Pre/Post Plasmid Curing and Killing Kinetic Reactivity of Discorea bulbifera Linn Against Multiple Antibiotics Resistant Clinical Isolates, Using Escherichia coli as A Case Study
Oludare Temitope Osuntokun1*, Agbi Mayowa1, Thonda OA2 and Aladejana OM2
1 Department of Microbiology, Adekunle Ajasin University, Nigeria
2Department of Biological Science, Kings University, Nigeria
Submission: July 16, 2019; Published:August 27, 2019
*Corresponding author:Oludare Temitope Osuntokun, Department of Microbiology, Faculty of Science, AdekunleAjasin University, Akungba Akoko, Ondo State Nigeria.
How to cite this article:Oludare Temitope Osuntokun, Agbi Mayowa, Thonda OA, Aladejana OM. Pre/Post Plasmid Curing and Killing Kinetic Reactivity of Discorea bulbifera Linn Against Multiple Antibiotics Resistant Clinical Isolates, Using Escherichia coli as A Case Study. Int J cell Sci & mol biol. 2019; 6(2): 555685.DOI: 10.19080/IJCSMB.2019.06.555685
Abstract
The emergence and spread of multidrug-resistant (MDR) bacteria to commonly used antibiotics is a severe health problem and a major challenge to global drug discovery. Plasmid-mediated multidrug resistance is one of the most imperative tribulations in the treatment of infectious diseases. The use of plasmid curing agents in association with antibiotics may serve as a feasible way to control the development and spread of antibiotic resistance encoded by antibiotic resistance plasmids (R-plasmids). This has encouraged scientists to constantly search for various plasmid curing agents and the introduction of this new approach to combat the problem of antibiotic resistance. Investigation on the antimicrobial properties of Discorea bulbifera was carried out against certain clinical isolates. The antimicrobial activities of ethanol extracts of this plant against some multiple resistant isolates was carried out using agar well diffusion method. In addition to antimicrobial effect exhibited by ethanol extract of D. bulbifera, the plant extract also exhibited appreciable killing rate, leakage of potassium ions and plasmid curing of E. coli, which was an indication of bacterial cell membrane disruption by the antimicrobial compounds present in this extract. The result of the phytochemical screening of D. bulbifera revealed that the whole tuber contains saponins, tannins, flavonoids, terpenoids and cardiac glycosides. This study showed that Discorea bulbifera ethanol extract exerted its cidal effects on the test cells through disruption of their cell membranes. Each of these bioactive agents has two or more health promoting effects, and this may be responsible for their antibacterial potentials. The results from this study has shown that resistance in Escherichia coli is plasmid mediated as a result of its loss after curing. The plasmid curing of E. coli with Sodium dodecyl sulphate (SDS) and ethanolic extract of D. bulbifera caused the elimination of resistance plasmid and revert the resistant strain into susceptible form.
Keywords: Pre/Post plasmid curing; Killing kinetic reactivity; Discorea bulbifera; Multiple antibiotics resistant clinical isolates; Escherichia coli
Introduction
Antimicrobial resistance genes (ARGs), are frequently located on plasmids, which are self-replicating elements of DNA. They are often transmissible between bacteria, and some have spread globally. Plasmid curing is the elimination of plasmid DNA from bacterial isolates in order to determine the relationship of multidrug resistance with plasmid DNA [1]. Plasmid-mediated multidrug resistance is one of the most upcoming problems in the treatment of infectious diseases, as bacteria have reached resistance to most of the antibiotics that are available for treatment. Antibiotic resistance in bacteria may be an inherent trait of the organism that renders it naturally resistant, or it may be acquired by means of mutation in its own DNA or acquisition of resistance conferring DNA from another source. Thus, elimination of R-plasmids makes the antibiotic therapy effective. Novel strategies to fight antimicrobial multidrug resistance are required, so plasmid curing, and antiplays mid strategies could reduce antimicrobial resistance genes and sensitize bacteria to antibiotics [2]. One of the ways to minimize plasmid transmission of antibiotic resistance is to eliminate the plasmids. This process is known as plasmid curing and many compounds have been shown to be capable of causing this effect [2].
Majority of the plasmid curing agents are of synthetic origin such includes acridine dyes, ethidium bromide and sodium dodecyl sulphate, acridine orange, and acriflavine are unsuitable due to their toxicity or mutagenic nature. There is constant need of developing novel curing agents which are more effective and at the same time non-toxic. Alternatively, the solution to this problem would be the use of plant derived secondary compounds which would show promising activity against multiple-drug resistant bacteria and cause reversal of antibiotic resistance. The naturally occurring quinones constitute a major class of bioactive compounds which can serve as potential plasmid eliminating agents. Recently, it was shown that 24% of non-antibacterial drugs impact growth of members of the human microbiome. Various herbal extracts from plants like Cinnamomum verum, Zingiber officinale, Nigella sativa, Pipernigrum, Plumbago zeylenica, etc. containing phenol (eugenol), tannins, flavonoids, terpenoids, napthoquinones, alkaloid, saponin could be used for plasmid curing [3]. Elimination of bacterial plasmids from bacterial species grown as pure or mixed bacterial is achieved in the presence of sub-inhibitory concentration of non-mutagenic heterocyclic compounds. Amphiphilic compounds having a planar ring system act as antiplasmid compounds with substitution in the L-molecular region. Super helical structure of plasmid DNA binds more to the heterocyclic compound than linear or open circular form; So, it exhibits more antiplasmid activity and helpful to reduce the spread of antibiotic resistance plasmid in the ecosystem [4].
