Effect of Exercise on Hematological Values and its Relationship with Erythrocyte Membrane Damage in Horses
Cesar Savignone, Pedro Zeinsteger, Javier Barberón, Belen Ventura and Alejandro Palacios*
Department of Biochemistry, National University of La Plata, Argentina
Submission: July 18, 2019; Published:August 30, 2019
*Corresponding author:Alejandro Palacios, Department of Biochemistry, Faculty of Veterinary Science, National University of La Plata, CC296, B1900AVW, La Plata, Argentina
How to cite this article:Cesar Savignone, Pedro Zeinsteger, Javier Barberón, Belen Ventura, Alejandro Palacios. Effect of Exercise on Hematological Values and its Relationship with Erythrocyte Membrane Damage in Horses. Int J cell Sci & mol biol. 2019; 6(3): 555687.DOI: 10.19080/IJCSMB.2019.06.555687
Abstract
Physical exercise is considered a stress for the animal organism because it generates different types of responses, as are the negative effects caused by free radicals. In this paper, the hematologic response level of red blood cells and the presence of oxidative alterations in their membranes, in horses subjected to a test of high-intensity exercise were evaluated. The results indicate a significant increase in the number of red blood cells, hematocrit and hemoglobin concentration, reaching maximum values when the horses reach fatigue during exercise test, descending during aerobic recovery. The three parameters behaved similarly. In relation to damage the membrane of red blood cells, it was estimated by the degree of peroxidation, measured by chemiluminescence, over the exercise period. The values produced by peroxidative damage are high at the time of exercise exhaustion, remaining stable after recovery. The correlation between the studied hematological values and the degree of damage of the erythrocyte membrane shows that, despite of the increased circulating red blood cells because of splenic contraction, the erythrocytes are damaged due to the predominant oxidizing environment during the maximal exercise
Keywords: Horse; Red blood cells; Exercise; Chemiluminescence; Hematocrit
Introduction
Exercise generates different responses in an individual depending on the type and duration, being a stress situation to the organism that tests its adaptability [1]. Regarding the hematological system, several authors (both in humans and different animal species research) describe changes in blood volume, population of white and red cells and in the count and shape of platelets, considering such changes being related to exercise [2-6]. It has been determined that the increase in the number of erythrocytes during exercise is mainly due to a process of splenic contraction by sympathetic stimulation [6], as a result of processes of hypoxia and acidosis generated during intense exercise, which in turn leads to a stronger splenic contraction [7]. Studies in different animal species show, when considering erythrocytes and after a vigorous and prolonged exercise, increases in total cell mass within a proportionally greater plasma volume [8-13]. Horses can rapidly increase the oxygen carrying capacity of blood by increasing hemoglobin concentration through splenic contraction [10,14]. It can be stated that, when considering parameters related to erythrocytes (cell count, hematocrit and hemoglobin concentration), values are often increased in the early stages of an exercise (splenic contraction). After long-term exer cise, the expansion of blood volume and the subsequent dilution effect (body fluids redistribution), cause a decrease in the relative values of these parameters, although they vary according to the degree of dehydration caused by the exercise itself [15].
In aerobic organisms, oxygen (O2) is essential for life as it is fundamental for energy metabolism, but it can also be toxic and is implicated in numerous diseases and degenerative conditions [16]. Oxygen is used as the terminal oxidant in the mitochondrial respiratory chain, however, the presence of intracellular O2 can result in the occurrence of redox reactions which may damage biomolecules [17]. Formation of free radicals during exercise depends on the intensity, frequency, duration and type of it. High levels of oxygen consumption during exercise have also been implicated as a contributing factor to oxidative stress [18-21]. Circulating erythrocytes are regularly exposed to stress conditions and are particularly vulnerable because they have no mechanisms of membrane repair or regenerative capacity [22]. During exercise, the oxidation of oxyhemoglobin to methemoglobin generates a great number of free radicals by release of O2-, a condition that is directly related to the type of exercise and the oxygen need for in tissues [22]. Oxidative damage can only be verified by direct measurement of different markers of this process. Peroxidation is by far the biomarker of oxidative damage most extensively studied after exercise [23]. Several assays in both human and veterinary medicine have been developed to study peroxidation in red blood cells, such as the addition of various pro-oxidants like cumene hydroperoxide [24] tert-butyl hydroperoxide (t-BHP) [25-27] and fatty acid hydroperoxides [13,28] which have been made from suspensions of red cell ghosts [13,14,25- 28] and also from lysed cells [29-32]. Aims of this study were to analyze de oxidative alterations in the erythrocyte membrane of horses submitted to a high intensity exercise test by estimating the degree of peroxidation by chemiluminescence and to evaluate the relationship between these changes and the variations in the hematological values during different sates of exercise.
