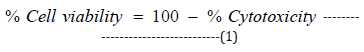
where; % Cytotoxicity = {(O.D. of Control cells – O.D. of cells treated with test formulation)/ OD of Control cells} *100
Estimation of an immunomodulation activity
The single cell suspension of THP-1 cells was prepared in RPMI and 10% FBS using a hemocytometer. The cells were seeded density of 0.25 X 106 cells/well/0.5mL in 48-well plates and incubated in a CO2 incubator for 24 hours and 95% humidity. The cells were centrifuged to obtain the pellet. Supernatants were removed and cells were resuspended in medium RPMI-1640 + 0% FBS. After treatment in all the experimental test groups, the cells were incubated in a 5% CO2 incubator for 24 hours. After 24 hours of incubation, culture supernatants were collected from each well and stored at -20°C until analysis. Further, the level of cytokines (IL-6 and TNF-α) in culture supernatants of THP-1 cells was determined using ELISA as per manufacturer’s instructions. In general, ELISA assay was performed using assay diluents, which was added to each well followed by sample addition (supernatants of cells) were directly pipetted into the wells and incubated for 2 hours at room temperature (RT). After washing away any unbound substances for a total of 5 times, 200μL of IL-6/TNF-α conjugate was added to each well and incubated for 2 hours at RT. Following a wash (5 times) to remove any unbound conjugate, 200μL of substrate solution was added to the wells and incubated for 30 minutes at RT in dark. The reaction was stopped by adding 50μL stop solution to each well. The optical density of the sample was measured at 450nm. Further, the concentration of IL-6 & TNF-α (pg/ml) was determined using the standard curve. Inhibitory effect of the Test Items on secretion of IL-6 & TNF-α against LPS stimulated level was determined as follows:
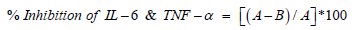
Where A = Concentration of cytokines (pg/mL) in the Control cells (stimulated with LPS alone) and B = Concentration of cytokines (pg/mL) in cells treated with test formulation/positive control + LPS
Statistical Analysis
The data were represented as mean ± standard error of mean (SEM) and subjected to statistical analysis using Sigma-Plot statistical software (Version 11.0). For multiple comparison Oneway analysis of variance (ANOVA) followed by post-hoc analysis by Dunnett’s test and for between two groups comparison Student’s t-test was performed. The p≤0.05 was considered as statistically significant.
Results and Discussion
MTT assay- non-cytotoxic effect of the test formulation
The cytotoxic effects of test item were tested on TMP-1 cell. The results were compared with respect defined positive controls such as calcitriols. The TMP-1 cell was treated with the test formulation for 24 hours. The effect on viability of cells was determined after 24 hours of treatment by MTT assay (Figure 1). The THP-1 cells were treated with the test formulation and in various experimental test group for 24 hours. Calcitriol (PC) demonstrated 121.2%, 128.1%, and 110.2% cell viability in the concentration 100 nM, 500 nM, and 1 μM respectively. Following test formulation resulted in more than 70% cell viability in the concentration range of 0.1μg/mL to 50μg/mL. The results of percentage cell viability range in all the tested cell lines showed the cell viability range of 77% to 134% in different test item groups with RPMI. These data suggests that the test formulation along with RPMI groups were found safe at all the tested concentrations range up to maximum of 10μg/mL against the tested TMP-1 cells.
Assessment of immunity assay on cytokines assay
Immunomodulatory effect of the test formulation was determined in THP-1 cells stimulated with LPS. The cells were cotreated with the test formulation and stimulated with 10μg/mL of LPS for 24 hours. The levels of IL-6 and TNF-α were determined by ELISA.