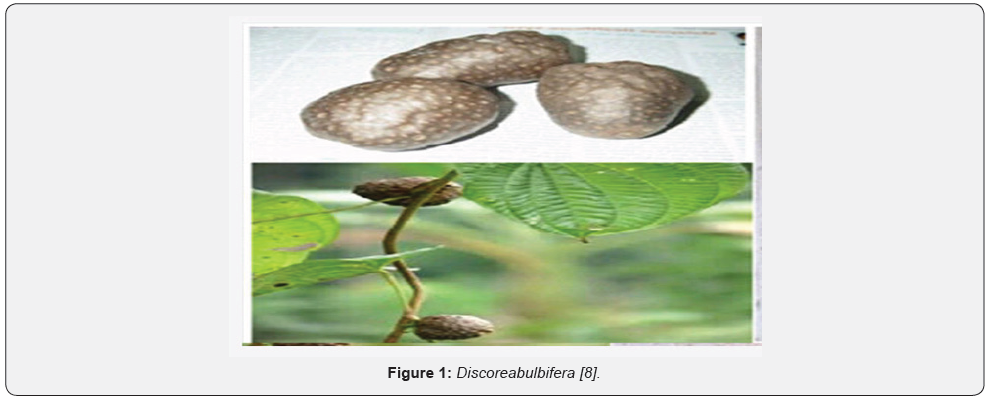
Discorea bulbifera (commonly known as the air potato, air yam, bitter yam, cheeky yam, potato yam) is a species of true yam in the yam family, Discoreaceae. It is native to Africa, Asia and northern Australia. It is a perennial vine with broad leaves and two types of storage organs. The plant form bulbils in the leaf axils of the twining stems, and tubers beneath the ground. It typically climbs to the top of trees and has a tendency to take over nature plants. Discorea bulbifera is a monocotyledonous, dioecius, herbaceous perennial vine. Its annual stems, which arise from tubers, twine counterclockwise [5]. Discorea bulbifera is a vigorously twining, long-stemmed perennial vine with non-spiny stems to 20m or more in length, freely branching above; internodes round or slightly angled in cross section and they twine counterclockwise. Plant has two types of storage organs. The plant forms bulbils in the leaf axils of the twining stems, and tubers beneath the ground. Tubers are like small, oblong potatoes with bitter taste. Conspicuous aerial tubers (called bulbils) are pale, round to globose in shape, up to 13cm wide and in inflorescence that give D. bulbifera the common name “air potato.” The leaves are attractive, alternate, broadly heart-shaped, attached by long petioles. Leaves 10-15x7.5-10cm, ovate-suborbicular, base deeply cordate, apex acuminate to shortly caudate, membranous, glabrous, basally 9-11-ribbed; petiole to 20cm long. Flowers rarely occur in D. bulbifera; where occurring, they are small, pale green and fragrant, and arising from leaf axils. Male flowers in slender, axillary panicled spikes, pendulous, to 18cm long; bracteoles ovate, acute [6].
Their skin is purplish black or earth colored, usually coated with abundant small feeding roots, but smooth in some cultivated varieties having flesh of white to lemon yellow, sometimes marked with purple flecks and very mucilaginous. A few root and root scars present in tubers, outer surface dark brown, inner yellow to light brown; odour- indistinct; taste-bitter [7] Figure 1.
Materials and Methods
Source and collection of plant samples
Tubers of Discorea bulbifera Linn (Aerial yam) were obtained from Akungba-Akoko, Ondo-State, Nigeria. Several tubers of D. bulbifera were harvested, packed in clean sterile manila papers, labelled with a voucher specimen and transported to the Laboratory of Department of Microbiology, Adekunle Ajasin University Akungba, Ondo State for analysis
Authentication of plant samples
The plants were authenticated at the Department of Plant Science and Biotechnology, Adekunle Ajasin University, Akungba Akoko, Ondo State, Nigeria.
Preparation of plant samples: Plant materials were first washed thoroughly with sterile distilled water and appropriately air dried at room temperature for two weeks to ensure the samples lose most of their moisture content [8].
Preparation of plant ethanolic extract: The tuber of Dioscorea bulbifera was collected and washed well in tap water first and then with the distilled water. The cleaned tubers were sliced and allowed for the complete shade drying. 200 grams of clean dried sample was soaked in 400ml of absolute ethanol making ratio of 1:2 and kept for four days [9].
Extraction of plant material: Extracts were collected and concentrated under reduced pressure using rotary evaporator at 40°C, then reconstituted with 20%dimethysulphoxide (DMSO). The stock extracts were kept in the refrigerator at 4°C for further use [10].
Standardization of plant extracts: At aseptic condition, the extracts were reconstituted by adding 1g of each extract to 2.5ml of DMSO and 7.5ml of sterile distilled water, making it 100mg/ ml. For each extract, 5ml of distilled water is measured into three sterile bijou bottles. In bijou bottle A, 5ml from the 100mg/ml bijou bottle was drawn and added, making it 50mg/ml. The serial concentration was prepared to get concentration of 50mg/ml, 25mg/ml and 12.5mg/ml respectively [11].
Test organisms and source of test microorganisms
The test organisms used were standard strains of bacteria and clinical isolates. They include Bacillus subtilis, Staphylococcus aureus, Streptococcus pyogenes, Escherichia coli, Proteus mirabilis, Shigelladysentriae, Pseudomonas aeruginosa, Klebsiella pneumoniae and Salmonella typhi. These organisms were obtained from the stock culture in the Laboratory of the Department of Microbiology, AdekunleAjasin University, Akungba-Akoko, Ondo State, Nigeria.
Standardization of test organisms: Slants of the various test organisms were sub cultured at aseptic condition, using a sterile wire loop, approximately one isolated colony of each pure culture was transferred into 9ml of sterile nutrient broth and incubated for 24hours. After incubation, 0.1ml of the broth culture was transferred into 9.9ml of sterile distilled water contained in each test tube using a sterile needle and syringe, and then mixed properly. The broth now serves as a source of inoculum containing approximately 106cfu/ml of bacterial suspension [12].
Antibacterial screening of the extract
All the test organisms were sub-cultured onto sterile Mueller Hinton agar plates and incubated at 37°C for 18-24 hours. Five distinct colonies for each organism were inoculated onto sterile Mueller Hinton broth and incubated for 3-4hours. All inocula were standardized accordingly to match the 0.5 McFarland standards, and this standard was used for all susceptibility tests. All the extracts were reconstituted accordingly into the following concentrations; 100, 50, 25, 12.5mg/ml, using Dimethl sulphoxide (DMSO). The susceptibility testing was investigated by the agar diffusion method according to the method of Osuntokun and Oladele [13]. All experiments were performed in duplicates [13].