Materials and Methods
Experimental animals
Five adult horses, 3 purebred Arabians and 2 half-Arabians, were used. Animals were in excellent nutritional and health status and had an average weight of 440kg. They were housed in boxes at the facilities of the Hospital School of the Faculty of Veterinary Sciences, National University of La Plata, all throughout the test period. Animals were fed based of body weight (2.5% dry matter). Diet exhibited a 50:50 ratio of hay (roll pasture) and concentrated (balanced feed with 12% protein and 2.75Mcal/ kg); water was administered ad-libitum.
Standardized exercise test
Standardized exercise tests are tests of physical exertion with controlled speed, gradual in many cases, which allow to reproduce or simulate a maximum effort situation under controlled conditions. Animals performed the test at the Laboratory of Physiology and Pathophysiology of Sport Horse, Faculty of Veterinary Sciences, National University of La Plata. For this purpose, a horse treadmill (Kagra, model Mustang 2200) was used, located in a building with the following dimensions: length 14.6m, width 9 meters and 10 meters high. The facility has a non-slip rubber floor to prevent horses from slipping, two frontal fans to simulate wind drag when the animal runs on a track, and a wall clock that records barometric pressure (mbar), temperature (°C) and humidity (%), to know the environmental conditions where the animal performs physical effort.
Technical characteristics of the treadmill are:
A. Length: 10.3 meters.
B. Width: 3.64 meters.
C. Floor to band height: 0.55 meters.
D. Maximum height: 4.3 meters.
E. Weight: 3770 kg.
F. Total dimensions: 4 meters long and 1.2 meters wide.
G. Machine speed: range 0-15 m/sec.
H. Incline percentage: range 0 to 11%.
I. Permanent load factor: up to 700 kg.
Exercise protocol
Prior to the start of the exercise tests all the horses had an adaptation period to the treadmill consisting of ascending and descending, walking, trotting and galloping. Standardized exercise test consisted of 1min preheating at a speed of 1.5m/sec. and then 4min at 4m/sec, followed by a 3% slope steps of 1min with increasing intensities (5; 6; 7; 8; 9; 10; 11; 12; 13 m/sec, etc.) until fatigue. Then, the recovery phase was as follow: 4 and 1.5m/s without slope during 4 and 1min, respectively [14]. Three tests were performed on each animal with at intervals not less than one week.
Blood sampling
For blood collection during tests, an antiseptic was applied to the skin and jugular catheterization was performed with a #14 Abbocath which was fixed with a suture to the skin. Then the catheter was connected to a flexible tube (both heparinized) long enough to gather blood sample without interrupting the exercise.
Blood samples were taken:
A. Before exercise (T0 or rest)
B. At fatigue (T1 or exercise)
C. After the recovery phase (T2 or recovery) [14].
Samples (5ml blood) were placed in heparinized tubes, immediately homogenized and processed at the end of the extraction of the three samples.