Determination of IL-6 against LPS stimulation
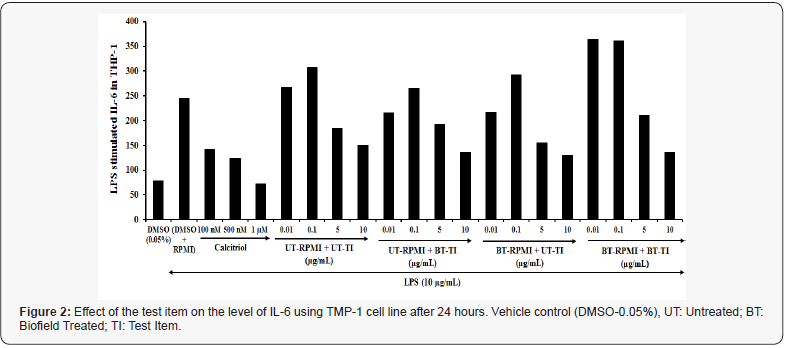
The result of IL-6 was determined among all the experimental test groups, and the results are demonstrated in (Figure 2). The data showed that the effect of the test formulation on secretion of IL-6 against LPS stimulation was significantly inhibited by the test formulation at various concentrations. Stimulation of THP 1 cells with inflammatory stimulus; LPS (10μg/mL) resulted in enhanced secretion of IL-6 by 210% as compared to the untreated cells. Calcitriol in the concentration range 100 nM, 500 nM, and 1 μM showed inhibition of IL-6 from 42.1%, 49.2%, and 70.5% as compared to the control cells (LPS stimulated). However, the experimental test group’s viz. untreated medium and Biofield Treated Test item (UT-RPMI+BT-TI) showed a significant inhibition of IL-6 by 11.8%, 20.8%, and 44% in the 0.01, 5 and 10μg/mL respectively as compared with the vehicle control, while 14.4% inhibition at 10μg/mL as compared with the untreated test formulation group. Biofield Treated medium and untreated Test item (BT-RPMI+UT-TI) showed a significant inhibition of IL-6 level 48.7% and 21.1% at 5 and 10μg/mL, respectively as compared with the untreated test formulation and RPMI group. However, the Biofield Energy Treated medium and Biofield Energy Treated Test item (BT-RPMI+BT-TI) showed a significant inhibition of IL-6 level by 422.9%, 84.2%, and 14.7% at 0.01, 0.1, and 10μg/mL, respectively as compared with the untreated test formulation and RPMI group. High level of IL-6 results in various level of stress, which leads to various immunological disorders. Thus, alteration and management in the level of cytokines has wide range of implicated in major depressive and immunological illness. This is also regarded as the innate inflammatory response to many physical stressors (e.g., depression, traumatic stress, infection, inflammation) [27,28]. Overall, all the experimental test groups showed a significant benefit with improved immunity by inhibition of IL-6 level at all the tested concentrations compared with the untreated test medium.

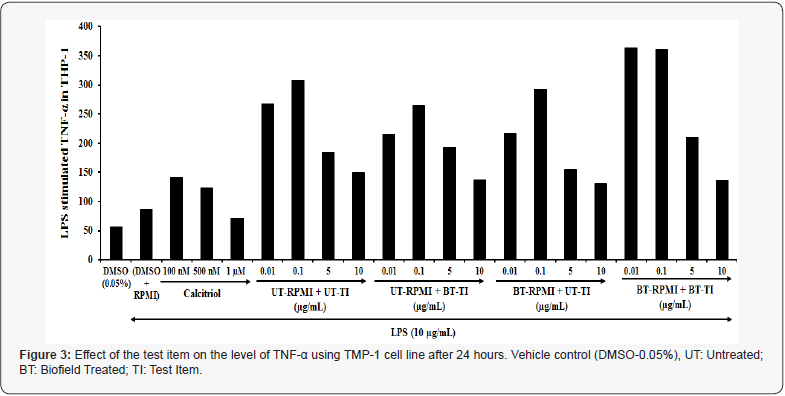
Determination of TNF-α against LPS stimulation
Similarly, TMP-1 cells also showed a significant inhibition of TNF-α as compared with the untreated test medium. The data showed a significant inhibition of TNF-α at various experimental concentrations on TMP-1 cell line (Figure 3). The positive control, calcitriol in TMP-1 cells showed a significant inhibition of TNF-α by 57.90%, 49%, and 28.40% at 100nM, 500nM, and 1μM, respectively. However, the experimental test group’s viz. untreated medium and Biofield Treated Test item (UT-RPMI+BT-TI) showed a significant inhibition of TNF-α by 26.1%, 29.2%, and 155.2% at 0.01, 0.1, and 5μg/mL respectively as compared with the untreated group, while Biofield Treated medium and untreated Test item (BT-RPMI+UT-TI) showed a significant inhibition of TNF-α by 50% and 14.9% at 0.01 and 10μg/mL, respectively as compared with the untreated test formulation and RPMI group. However, the Biofield Energy Treated medium and Biofield Energy Treated Test item (BT-RPMI+BT-TI) showed a significant inhibition of TNF-α by 46.4%, 17.5%, 21.2%, and 228.4% at 0.01, 0.1, 1, and 10μg/mL, respectively as compared with the untreated test formulation and RPMI group. Overall, all the experimental test groups showed a significant inhibition of TNF-α in the TMP-1 cells under LPS induced condition at all the tested concentrations compared with the untreated test medium. TNF-α, a proinflammatory cytokine is responsible for various inflammatory reactions in the body. These cells signaling protein are highly engaged in systemic inflammation along with its important role in acute phase reaction. TNF-α also mediates and regulates immune responses, inflammation, and immune activation, that may involve in depressive symptoms in many cases. Thus, its alteration may result in various immunological disorders [29,30]. In this research plan, the results showed the significant inhibition of IL-6 and TNF-α level in TMP-1 cell line, which helps in slowdown of the disease progression, disease-related all other symptoms/ complications and reduced the chances of disease susceptibility. Based on the overall data, it suggests that the Biofield Energy Healing Therapy was found to be most effective and benefited to prevent and protect from the occurrence of any type of diseases and can be used as significant way for energy boosting in various disease states that will ultimately improve the overall health and quality of life in human.