Plasmid isolation
Bacterial cells were grown on nutrient agar and harvested with 200 L of buffer 1A (400Mm Tris, 200Mm Na EDTA and acetic acid to pH 8.0). The cells were then vortex and 400L of lysing solution (4 % SDS, 100 Mm Tris) were added, centrifuged at 14,000g for 5min. the supernatant were collected in fresh Eppendorf tubes and 700L of chloroform were added and vortexed. After which it was left on ice for 1 hour and centrifuged at 14,000g for 5 minutes. The aqueous layers were collected in fresh Eppendorf tubes. To 500L of aqueous layer, 1ml absolute ethanol was added and tubes keep in ice for 1 hour. Tubes were centrifuged again at 14000g for 5 minutes and the supernatant were decanted. The pellets were washed in 70% ethanol and air-dried. Afterwards, 100 L of buffer 3C was added to the pellets [14].
Plasmid profiling
The E. coli culture were inoculated in nutrient broth and placed at 37°C for 24 hours. E. coli culture (0.5mL) was transferred in a microfuge tube and same volume of phenol: chloroform: isoamyl- alcohol (25:24:1) were added. The phenol was saturated with Tris EDTA buffer (10mM Tris, 1mM EDTA with final pH7.5) before mixing with chloroform and Isoamyl alcohol. The microfuge tube containing mixtures were vortexed at the maximum speed for one minute. Then the tubes were centrifuged at 12,000rpm for 5 minutes. After centrifugation, the upper aqueous phase (0.45mL), leaving the interphase intact, were collected in another microfuge tube containing 0.5mL isopropanol. The microfuge tubes were mixed well and centrifuged immediately at 12,000rpm for five minutes [15].
Gel electrophoresis: The resultant plasmids were separated using gel electrophoresis. Agarose gel (0.8%) in 1X TBE buffer were prepared and placed in Gel electrophoresis tank containing 1X TBE buffer. The current was supplied about 85V for 15 minutes and the resulting bands were visualized under UV trans illuminator and compared with 100bp and 1kb ladder [16].
Plasmid curing
The isolates that was observed to harbour plasmid was subjected to plasmid curing. The chemical agent used for the plasmid curing was SDS and Discorea bulbifera extract. Escherichia coli isolates was cultured in Mueller Hinton agar for18 hours at 37°C in duplicate test tubes. Loopful culture (0.5McFarland) was inoculated in a microfuge tube containing freshly prepared 1mL TSB. The microfuge tube was placed on thermostat with set temperature of 45°C for 18 hours as mentioned previously [17]. E. coli culture was inoculated in test tubes (Triplicate) containing 10 % SDS and 100mg/ml of ethanolic extract of Discorea bulbifera in Mueller Hinton agar on different plates. The tubes were placed at 37°C for 24 hours. The curing of plasmid in the isolate was confirmed by antibiotic sensitivity testing using ethanol extract of Discorea bulbifera [18].
Secondary metabolites screening of Discorea bulbifera extract
Preliminary test / Preparation test: Discorea bulbifera filtrate were prepared by boiling 20 g of the fresh Discorea bulbifera extract in distilled water. The solution was filtered through a vacuum pump. The filtrate was used for the phytochemical screening for flavonoids, tannins, saponins, alkaloids, reducing sugars, anthraquinones and anthocyanosides [19].
Quantitative method of analyses
A. Saponins: About 20 grams each of dried Discorea bulbifera extract were ground and, put into a conical flask after which 100ml of 20 % aqueous ethanol were added. The mixture was heated using a hot water bath. At about 55°C for 4 hours with continuous stirring, after which the mixture was filtered, and the residue was re-extracted with further 200ml of 20% ethanol. The combined extracts were reduced to 40ml over a water bath at about 90°C. The concentrate was transferred into a 250ml separatory funnel and 20ml of diethyl ether were added and then shaken vigorously. The aqueous layer was recovered while the ether layer was discarded. The purification process was repeated three times. 60ml of n-butanol were added. The combined n-butanol extracts were washed twice with 10ml of 5% aqueous sodium chloride. The remaining solution were heated in a water bath. After evaporation, the samples were dried in the oven to a constant weight; the saponin content was calculated as percentage of the starting material [20].
B. Flavonoids: About 10g of the Discorea bulbifera sample were extracted repeatedly with 100ml of 80% aqueous methanol, at room temperature. The whole solution was filtered through Whatman filter paper No 42. The filtrate was later transferred into a crucible and evaporated into dryness over a water bath; the dry content was weighed to a constant weigh [21].
C. Cardiac glucosides: Legal test and the killer-kiliani was adopted, 0.5g of the extract were added to 2ml of acetic anhydrate plus H2S04[22].
D. Tannins: About 500mg of the Discorea bulbifera sample were weighed into a 50ml plastic bottle. 50ml of distilled water was added and shaken for 1 hour on a mechanical shaker. This was filtered into a 50ml volumetric flask and made up to the marked level. Then, 5ml of the filtrate was transferred into a test tube and mixed with 2ml of 0.1M Fe Cl in 0.1M Hcl and 0.008M potassium ferrocyanide. The absorbance was measured at 120nm within 10 minutes. The tannins content was calculated using a standard curve of extract [23].
E. Alkaloids: Five grams of the Discorea bulbifera sample were weighed into a 250ml beaker and 200ml of 10% acetic acid in ethanol was then be added, the reaction mixture were covered and allowed to stand for 4 hours. These were filtered and the extract was concentrated on a water bath to one-quarter of the original volume. Concentrated ammonium hydroxide was added dropwise to the extract until the precipitation is complete. The whole solution was allowed to settle, and the precipitate was collected, washed with dilute ammonium hydroxide and then filtered; the residue being the alkaloid, which was dried and weighed to a constant mass [24].
F. Phlobatannins: About 0.5 grams of each Discorea bulbifera were dissolved in distilled water and filtered. The filtrate was boiled in 2% HCl, red precipitate shows the present of phlobatannins [25].
