The Advantages of Topical a Cell Delivered Concert with Minimal Depth FUE Procedures: A Single Case Study
Megan A Cole and John P Cole*
Megan A Cole and John P Cole*
Submission: November 13, 2019; Published:December 02, 2019
*Corresponding author:John P Cole, Department of Forhair Clinic, Alpharetta, GA 30009, USA
How to cite this article:Megan A Cole, John P Cole. The Advantages of Topical a Cell Delivered Concert with Minimal Depth FUE Procedures: A Single Case Study. Int J Cell Sci & Mol Biol. 2019; 6(4): 555695. DOI: 10.19080/IJCSMB.2019.06.555695
Keywords:Matri stem; Micro matrix; A cell; Forhair; Superseding; Regenerative cell; Prostaglandin; Transection; Skeletal myoblasts
Opinion
Minimal depth follicular unit extractions (FUEs) are defined as those in which the punch depth is restricted to 1.8-3.0 mm below the scalp surface, corresponding anatomically with the reticular dermis–subcutaneous adipose boundary and superseding the depth of the average hair follicle (HF) by as much as 2.4mm [1]. Conceivable advantages inherent to the minimal depth FUE approach arise from the retention of follicle-adjacent and -associated tissues in an undisturbed state, ready to interact with post-surgical topical treatments. In particular, regenerative cell populations, including epidermal stem cells within the bulge region and mesenchymal stem cells in the dermal papilla, remain intact. Given the appropriate stimulation, such cell populations should be capable of efficiently regenerating a HF at the graft site in its entirety. Herein, we propose that topical application of A Cell, a porcine-derived extracellular matrix (ECM) product, to minimal depth FUE graft sites activates stem cell populations in those areas to establish a new HF. Indeed, a myriad of research findings support the idea that ECM-directed cues can determine stem cell activity and differentiated fate. For example, skeletal myoblasts cultured on skeletal muscle ECM display accelerated growth and differentiation compared to controls [2,3]. Conversely, mesenchymal stem cells cultured on a matrix of endothelial cell origin exhibit a vascular cell phenotype upon differentiation [4]. Additional research findings suggest that stem cells require the cell-generated physical forces supplied by matrix adhesive proteins to induce differentiation [5]. Moreover, ECM constituents, namely hyaluronic acid (HA), may further influence cell behavior in the wound response cascade via activation of anti-apoptotic pathways [6]. Clinically, ECM coatings and patches have been used to improve cell performance in bioartificial kidneys and initiate full epithelialization of severe, chronic wounds in as few as 13 days, respectively [7,8].
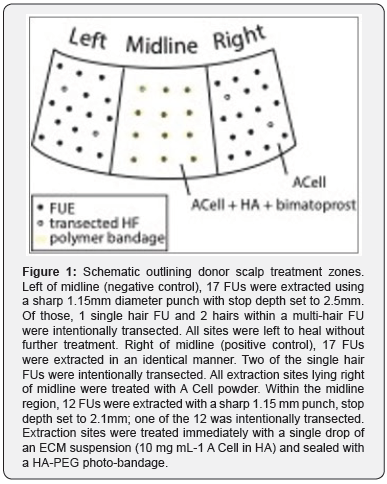
In the following case study, minimal depth FUE sites created in the occipital scalp of a 29-year-old Caucasian male (Norwood Class 3A) were treated with granularized ECM (MatriStem MicroMatrix, A Cell), alone or as a suspension in HA. Hair regrowth was evaluated at 3 months and compared to the negative control region of the scalp where no treatment was applied. In each region, intentional HF transections were made in order to assess the extent to which ECM therapy may be considered effective. Donor scalp treatment zones, HF extraction counts, and HF transection details are provided in Figure 1. In the midline occipital scalp, a hydrogel sealant was created over the ECM/HA treatment using a liquid bandage formulated with a water-soluble, poly (ethylene glycol)-based photopolymer system dispersed in a mixture of HA gel and normal saline. Upon exposure to high intensity visible light (Smart Lite Max LED Curing Light, Dentsply), the bandage polymerized into a breathable hydrogel sealant that confined the serosanguinous exudate to the extraction site. The seal provided by the bandage was broken as the HF elongated, and 1 week after the procedure, intact bandages were removed entirely. Extraction sites were then treated with a single drop of bimatoprost 0.03% ophthalmic solution, a prostaglandin up-regulator, once daily every Monday through Friday over the course of one month.