Sample processing
Hematology: First processing stage consisted of hemogram determinations (RBC count, hematocrit and measurement of hemoglobin concentration). Tests were performed at the Central Laboratory, Faculty of Veterinary Science, National University of La Plata, using a Sysmex KX 21 automated hematology analyzer
Preparation of erythrocytes
Red cells were isolated from whole blood by centrifugation (1000g for 10min at 4°C). The buffy coat and plasma were discarded, and erythrocytes were washed three times in isotonic phosphate buffer (PBS 5mM pH 7.4, 150mM NaCl). The erythrocyte pellet was suspended in isotonic phosphate buffer. Preparation of suspension of erythrocyte lysates was carried out according to the method of Dodge et al. [33]. Briefly, packed, washed erythrocytes were lysed by adding 10vol of 5mM phosphate buffer pH 7.4 (at 4°C) while mixing and after leaving on ice for 30min. Finally, the suspension was homogenized.
Peroxidation of erythrocyte analyzed by chemiluminescence
Peroxidation of erythrocyte lysates with final concentration of 0.25mg/ml total hemoglobin was initiated by adding of t-BHP (80mM) to each vial (T0, T1 and T2). Erythrocyte lysates preparations, which lacked t-BHP, were carried out as control simultaneously. Membrane light emission was determined over a 40min period at 37°C, chemiluminescence was recorded as count per minute (cpm) every 10min and the sum of the total chemiluminescence was used to calculate total cpm. Chemiluminescence was measured as counts per min in liquid scintillation analyzer Packard 1900 TR equipment with a program for chemiluminescence [34].
Statistical Analysis
Data obtained were considered independently for each of the determinations and are presented as the means ± SE. Test Student’s t was performed, assuming a single distribution and equal variances between samples to analyze the presence or absence of significant changes (P<0,05) between the means of the different experimental values (T0, T1 and T2). Possible associations between variables analyzed (hematological values and peroxidation) were performed through Pearson correlation coefficient (SSPS® Version 11).
Results
Hematologic values
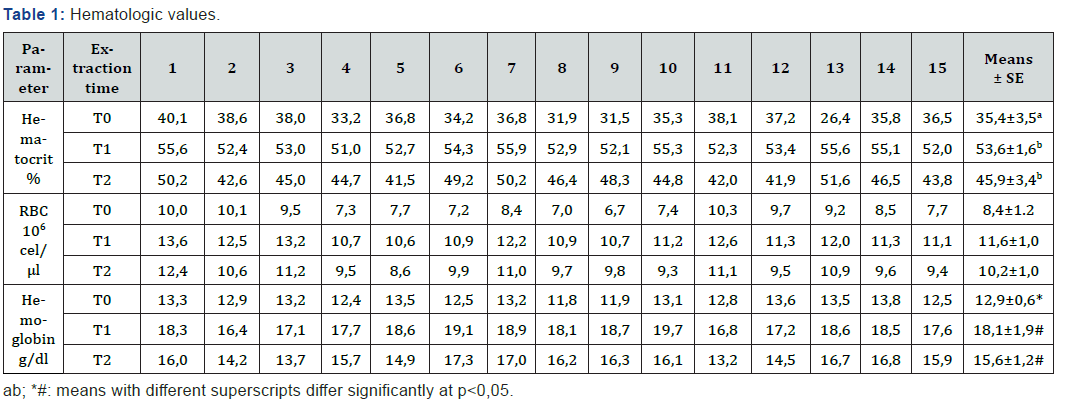
Table 1 and Figure 1 show the parameters of the red series evaluated for this study. Table 1 shows the individual results of the three blood parameters analyzed for each of the samples obtained during the exercise test. In T0 the average number of erythrocytes was 8,44x106 cell/μl (±0,32), 11,65x106 cells/μl (±0,25) in T1 and 10,15x106 cells/μl (±0,26) in T2 (Figure 1). Hematocrit increased during the initial stage of exercise test, with a baseline of 35,4% (±0,9) at T0 that increased to 53,6% (±0,4) in T1, and finally descended to 45,9% (±0,9) in T2 (Figure 1). Regarding hemoglobin concentration, parameter showed a similar trend, ranging from 12,93 g/dl (±0,2) to 18,09 g/dl (±0,2) and 15,63 g/dl (±0,3) for T0, T1 and T2, respectively, (Figure 1). Although the three parameters presented a similar distribution throughout the exercise routine, the initial values (T0) were only different from those observed in T1 for hematocrit (p=0,021), and for hemoglobin concentration (p=0,010); the differences observed for the total number of red blood cells are not statistically significant. No significant differences were found when analyzing the values obtained after finalization of the test period (T2), in relation to the initial values (T0) and data from the samples obtained at the time of fatigue (T1).