Results
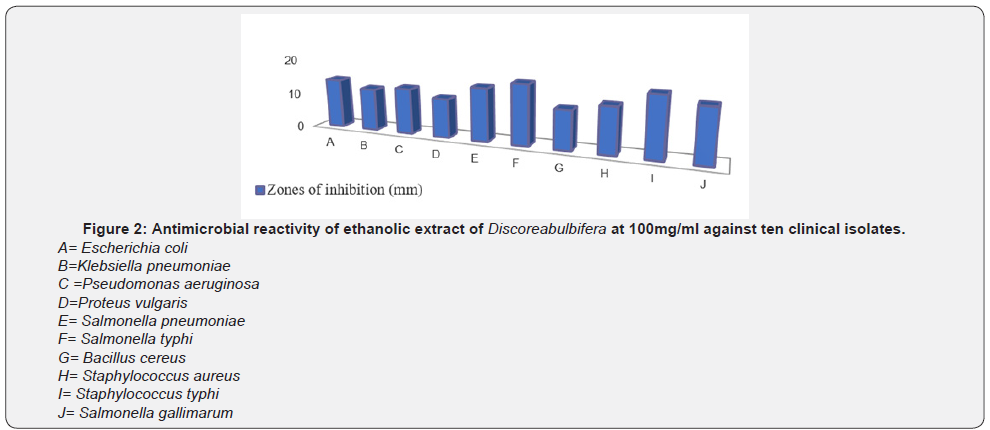
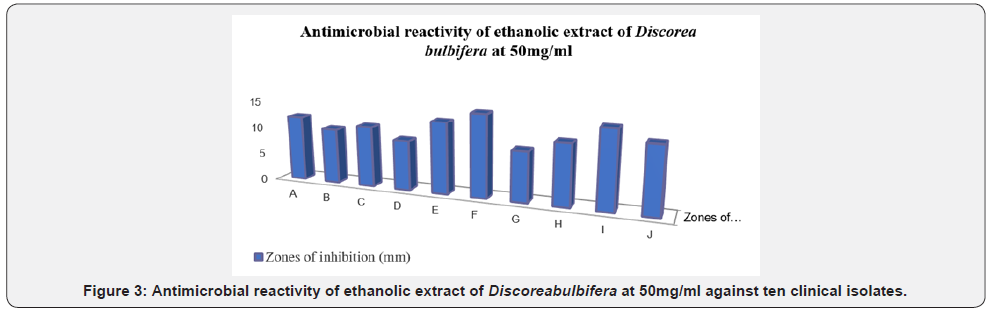
Figure 2-5 show the zones of inhibition of ethanolic extract of Discorea bulbifera bacterial growth at different concentration (100mg/ml, 50mg/ml, 25mg/ml and 12.5mg/ml) respectively. The antibacterial activities were expressed as the zone of inhibition diameters (mm) produced by the plant extract. The ethanolic extract of Discorea bulbifera inhibited some of the bacteria with a measurable zone of inhibition. The extract had higher inhibitory activity on Staphylococcus aureus (Gram-positive bacteria) and Bacillus cereus (Gram-positive bacteria) and with zones of inhibition of 15mm and 17mm at 100mg/ml respectively. The Table 1 also shows the MIC and MBC values. Both extracts had MIC values ranging from 50-100mg/ml, and the MBC values of 100mg/ml. The ethanol extract of Discorea bulbifera showed minimum inhibitory concentration of 50mg/ml to Escherichia coli, Staphylococcus aureus and Bacillus cereus. The extract also showed minimum inhibitory concentration of 100mg/ml to Klebsiella pneumonia, Salmonella typhi, Pseudomonas aeruginosa and Proteus vulgaris. Only three of the bacteria isolate (Escherichia coli, Staphylococcus aureus and Bacillus cereus) showed MBC value of 100mg/ml (Table 1). The potassium ion efflux (leakage) from multi-drug resistant Escherichia coli cellstreated with different concentrations (100mg/ml, 30mg/ml, 10mg/ml and 3mg/ml) of ethanolic extract of Discorea bulbifera in different time ranges between 0min, 15min, 30min, 60min and 90minutes (Table 2). Each point represents the amount of potassium ion leaked (μg/mL) from the cells at a particular time interval in the presence of the fraction.

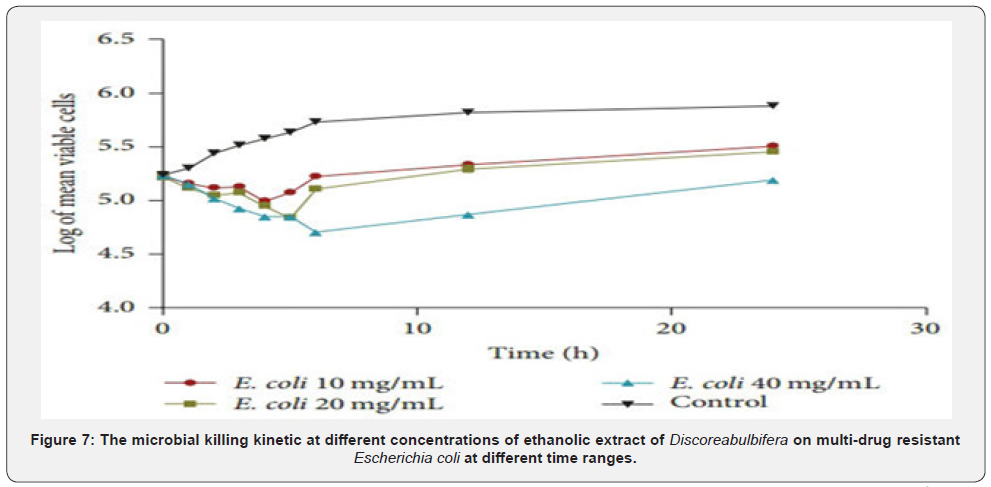
The potassium ion leakage of 100mg/ml concentration of the extract for 15min was 1.26μg/ml, and 1.40(μg/ml) at 90 minutes. The highest effect of potassium efflux was exhibited by the 100mg/ml concentration of the extract, having ranges of 1.22 to 1.40. The 3mg/ml concentration has the least reactivity of 0.21 at 90minutes. The rate of potassium ion leakage assay showed that the percentage of ion leakage increases with increased concentration and duration. The quantity of ion leaked out of the cells also depict the sensitivity of the bacteria isolates to the extract. Figure 6 shows the extent and rate of kill of Escherichia coli by ethanol extract of Discorea bulbifera at different concentrations of 40mg/ml, 20mg/ml and 10mg/ml. The microbial killing kinetic of different concentrations of ethanolic extract of Discorea bulbifera on multidrug resistant Escherichia coli in different time ranges between 15min, 1 hour, 3 hours and 6 hours. The rate of the organism killed by 10mg/ml concentration in 15min was 4.24 while the rate of cell killed at 30min rose to 4.29. After 60min of contact time with this fraction, the rate of the organisms killed was 4.34. Each point represents the mean log10 survival of bacterial cells at a particular time interval in the presence of the fraction. After 90min of the contact time interval, the percentage of the organisms killed has increased to 5.12, while it rose to 5.16 after 120min of contact time. The extent and rate of kill of the fraction at 40mg/ml concentration followed the same trend with the concentrations aforementioned. As the concentrations of the fraction increased with increase in time, the percentage of the organisms killed also increased.