MicroMatrix, A Cell), alone or as a suspension in HA. Hair regrowth was evaluated at 3 months and compared to the negative control region of the scalp where no treatment was applied. In each region, intentional HF transections were made in order to assess the extent to which ECM therapy may be considered effective. Donor scalp treatment zones, HF extraction counts, and HF transection details are provided in Figure 1. In the midline occipital scalp, a hydrogel sealant was created over the ECM/HA treatment using a liquid bandage formulated with a water-soluble, poly (ethylene glycol)-based photopolymer system dispersed in a mixture of HA gel and normal saline. Upon exposure to high intensity visible light (Smart Lite Max LED Curing Light, Dentsply), the bandage polymerized into a breathable hydrogel sealant that confined the serosanguinous exudate to the extraction site. The seal provided by the bandage was broken as the HF elongated, and 1 week after the procedure, intact bandages were removed entirely. Extraction sites were then treated with a single drop of bimatoprost 0.03% ophthalmic solution, a prostaglandin up-regulator, once daily every Monday through Friday over the course of one month.
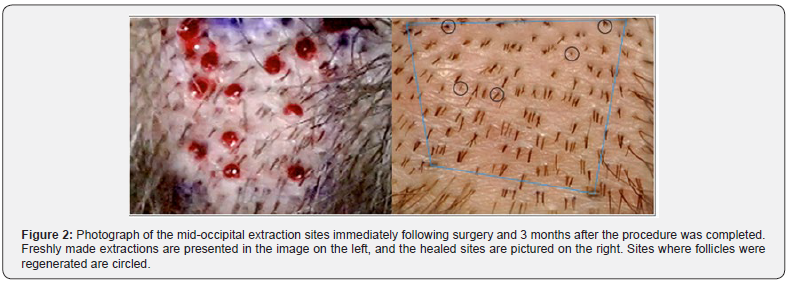
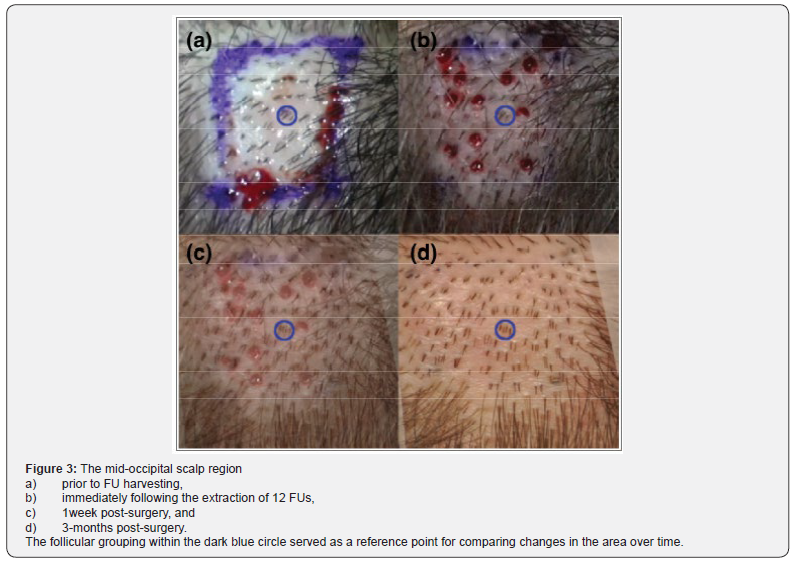
In this study, wound healing was excellent in all three regions; there was no visible difference in the healing between control and ECM-treated extractions sites at the macroscopic level. Nevertheless, the rate of hair regeneration in extraction sites varied with respect to incision depth and extraction site treatment protocol. Hair regeneration was lowest in the negative control region, where, similar to the positive control region, incision depth was set to 2.5 mm. Thus, one may conclude that treatment of extraction sites with ACell had a positive effect on hair regeneration since hair regeneration was observed in 24% of sites in the positive control region at 6 months.