Conclusion
Immunological activity was tested for cytokines assay on TMP- 1 cells. The experimental results with respect to IL-6 and TNF-α activity using Biofield Energy Treated/Blessed test formulation showed a significant inhibition as compared with the untreated test formulation. However, the cell viability MTT test of TMP-1 cell line assay showed a significant improved the cell viability with more than 77% cell viability among the different test groups, while Biofield Energy Treated/Blessed test item also showed significantly improved the cell viability up to 134% as compared with the untreated test group. The percentage cell viability in all the tested cell lines showed the cell viability range of 77% to 134% in different test item groups with RPMI-1640. Thus, based on MTT data indicated that the test formulation and the medium, RPMI was found safe and nontoxic in all the tested concentrations. Further, the level of IL-6 was significantly inhibited by 48.7% and 21.1% at 5 and 10μg/mL, respectively in the BT-RPMI+UT-TI, while 11.8%, 20.8%, and 44% in the 0.01, 5 and 10μg/mL respectively in the UT RPMI+BT-TI group, and 422.9%, 84.2%, and 14.7% inhibition of IL-6 at 0.01, 0.1, and 10μg/mL, respectively in the BT-RPMI+BT-TI as compared with the untreated test formulation and RPMI group. On the hand, TNF-α inhibition was reported in the UT-RPMI+BTTI group by 26.1%, 29.2%, and 155.2% at 0.01, 0.1, and 5μg/mL respectively, as compared with the untreated group. BT-RPMI+UTTI group showed a significant inhibition of TNF-α by 50% and 14.9% at 0.01 and 10μg/mL, respectively, while BT-RPMI+BT-TI group showed a significant inhibition of TNF-α by 46.4%, 17.5%, 21.2%, and 228.4% at 0.01, 0.1, 1, and 10μg/mL, respectively as compared with the untreated test formulation and RPMI group. Overall, the Biofield Energy Treated/Blessed (the Trivedi Effect®) test formulation showed a significant impact on cytokine level in TMP-1 cell line, which play a vital role in maintaining various immune related disorders, increase alertness, energy, attention, allergy, Alzheimer, cardiovascular, cancer, diabetes, eye, immune, inflammatory, or Parkinson. Therefore, the Consciousness Energy Healing based test formulation might be suitable alternative nutritional supplement, which could be useful for the management of various immune-related disorders that may be caused by poor nutrition, genetics, or problems with the rate of bone growth or rebuilding. Thus, in conclusion this therapy might also reduce the severity of any type of acute/chronic disease (auto-immune related and inflammatory disorders) progression rate and can be used in both before and after the manifestation of any disease symptoms in healthy, unhealthy, and ill peoples.
Acknowledgement
The authors are grateful to Dabur Research Foundation, Trivedi Science, Trivedi Global, Inc., and Trivedi Master Wellness for the assistance and support during the work.
References
- Zhang JM, An J (2007) Cytokines, inflammation, and pain. Int Anesthesiol Clin 45(2): 27-37.
- Abd El Kader SM, Al Shreef FM (2018) Inflammatory cytokines and immune system modulation by aerobic versus resisted exercise training for elderly. Afr Health Sci 18(1): 120-131.
- Singh N, Tailang M, Mehta SC (2016) A Review on Herbal Plants as Immunomodulators. Int J Pharm Sci Res 7(9): 3602-3610.
- Zhang JM, An J (2007) Cytokines, Inflammation and Pain. Int Anesthesiol Clin 45: 27-37.
- Coppack SW (2001) Pro-inflammatory cytokines and adipose tissue. Proc Nutr Soc 60: 349-356.
- Ding Lei Su, Zhi Min Lu, Min Ning Shen, Xia Li, Ling Yun Sun (2012) roles of pro- and anti-inflammatory cytokines in the pathogenesis of SLE. J Biomed Biotech 15.