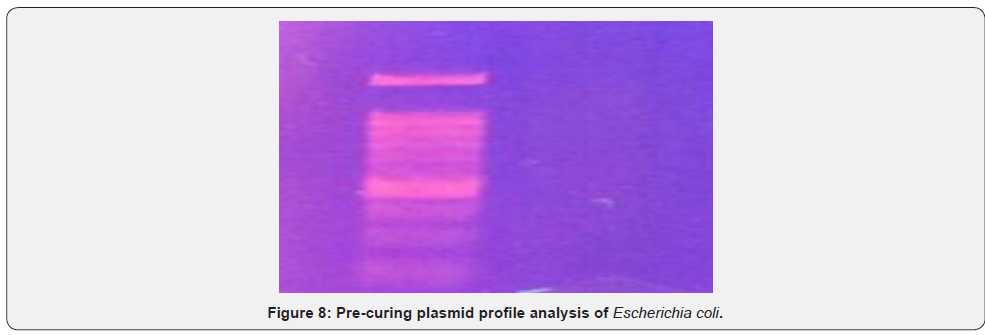
Plasmid mediated analysis of different multi drug resistant E. coli isolates was observed by agarose gel electrophoresis which showed plasmid bands in different combinations (Pre-curing plasmid profile of E. Coli) (Figure 7). Plasmid mediated analysis of different multi drug resistant E. coli isolate was observed by agarose gel electrophoresis which do not show plasmid band (Post-curing plasmid profile of E. Coli) (Figure 8)
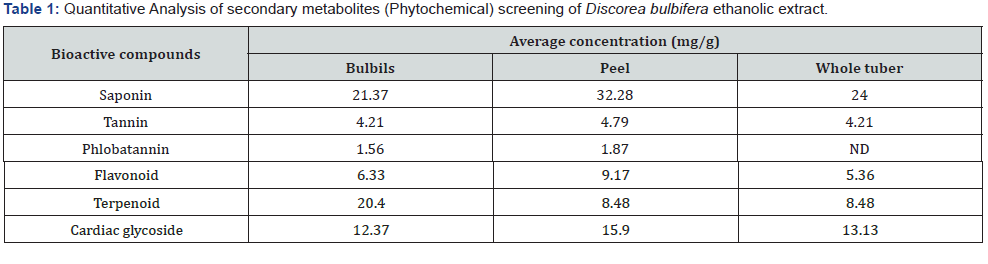
The Secondary metabolites screening of Discorea bulbifera extract (phytochemical screening) for the presence of bioactive components in the tuber of D. Bulbifera samples are presented in Table 1. The analysis indicated the presence of saponin, tannin, flavonoids, terpenoid, phlobatannin and cardiac glycosides. The peels of the tubers were observed to contain higher amount of these bioactive compounds in the following order; saponin (32.28mg/g), followed by terpenoids(22.90mg/g), cardiac glycosides (15.90 mg/g), flavonoid (9.17 mg/g), tannin (4.79 mg/g) and phlobatannin (1.87 mg/g) was the least among the bioactive ingredients detected in the peel of D. bulbifera.
The susceptibility of E. coli to different concentrations (100mg/ml, 50mg/ml, 25mg/ml and 12.5) of Discorea bulbifera ethanolic extract forming different zones of inhibition. The reactivity of the ethanolic extract of Discorea bulbifera against multiple drug resistant E. coli before plasmid isolation and curing were observed. The ethanolic extract inhibited the bacteria forming a measurable zone of inhibition. The 12.5mg/ml concentration of the extract has no reactivity against the plasmid E. coli. The concentration of 25mg/ml and 50mg/ml showed inhibition zones of 9mm and 11mm respectively. The plant extract also inhibited the growth of E. coli with zones of inhibition of 15mm at 100mg/ ml. The 100mg/ml concentration showed the highest reactivity against the bacteria isolate (Figure 9).
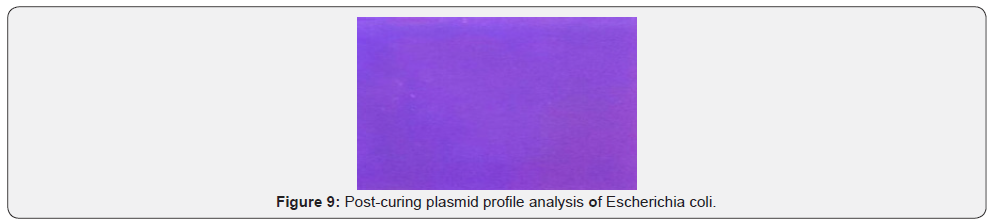
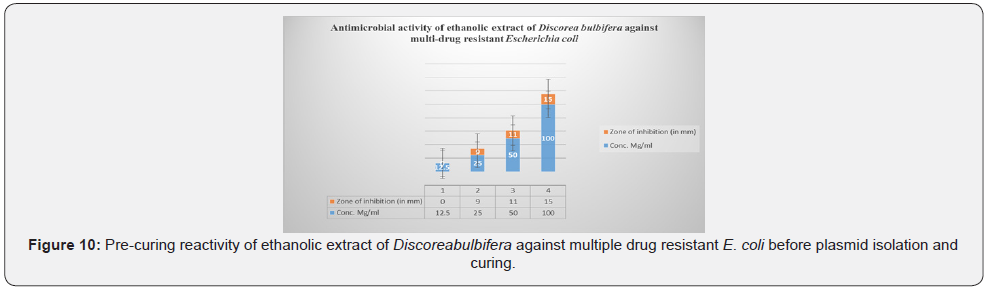
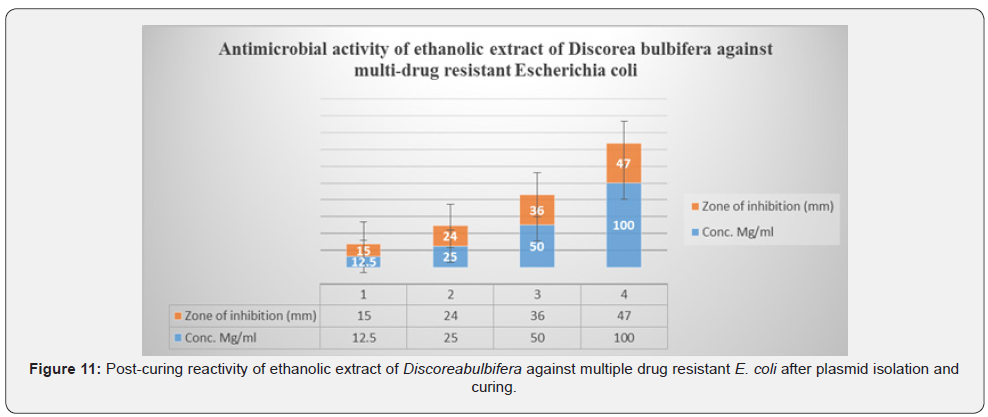
Figure 10 shows the zones of inhibition of E. coli growth at different concentration of ethanolic extract of Discorea bulbifera and the reactivity of the ethanolic extract of Discorea bulbifera against multiple drug resistant E. coli before plasmid isolation and curing. The antibacterial activities were expressed as the zone of inhibition diameters (mm) produced by the plant extract. The ethanol extract of Discorea bulbifera inhibited the bacteria forming a measurable zone of inhibition. The 12.5mg/ml concentration of the extract inhibited the bacteria growth with 15mm clear zone of inhibition on the plasmid-cured E. coli. The concentration of 25mg/ml and 50mg/ml showed inhibition zones of 24mm and 36mm respectively. The plant extract also inhibited the growth of E. coli with 47mm zone of inhibition at 100mg/ml. The 100mg/ml concentration showed the highest reactivity against the bacteria isolates.