Degree of peroxidation
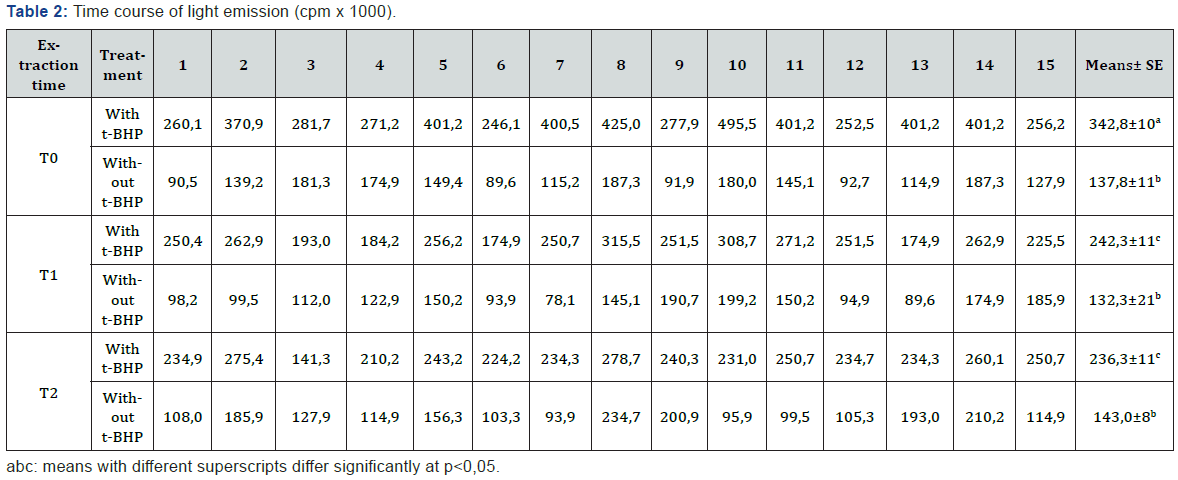
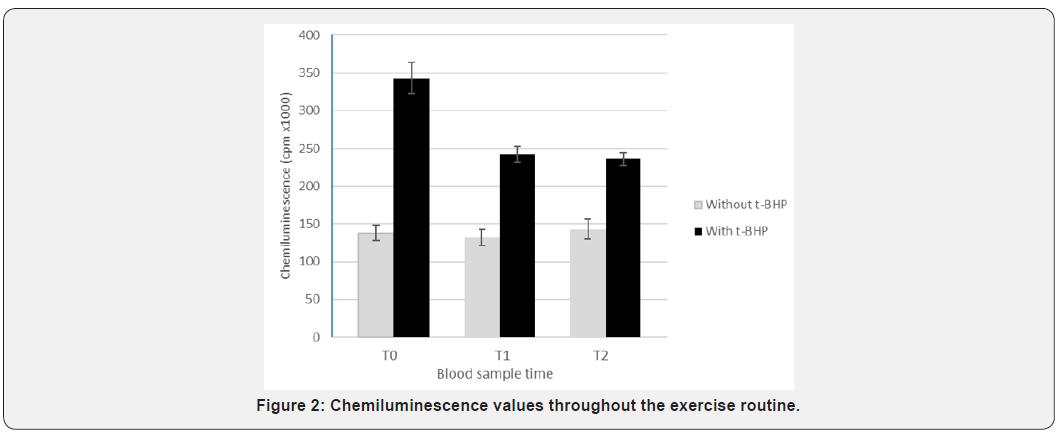
The addition of t-BHP to the suspension of erythrocyte lysates resulted in their peroxidation, as evidenced by the light emission (Table 2). When comparing the values obtained at the three times (T0, T1 and T2), with or without the addition of t-BHP, these were always statistically different (p<0,001). The total chemiluminescence values (total cpm) in samples with the addition of t-BHP were 342.830cpm (±21.013), 242.258cpm (±11.367) and 236.256cpm (±8.245) for T0, T1 and T2 respectively. These results are shown in Figure 2. The values were significantly different between T0 and T1 (p=0,011) and between T0 and T2 (p=0,003). There were no differences between T1 and T2.