In this study, wound healing was excellent in all three regions; there was no visible difference in the healing between control and ECM-treated extractions sites at the macroscopic level. Nevertheless, the rate of hair regeneration in extraction sites varied with respect to incision depth and extraction site treatment protocol. Hair regeneration was lowest in the negative control region, where, similar to the positive control region, incision depth was set to 2.5 mm. Thus, one may conclude that treatment of extraction sites with ACell had a positive effect on hair regeneration since hair regeneration was observed in 24% of sites in the positive control region at 6 months. that established by A Cell alone. Consequently, the HF regenerative potential of the topical combination treatment used on midline incisions may have surpassed the impact the A Cell treatment alone even if the extractions had both been conducted with the 2.5 mm stop. Based on this case report, A Cell has a positive impact on hair follicle regeneration when used in combination with minimal depth FUE procedures, and a follow-up, controlled study is warranted to evaluate the potential for ECM products to induce follicle neogenesis. The reduced percentage of follicle regeneration at deeper extraction depths, presumably from compromises to the local population of CK 15+ stem cells, would fully substantiate the theory that stem cells originating from the extraction site rather than from an adjacent location are the primary contributor to follicle regeneration had both depths received identical topical treatments. Given the discrepancy in treatments, the source of stem cells leading to follicle neogenesis should be studied traced, ideally in a systematic experimental process that incorporates HA gel, bimatoprost ophthalmic solution, and a wound sealant with a granular ECM product.
References
- Jimenez F, Izeta A, Poblet E (2011) Morphometric analysis of the human scalp hair follicle: practical implications for the hair transplant surgeon and hair regeneration studies. Dermatol Surg 37(1): 58-64.
- Stern MM, Myers RL, Hammam N, Stern KA, Eberli D, Kritchevsky SB, et al. (2009) The influence of extracellular matrix derived from skeletal muscle tissue on the proliferation and differentiation of myogenic progenitor cells ex vivo. Biomaterials 30(12): 2393-23
- Wilschut KJ, Haagsman HP, Roelen BAJ (2010) Extracellular matrix components direct porcine muscle stem cell behavior. Exp Cell Res 316(3): 341-52.
- Lozito TP, Taboas JM, Kuo CK, Tuan RS (2009) Mesenchymal stem cell modification of endothelial matrix regulates their vascular differentiation. J Cell Biochem 107(4): 706-13.
- Reilly GC, Engler AJ (2010) Intrinsic extracellular matrix properties regulate stem cell differentiation. J Biomech 43(1): 55-62.
- Toole BP (2004) Hyaluronan: From extracellular glue to pericellular cue. Nat Rev Cancer 4(7): 528-5
- Zhang H, Tasnim F, Ying JY, Zink D (2009) The impact of extracellular matrix coatings on the performance of human renal cells applied in bioartificial kidneys. Biomaterials 30(15): 2899-2911.
- Kimmel H, Rahn M, Gilbert TW (2010) The clinical effectiveness in wound healing with extracellular matrix derived from porcine urinary bladder matrix: A case series on severe chronic wounds. J Am Col Certif Wound Spec 2(3): 55-59.
- Plikus MV (2012) Epithelial stem cells and implications for wound repair. Semin Cell Dev Biol 23(9): 946-9
- Ito M (2007) Wnt-dependent de novo hair follicle regeneration in adult mouse skin after wounding. Nature 447(7142): 316-3






