Discussion
The varied antibacterial activity recorded for tuber of this yam could be attributed to different metabolites constituted by this plant parts, following the fact that these phytochemicals are routinely described as the major antibacterial factors found in plant [26]. Furthermore, the minimum inhibitory concentration obtained for the ethanolic extract of this tuber in the present study contradict the general believe that plant extracts are more effective against Gram-positive than Gram-negative bacteria [27], as both Gram-positive and Gram-negative isolates tested against the extract were inhibited at concentrations ranging between 50mg/ ml and 100mg/ml. The significant activity displayed by this tuber also reinforce the hypothesis that D. Bulbifera could be explored as potential antimicrobial drug. Moreover, [28] also emphasized that active compounds from D. bulbifera are substrates of multidrug resistant (MDR) bacteria efflux pumps, suggesting a possible use as an inhibitor in the fight against these strains. Although, the mechanisms of antibacterial activity of the tuber (D. bulbifera) is not evaluated in this present study, membrane disruption and formation of complex with bacterial cell wall are suspected mechanisms because of the terpenoids and flavonoids content of the tuber [29]. Potent bacterial killing was evident as extract concentration gets higher and time increases. This observation was similar to the antibacterial effect of Hemides musindicus [28]. The ability of this fraction to completely inhibit these pathogens at minimal contact time and at low concentrations is an indication that bioactive compounds in Discorea bulbifera could be used for the development of antimicrobial compounds for treatment of infections caused by pathogens. Such antimicrobials could be useful in combating infections caused by multidrug resistant microorganisms.
In addition to killing rate exhibited by ethanol extract of D. bulbifera, other modes of action including leakage of potassium ions and plasmid curing potential were studied. The fraction exhibited appreciable leakage of potassium ions and plasmid curing of E. coli (Figures 5), which is an indication of bacterial cell membrane disruption by the antimicrobial compounds present in this extract. The ethanolic extract exhibited appreciable potentials to cause potassium ion efflux from the test bacterium. The concentrations of potassium ions leaked from the test bacterium increased with increase in concentration of the fractions as well as increase in contact time of the cells with the fractions [29]. The cytoplasmic membrane damage caused by antimicrobial agents could have caused the cations to be freely transported out of the cell and thus led to the death of the cell. Potassium ion is involved in the maintenance of a constant internal pH and membrane potential, therefore the efflux of potassium out of the membrane will have detrimental effect on the cell functions and lead to the cell death. The results obtained from this study showed that Discorea bulbifera ethanol extract exerted its cidal effects on the test cells through disruption of their cell membranes. According to Zasloff, antibacterial agents could act by disrupting the cytoplasmic membrane of bacteria and as such cause destabilization and permeabilization. This observation supports the mode of action exhibited by ethanol extract obtained from Discorea bulbifera [30].
The result of the phytochemical screening of the extract of D. Bulbifera revealed that the whole tuber contains saponins, tannins, flavonoids, terpenoids and cardiac glycosides. Each of these bioactive agents has two or more health promoting effects, and this may be responsible for their antibacterial potentials [31]. reported that plant extracts containing bioactive agents with antimicrobial properties have been found useful in treating bacterial and fungal infections. Saponins were detected in the tuber of D. bulbifera in this present study with the highest concentration among other bioactive compounds. According to [32], presence of saponin in D. Bulbifera as observed in this study, suggest that the tuber may have hypocholesterolenic effect, in that, saponins reduces the uptake of certain nutrient including glucose and cholesterol at the gut through intralumeral physicochemical interactions. This action tends to lessen the metabolic burden that would have been placed on the liver- hypocholesterolemic effect. Saponins have also been reported to possess the properties of precipitating and coagulating red blood cells. Therefore, in medicine, D. bulbifera can be applied as antibleeding agent to arrest lost of blood in case of injuries. Vitamins and some vital minerals such as zinc and iron are reported to form insoluble complexes with saponin [33]. This also may have contributed to the antimicrobial activity of D. bulbifera against some pathogenic microorganism that requires these vitamin and mineral for their metabolic processes.
The mode of antimicrobial activities confers on plants by tannins, which include their ability to inactivate microbial adhesions, enzymes and cell envelope transport proteins [34], are all indicative of the antimicrobial efficacy of the extracts of D. Bulbifera against the test microorganisms in this study. A number of terpenoid as a bioactive compound has been isolated and have been shown to elicit antibacterial and antiprotozoal properties. Therefore, the considerable amounts of terpenoid in the tuber of D. bulbifera indicated that the tuber elicit medicinal benefits. Flavonoids are important class of polyphenols, structurally made of more than one benzene ring, which are found in plants [35]. It has long been reported by [35] that alkaloids and flavonoids are responsible for the antimicrobial activities in higher plants. Therefore, the considerable amount of flavonoids detected in the sampled tuber indicated the pharmacological properties embedded in the tuber of D. bulbifera.