Correlation between variables
A high positive correlation was found by comparison of the three hematologic variables analyzed (hematocrit, red blood cell count and hemoglobin level), regardless the time of sample collection. Conversely, individual comparison of these variables with the values of chemiluminescence after the addition of t-BHP, resulted negative. These results show that, despite the increment caused by the exercise test in the number of red blood cells and their associated variables (hematocrit and hemoglobin), membranes of these cells demonstrated a greater damage, as evidenced by the lower degree of chemiluminescence.
Discussion
The organism responds to physical demands, such response is related to the type and duration of the activity. This response is manifested in several organic systems and the hematological system is not the exception, in particular the red blood cells studied in this work. The literature mentions that hemoconcentration takes place at the beginning, being this attributable to dehydration as a consequence of fluid loss through perspiration. Also, an increase in hemoglobin concentration, hematocrit and number of erythrocytes while plasma volume decreases, as well as total blood volume. When physical challenge is more demanding or occurs over a longer time, these variables tend to increase [6]. The increments in hemoglobin, hematocrit and red blood cells observed in this work, leads to hemoconcentration, determined by the mobilization of erythrocytes from the spleen [35] and the fluid output to the extravascular space [36]. The increments in these variables were similar
In horses during rest, about 33% of the erythrocytes are stored in spleen. In the present study increments were observed for the three hematological variables analyzed when horses reached fatigue (T1); values decrease after recovery but remain higher compared to T0. In relation to the damage of red blood cell membranes, there is increasing evidence of changes induced by exercise in the oxidant / antioxidant balance, depending on the type, intensity and duration of exercise [20]. This is due to the production of reactive oxygen species, which cause cell and tissue damage [37]. Many studies have documented the oxidative stress induced by exercise by measuring oxidative damage to cellular components. Among the biomarkers of oxidative damage, peroxidation is a widely used method [23]. In this study, peroxidation of erythrocyte membranes was evaluated by measuring the light emission in suspensions of lysed erythrocytes of horses subjected to high-intensity exercise, exposed to an oxidant (t-BHP). It is established that an increase in the peroxidation rate produces a parallel increase in the photoemission. Since oxygen radicals are produced continuously in erythrocytes by autoxidation of hemoglobin and that erythrocytes have mechanisms for protection against oxidative damage, including catalase [38] superoxide dismutase [39] and low molecular weight antioxidants such as ascorbate [40] we must consider that the existence of changes in erythrocyte membranes, evaluated by peroxidation, is a consequence of physical exercise. Results clearly suggest the pro-oxidizing environment prevailing in blood during high-intensity exercise, probably associated with the release of ROS induced by such circumstance [41-43].
When relating the tested hematological parameters (number of RBC, hematocrit and hemoglobin) with the chemiluminescence values obtained in the three periods analyzed, an inverse correlation is observed. The latter may be attributable to the fact that recirculating erythrocytes show considerable damage in their membranes, due to the oxidative environment prevailing in the stage of maximum exercise, which is maintained during the recovery stage, although there is an increase in hematological values mainly due to splenic contraction.
Acknowledgement
This work was supported Grant 11/V227 of Secretaría de Ciencia y Técnica, National University of La Plata, Argentina.
Compliance with ethical standards
Procedures involving animals were in accordance with the recommendation of the Bioethics Committee of UNLP (National University of La Plata).
Conflict of Interest
All authors have no conflict of interest in this study.
References
- Posada Arias S, Garcia Naranjo R, Saldarriaga Restrepo A (2013) Hematological pre and post-values by gender and age exercise in dogs that do agility in Antioch. Vet Med Rev 25: 49-62.