- Velasco Ramirez SF, Rosales Rivera LY, Ramirez Anguiano AC, Bitzer Quintero OK (2013) Cytokines and the nervous system: The relationship between seizures and epilepsy. Rev Neurol 57: 171-177.
- Byrne JH, Voogt M, Turner KM, Eyles DW, McGrath JJ, Burne TH (2013) The impact of adult vitamin D deficiency on behaviour and brain function in male Sprague-Dawley rats. PLoS One 8(8): e71593.
- Rayman MP (2000) The importance of selenium to human health. Lancet 356: 233-241.
- Beard JL, Connor JR (2003) Iron status and neural functioning. Ann Rev Nutr 23: 41-58.
- Peres FF, Lima AC, Hallak JEC, Crippa JA, Silva RH, et al. (2018) Cannabidiol as a Promising Strategy to Treat and Prevent Movement Disorders? Front Pharmacol 9: 482.
- Nagarkatti P, Pandey R, Rieder SA, Hegde VL, Nagarkatti M (2009) Cannabinoids as novel anti-inflammatory drugs. Future Med Chem 1(7): 1333-1349.
- Kang S, Min H (2012) Ginseng, the 'Immunity Boost': The Effects of Panax ginseng on Immune System. J Ginseng Res 36(4): 354-368.
- Maizes V, Rakel D, Niemiec C (2009) Integrative medicine and patient-centered care. Explore (NY) 5: 277-289.
- Bischof M, Del Giudice E (2013) Communication and the emergence of collective behavior in living organisms: a quantum approach. Mol Biol Int 2013: 987549.
- Cassidy CM (2004) What does it mean to practice an energy medicine? J Altern Complement Med 10(1): 79-81.
- Barnes PM, Bloom B, Nahin RL (2008) Complementary and alternative medicine use among adults and children: United States. Natl Health Stat Report 12: 1-23.
- Fan K wai (2005) National Center for Complementary and Alternative Medicine Website. J Med Libr Assoc 93: 410-412.
- Wisneski L, Anderson L (2009) The Scientific Basis of Integrative Medicine. Boca Raton, FL: CRC Press 205.
- Trivedi MK, Branton A, Trivedi D, Jana S (2021) Effect of consciousness energy healing treatment on the metal profile and properties of tellurium. Eng Technol Open Acc 3(5): 555623.
- Mahendra KT, Alice B, Dahryn T, Snehasis J (2021) Consciousness energy healing treatment impacted the isotopic abundance ratio of 6-Mercaptopurine (6-MP). Nov Appro Drug Des Dev 5(5): 555673.
- Trivedi MK, Branton A, Trivedi D, Nayak G, Mondal SC, Jana S (2015) Morphological characterization, quality, yield and DNA fingerprinting of biofield energy treated alphonso mango (Mangifera indica L.). Journal of Food and Nutrition Sciences 3: 245-250.
- Trivedi MK, Jana S (2021) Anti-aging activity of biofield energy treated novel proprietary test formulation by assessment of vital biomarkers in cerebrospinal fluid (CSF) in Sprague Dawley rats. On J Neur & Br Disord 5(2).
- Trivedi MK, Jana S (2021) Evaluation of biofield energy healing treatment based proprietary test formulation on gut health potential in colon cancer cell line (HT-29). J Pharmacol Clin Res 8(4): 555743.
- Trivedi MK, Branton A, Trivedi D, Jana S (2021) Isotopic abundance ratio analysis of consciousness energy healing treated folic acid. Food Nutr Current Res 4(2): 290-295.
- Trivedi MK, Branton A, Trivedi D, Jana S (2020) The consciousness energy healing treatment and its impact on the isotopic abundance ratio analysis of flutamide. Drug Des Int Prop Int J 3(5).
- Bob P, Raboch J, Maes M, Susta M, Pavlat J, et al. (2010) Depression, traumatic stress and interleukin 6. J Affect Disord 120(1-3): 231-234.
- Tanaka T, Narazaki M, Kishimoto T (2014) IL-6 in inflammation, immunity, and disease. Cold Spring Harb Perspect Biol 6(10): a016295.
- Mohan VP, Scanga CA, Yu K (2001) Effects of tumor necrosis factor alpha on host immune response in chronic persistent tuberculosis: possible role for limiting pathology. Infect Immun 69(3): 1847-1855.
- Parameswaran N, Patial S (2010) Tumor necrosis factor-α signaling in macrophages. Crit Rev Eukaryot Gene Expr 20(2): 87-103.






