Plasmids frequently carry genes for antibiotic resistance, toxigenicity and can as well confer extremophiles status on microorganisms. The functions of these plasmids have classically been correlated with phenotypical properties, including drug resistance, carbohydrate metabolism, amino-acid metabolism, carotenoids, colic acid derivatives, organic acids and bacteriocins production [36]. Plasmids are useful markers in Recombinant DNA technology and as such this makes plasmids indispensable tool in Molecular Biology. The results from this investigative study have shown that resistance in Escherichia coli are plasmid based as a result of its loss after curing. The resistance showed by the isolates was plasmid mediated [37]. The plasmid curing of E. coli with Sodium dodecyl sulphate (SDS) and ethanolic extract of D. bulbifera causes elimination of resistance plasmid and revert the resistant strain into susceptible form [38]. Cured derivative was found to be more sensitive than wild type which was proved by replica plate technique. Agarose gel electrophoresis revealed presence of plasmids in wild type strain but absence of plasmids in the cured derivative which was the physical confirmation of plasmid curing effected by SDS and Discorea bulbifera extracts [39,40].
Recommendation
This finding resulted in the possibility of a new type of combination between antibiotics and potential drugs effective against plasmid encoded multiple antibiotic resistance. From the result obtained, from the antimicrobial activity of this plant, I recommend that:
I. Elimination of plasmid-determined antibiotic resistance in pathogenic strains of bacteria is of great practical significance both in the treatment of bacterial infection and in microbial genetics. Already ineffective antibiotics can become effective if plasmid encoded antibiotic resistance is eliminated from the population.
II. Many of the ineffective or outdated antibiotics could be revived if used in combination with such curing agents. This would be a novel approach towards controlling multidrug resistant bacterial infections especially in hospital environment.
III. I anticipate that future research should continue in this area, driven in large part by the need to prevent and treat resistant infections.
IV. Studies on in vivo plasmid curing will be crucial in developing methods to sensitize bacteria to existing antibiotics. In the future, it may be that doctors prescribe a plasmid curing agent to help ensure that the antibiotics taken by the patient are effective. Alternatively, a plasmid curing agent could be taken by an individual (e.g. on return from an area where plasmid-mediated drug-resistance is common) as a way of restoring drug-susceptible bacteria to the gastrointestinal microbiome.
V. The use of plasmid curing strategies in settings other than in humans and animals should not be under appreciated. For instance, another potential use of curing strategies could be on farms where livestock are often exposed to antibiotics, and harbour multiple MDR plasmids. Soil, waste water treatment, and aquaculture could all be treated with plasmid curing agents to reduce drug-resistance.
VI. Altogether, plasmid curing has come a long way, from the use of toxic compounds to novel designer curing methods based on incompatibility. Further research is now needed to uncover safe and effective means to cure plasmids, particularly in the face of the global antibiotic resistance crisis.
Conclusion
Development of bacterial resistance to multiple antibiotics has made treatment of infectious diseases increasingly difficult over the past few years. Many known antibiotics have now become ineffective owing to the development and spread of plasmid encoded high level of resistance in bacteria. However, such ineffective antibiotics can be rendered effective if R-plasmids encoding antibiotic resistance are eliminated from the bacterial population. The present study affirmed the fact that plant extracts D. bulbifera, represent a novel source for bioactive compounds employable as new antibacterial agents and safe plasmid curing agent. More so, it is conclusive that D. bulbifera is a rich source of relevant bioactive agents that do not only enhances the antibacterial properties of the tubers but also ascertain its health promoting qualities. It is therefore recommended that pharmaceutical industries should exploit the broad antibacterial potentials embedded in the extract of D. bulbifera to produce novels antibiotics of broad spectrum to circumvent the ever-emerging trend of multiple drug resistant pathogenic microorganisms. The present piece of work may prove to be beneficial for searching novel potential phytotherapeutic plasmid curing agents against multiple drug resistant bacterial strains and reversal of their plasmid-mediated-resistance.
References
- Adeniran AA, Sonibare MA (2013) In vitro potential anthelmintic activity of bulbils of Dioscoreabulbifera L. on earthworms and liverflukes. J Pharmacognosy Phytother 5(12): 196-203.
- Bakour S, Sankar SA, Rathored J, Biagini P, Raoult D, et al. (2016) Identification of virulence factors and antibiotic resistance markers using bacterial genomics. Future Microbiol 11(3):455-466.
- Branas P (2015) Molecular epidemiology of carbapenemase-producing Klebsiella pneumoniae in a hospital in Madrid: successful establishment of an OXA-48 ST11 clone. Int J Antimicrob Agents 46(1): 111-116.
- Chellat MF, Raguž L, Riedl R(2016) Targeting antibiotic resistance. Angew Chem Int Ed Engl 55(23): 6600-6626.
- Hawkey PM (2015) Multidrug-resistant Gram-negative bacteria: a product of globalization. J Hosp Infect 89(4): 241-247.
- James HD (2012) Phytochemicals: Extraction Methods, Basic Structures and Mode of Action as Potential Chemotherapeutic Agents. Phytochemicals. pp. 1-33.
- Fatemeh Jamshidi Kia, Zahra Lorigooini, Hossein Amini Khoei (2018) Medicinal plants: Past history and future perspective. Journal of Herbmed Pharmacology, 7 (1):1-7.
- Kado CI (2014) Historical events that spawned the field of plasmid biology. Microbiol Spectr 2(5).
- Katakweba AA, Muhairwa AP, Lupindu AM (2018) First report on a randomized investigation of antimicrobial resistance in fecal indicator bacteria from Livestock, Poultry, and humans in Tanzania. Microbial Drug Resistance 24(3): 260-268.
- Khameneh B, Diab R, Ghazvini K, Bazzaz BSF (2016) Breakthroughs in bacterial resistance mechanisms and the potential ways to combat them. Microb Pathog 95: 32-42.
- Khare CP (2016) Ayurvedic Pharmacopoeial plant drugs-Expanded therapeutics. CRC Press, Boca Raton, London, Newyork, USA pp. 227-228.