- Novosadova J (1970) The changes in hematocrit, hemoglobin, and plasma volume and after different proteins during types of exercise. Eur J Appl Physiol 36(3): 223-230.
- Myhre L, Hartung G, Nunneley S, Tucker D (1985) Plasma volume changes in middle aged male and female subjects during running marathon. J Appl Physiol 59(2): 559-563.
- Schmidt W, Maassen N, Tegtbur U, Braumann M (1989) Changes in plasma volume and red cell formation after a marathon competition. Eur J Appl Physiol 58(5): 453-458.
- Novosadova, Ordonez J, García-Die F, Estruch A, Gimferrer E (1993) Hematologic changes induced by exertion during a long-distance race. Blood 38(6): 443-447.
- Bonilla J (2005) Hematologic response to exercise. Journal of Health Sciences 3(2): 206-16.
- Leleu C, Cotrel C, Couroucé Malblanc A (2005) Relationship between physiological variables and race performance in French Standarbred trotters. Vet Rec 156(11): 339-342.
- Boucher JH, Ferguson EW, Wilhelmsem CL (1981) Erythrocyte alterations during endurance exercise in horses. J Appl Physiol 51(1): 32-34.
- Escribano B, Miller M, Zech MD (1995). Observations on body weight and condition of horses. Equine Vet J Sci 13: 310.
- Kästner SBR, Weishaupt MA, Fiege K, Auer JA (1999) Heart rate and hematological responses of quarter horses to a reining competition. Equine Vet J Sci 19(2): 27-31.
- Tyler Mcgowan CM, Golland LC, Evans DL, Hodgson DR, Rose RJ (1999) Haematological and biochemical responses to training and overtraining. Equine Vet J Sci 30(S30): 621-625.
- Gouveia A, Harkins H, Pagan MN (2003) Blood and muscle metabolic responses to draft work of varying intensity and duration in horses. Res Vet Sci 47(1): 102-109.
- Udilova N, Jurek D, Marian B, Gille L, Schulte Hermann R, et al. (2003) Induction of lipid peroxidation in biomembranes by dietary oil components. Food Chem Toxicol 41(11): 1481-1489.
- Muriel MG (2016) Determination of the kinetics of DNA damage of peripheral blood leukocytes in horses subjected to high physical exertion intensity. Doctoral thesis. Faculty of Veterinary Sciences, National University of La Plata, Argentina.
- López Chicharro J (2008) Assessment of energy expenditure during the year. In: Lopez Chicharro J. 3rd (Ed.), Pan American Medical Publishing. Buenos, Aires, Argentina. pp. 224-240.
- Marx JL (1985) Oxygen free radicals linked to many diseases. Science 235(4788): 529-531.
- Imlay JA, Linn S (1986) Radical DNA damage and oxygen toxicity. Science 240(4857): 1302-1309.
- Ji L (1996). Exercise, oxidative stress, and antioxidants. Am J Sports Med 24(6): 20-22.
- Ji L (1999) Antioxidants and oxidative stress in exercise. Society for Experimental Biology and Medicine 222(3): 283-292.
- Williams CA, Kronfeld DS, Hess TM, Saker KE, Waldron JE, et al. (2005) Comparison of oxidative stress and antioxidant status in endurance horses in three 80km races. Comp Ex Equine Physiol 2: 153-157.
- Kirschvink N, Moffarts B, Lekeux B (2008) The oxidant/antioxidant equilibrium in horses. Vet J 177(2): 178-191.
- Cimen M (2008) Free radical metabolism in human erythrocytes. Clin Chem Acta 390(1-2): 1-11.
- Clemens MR, Waller HD (1987) Lipid peroxidation in erythrocytes. Chem Phys Lipids 45(2-4): 251-268.
- Deaton CM, Marlin DJ (2003) Exercise-associated oxidative stress. Clin Pract Equine Tech 2(3): 278-291.
- Tesoriere L, Allegra M, D'Arpa D, Butera D, Livrea MA (2001) Reaction of melatonin with hemoglobin-derived oxoferryl radicals and inhibition of the hydroperoxide-induced hemoglobin denaturation in red blood cells. J Pineal Res 31(2): 114-119.
- Mawatari S, Murakami K (2001) Effects of ascorbate on membrane phospholipids and tocopherols of intact erythrocytes during peroxidation by t-butylhydroperoxide: comparison with effects of dithiothreitol. Lipids 36(1): 57-65.
- Zou CG, Agar NS, Jone GL (2001) Oxidative insult in sheep red blood cells induced by t-butyl hydroperoxide: the roles of glutathione and glutathione peroxidase. Free Radic Res 34(1): 45-56.
- Iglesias BF, Catala A (2005) Rat, caprine, equine and bovine erythrocyte ghosts exposed to t-butyl hydroperoxide as a model to study lipid peroxidation using a chemiluminescence assay. Res Vet Sci 79(1): 19-27.
- Mawatari S, Murakami K (1998). Analysis of membrane phospholipid peroxidation by isocratic high-performance liquid chromatography with ultraviolet detection. Anal Biochem 264(1): 118-23.
- Sajewicz W (2010) Effect of thiol drugs on tert-butyl hydroperoxide induced luminol chemiluminescence in human erythrocytes, erythrocyte lysate, and erythrocyte membranes. Chem Biol Interact 186(2): 144-151.
- Sajewicz W, Zalewska M, Milnerowicz H (2015) Comparative study on thiol drugs effect on tert-butyl hydroperoxide induced luminol chemiluminescence in human erythrocyte lysate and hemoglobin oxidation. Toxicol In Vitro 29(1): 148-54.
- Savignone C, Palacios A (2017) Equine erythrocyte lysed exposed to t-butyl hydroperoxide as a model to study the oxidative stress caused by exercise using a chemiluminescence assay. Glob J Med Res 17(1): 19-24.
- Savignone CA, Ventura B, G Mattioli, Palacios A (2016) Comparative study on tert-butyl hydroperoxide induced chemiluminescence in bovine, equine and canine erythrocyte lysate. Research & Reviews in Bio Sciences 11(1): 41-46.
- Dodge JT, Mitchell C, Hanahan D (1963) The preparation and chemical characteristics of hemoglobin-free ghosts of human erythrocytes. Arch Biochem Biophys 100(1): 119-130.
- Wright JR, Rumbaugh RC, Colby HD, Miles PR (1979) The relationship between chemiluminescence and lipid peroxidation in rat hepatic microsomes. Arch Biochem Biophys 192(2): 344-351.
- Persson S (1967) On blood volume and working capacity in horses. Acta Vet Scand 19: 9-18.
- Milne DW, Skarda RT, Gabel AA, Smith LG, Ault K (1976). Effects of training on biochemical values in standarbred horses. Am J Vet Res 37(3): 285-290.
- Clarkson PM, Thompson HS (2000) Antioxidants: what role do they play in physical activity and health? Am J Clin Nutr 72(2): 637-646.
- Agar NS, Sadrzadeh SM, Hallaway PE, Eaton JW (1986) Erythrocyte catalase. A somatic oxidant defense? J Clin Invest 77(1): 319-21.
- Fee JA, Teitelbaum HD (1972) Evidence That plays a role superoxide dismutase in red blood cells protecting against peroxidative hemolysis. Biochem Biophys Res Commun 49(1): 150-158.
- Meister A (1994) Glutathione-ascorbic acid antioxidant system in animals. J Biol Chem 269(13): 9397-9400.
- Murakami K, Mawatari S (1986) Oxidation of hemoglobin to methemoglobin in intact erythrocyte by a hydroperoxide induces formation of hemoglobin and glutathionyl binding of α-hemoglobin to membrane. Arch Biochem Biophys 417(2): 244-250.
- Umbarila Barreto LF (2007) Literature review of hematology and physiological findings in athletes’ horses in the form of full competition riding. Monograph for the title of veterinarian and animal scientist. Faculty of Agricultural Sciences, Veterinary Medicine and Animal Science, University of Applied and Environmental Sciences, Colombia.






