- Kobayashi J, Tanabiki M, Doi S, Kondo A, Ohshiro T, et al. (2015) Unique plasmids generated via pUC replicon mutagenesis in an error-prone thermophile derived from Geobacillus kaustophilus HTA426. Applied and Environmental Microbiology 81:7625-7632.
- Osuntokun OT, Olajubu FA (2014) Antibacterial and Phytochemical Properties of Some Nigerian Medicinal plant on Salmonella typhi and Salmonella paratyphi Isolated from Infected Hu man Stool in Owo local Government, Journal of Scientific Research &Reports 4(5): 441-449.
- Liu YY, Wang Y, Walsh TR (2016) Emergence of plasmid-mediated colistin resistance mechanism MCR-1 in animals and human beings in China: a microbiological and molecular biological study. Lancet Infect Dis 16(2): 161-168.
- Osuntokun Oludare temitope, Oluwafoise Bamidele Olugbenga (2015) Phytochemical screening of ten Nigerian medicinal plants. International Journal of Multidisciplinary Research and Development 2(4): 390-396.
- Maier L, Pruteanu M, Kuhn M (2018) Extensive impact of nonantibiotic drugs on human gut bacteria. Nature 555: 623-238.
- Meek RW, Vyas H, Piddock LJ (2015) Nonmedical uses of antibiotics: time to restrict their use? PLoS Biol 13(10): e1002266.
- Mühlen S, Dersch P. (2016). Anti-virulence strategies to target bacterial infections. Curr Top Microbiol Immunol;(March).
- Naas T, Oueslati S, Bonnin RA (2017) Beta-lactamase database (BLDB)-structure and function. J Enzym Inhib Med Ch. Pp. 32.
- Omodamiro OD (2015) Anti-inflammatory and diuretic activities of ethanol extract of Dioscoreabulbifera leaf. AJDDT 2(1): 29-38.
- Omojate GC, Enwa FO, Jewo AO, Eze CO (2014) Mechanisms of antimicrobial actions of phytochemicals against enteric pathogens-A Review. Journal of Pharmaceutical, Chemical and Biological Sciences 2(2): 77-85.
- Omolade Mary Adeosun, Daniel Juwon Arotupin, Odeyemi Adebowale Toba, Alaba Adewole Adebayo (2016) Antibacterial activities and phytochemical properties of extracts of Dioscoreabulbifera Linn (Air Potatoe) tubers and peels against some pathogenic bacteria. JPHYTO 5(1): 20-26.
- O’Neill J (2016) Tackling Drug-Resistant infection globally: Final Report and Recommendations the Review on Antimicrobial Resistance.
- Pal NP, Gaikwad SS, Jonsson V, Kristiansson E, Larsson DJ (2017) Untreated urban waste contaminates Indian river sediments with resistance genes to last resort antibiotics. Water Research 124: 388-397.
- Pandey AK, Kumar S (2013) Chemistry and biology activities of flavonoids: An Overview. Scient World J. pp. 162750.
- Patwardhan RB, Shinde PS, Chavan KR, Devale A (2015) Reversal of plasmid encoded antibiotic resistance from nosocomial pathogens by using plumbago auriculata root extracts. Int J Curr Microbiol App Sci. pp. 187-198.
- Raj A (2012) Antibiotic Resistance, Plasmid and RAPD Profiles of Multidrug-resistant Escherichia coli form Bacteria Isolated from Sewage Samples of Ghaziabad City, India. Environ RES Technol 2(4): 318-324.
- Sanjeet K, Gitishree D, Han Seung S, Jayanta KP (2017). Dioscorea spp. (A wild edible tuber): A study on its ethnopharmacological potential and traditional use by the local people of Similipal biosphere reserve, India. Frontiers in Pharmacol 8(52): 1-17.
- Stoesser N (2016) Evolutionary history of the global emergence of the Escherichia coli epidemic clone ST131. M Bio 7(2): e02162
- Subasini U, Thenmozhi S, Sathyamurthy D, Vetriselvan S, Victor Rajamanickam G (2013) Pharmacognostic and phytochemical investigations of Dioscoreabulbifera L. IJPLS 4(5): 2693-2700.
- Tang Y, Xue YB, Zhou L, Zhang JW, Yao GM (2014) New norclerodane diterpenoids from the tubers of Dioscoreabulbifera. Chem Pharm Bull (Tokyo) 62(7): 719-724.
- Tapondjou LA, Jenett Siems K, Bottger S, Melzig MF (2013) Steroidal saponins from the flowers of Dioscoreabulbifera var sativa. Phytochemistry 95: 341-350.
- Wang Y, Zhang R, Li J (2017) Comprehensive resistome analysis reveals the prevalence of NDM and MCR-1 in Chinese poultry production. Nat Microbiol 2:16260.
- Wilson AP, Livermore DM, Otter JA (2016) Prevention and control of multidrug-resistant Gram-negative bacteria: recommendations from a Joint Working Party. J Hosp Infect 92: S1-S44.
- Wonglumsom W, Sianglum W, Tiyasuttipan W, Sirisali S (2011) Plasmid Profiles and Antimicrobial Resistance Patterns of Escherichia coli. Rta Med J 64:175-80.
- Van Boeckel TP, Gandra S, Ashok A (2014) Global antibiotic consumption 2000 to 2010: an analysis of national pharmaceutical sales data. Lancet Infect Dis 14: 742-750.
- Van Boeckel TP, Glennon EE, Chen D (2017) Reducing antimicrobial use in food animals. Science 357: 1350-1352.
- Velez R, Sloand E (2016) Combating antibiotic resistance, mitigating future threats and ongoing initiatives. J Clin Nurs 25(13-14): 1886-1889.
- Vila J, Saez Lopez E, Johnson JR (2016) Escherichia coli: an old friend with new tidings. FEMS Microbiol Rev 40(4): 437-463.
- Yusuf Babatunde AM, Osuntokun OT, Ige OO, Solaja OO (2019) Secondary metabolite Constituents, Antimicrobial Activity and Gas chromatography-Mass spectrocopy Profile of Bombax buonopozense P. Beauv. (Bombacaceae) Stem bark Extract. Research Journal of Pharmacognosy and Phytochemistry 11(2): 87-92.






























