Urease: The Ultimate Therapeutic Target for Helicobacter Pylori
Mahbubul Hasan SM, Abhinandan Chowdhury and Chaman Ara Keya*
North South University, Bangladesh
Submission: December 18, 2017; Published: February 02, 2018
*Corresponding author: Chaman Ara Keya, North South University, Plot#15, Block#4, Bashundhara, Dhaka 1229, Bangladesh, Tel: +880-2-55668200; Fax: +880-2-55668202; Email: chaman.keya@northsouth.edu
How to cite this article: Mahbubul Hasan SM, Abhinandan Chowdhury Chaman Ara Keya1. Urease: The Ultimate Therapeutic Target for Helicobacter Pylori. Int J cell Sci & mol biol. 2018; 3(5) : 555625. DOI: 10.19080/IJCSMB.2018.04.555625
Abstract
Gastric ulcer and carcinoma is quite frequent throughout the globe. The most prevalent causative agent of gastric ulcer and carcinoma is a gram-negative bacterium, Helicobacter pylori (H. pylori). The ineffectiveness and side effects of approved drugs as well as antibiotic resistance is a major problem in the treatment of H. pylori. H. pylori possess uncommon urease enzyme which catalyzes the hydrolysis of urea. Urease is necessary for colonization of H. Pylori in the gastric mucosa and is a potent immunogen that elicits a vigorous immune response. As urease acts as both colonization and virulence factor, new inhibitor of urease from plant sources with no/less adverse effect is vital to efface H. pylori.
Keywords: Helicobacter pylori; Urease; Virulence; Inhibitor; Antibiotic resistance
Introduction
H. pylori infection is common all over the world. Surviving in stringent conditions of low pH in human stomach is not feasible for most of the microorganisms. However, unlike other organisms' gram-negative (H. pylori) can "customize" its surrounding to make it comfortable for survival. And this survival tactic of the bacterium leads to chronic gastritis and plays important role in peptic ulcer disease, gastric carcinoma, and gastric lymphoma [1,2].
Consequences of H. pylori infection
Gastric cancer had been marked as a prominent cause of cancer related death with above 95% relation with infection by H. Pylori [1]. 70 to 90% of the residents of developing countries act as host of H. pylori on the other hand as in advanced countries, the predominance of infection is less [3]. Among infected individuals, approximately 10% develop severe gastric lesions such as peptic ulcer disease, 1%-3% progresses to Gastric carcinoma. Gastric carcinoma represents the second most frequent cancer in the world [4,5]. Gastric carcinogenesis is a multifactorial process and it results from interaction of the several factors that are related to diet, environment, genetic susceptibility, and Helicobacter pylori infection. Substantial epidemiological evidence exists for an increased risk of gastric carcinoma with H. pylori infection [6]. This carcinogenesis by H. pylori consists of two pathways. The direct pathway effects on gastric epithelial cells, by alteration of DNA and cellular proteins. And the indirect pathway works by initiating inflammation by innate and adaptive immunity [1]. Action of the bacterial associated protein cagA, its peptidoglycan, VacA toxin, sialic acid-binding adhesion (SabA), outer inflammatory protein (OipA), duodenal ulcer promoting gene (dupA) and the flagella altogether plays eminent roles in development of carcinogenesis [2]. Apart from these, a crucial role of gastritis is played by the unique feature of H. pylori; the urease enzyme [2].
Unique H. Pylori Urease in Action
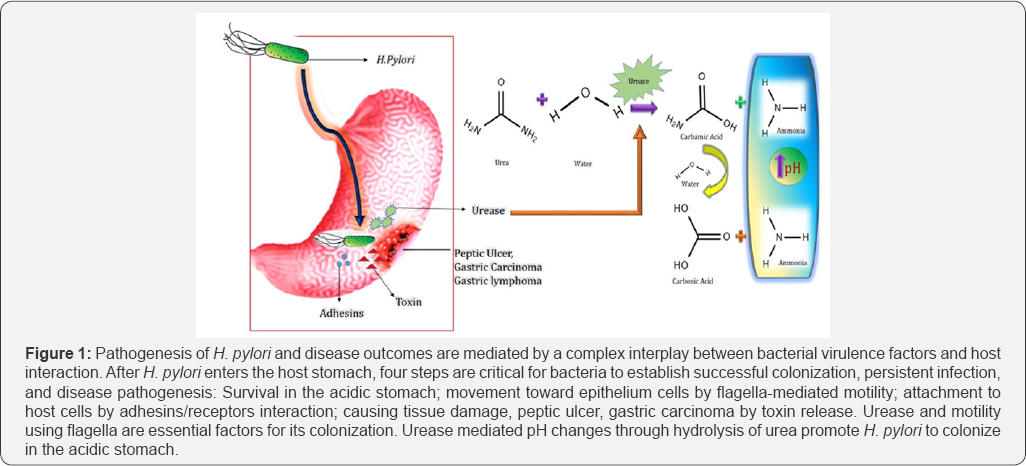
Urea aminohydrolase also known as urease is a nickel- containing hexameric molecule. It catalyzes the hydrolysis of urea into ammonia and carbamic acid (Figure 1). Simultaneously carbamic acid decomposes into carbonic acid and a second ammonia molecule. Ammonium molecule being a weak base elevates the pH, hence making the surrounding for H. pylori comfortable to colonize in harsh acidic condition [7,8]. Significant amount of ammonium also promote cytotoxic chemical such as monochloramine which causes tissue damage. Moreover, the carbonic acid which is another product of the catalytic process diminishes the antimicrobial activity of peroxynitrite, a metabolite of nitric oxide. Therefore protecting the bacteria in every way possible [9]. All these features of the bacteria become evident due to the presence of its exclusive urease enzyme. This enzyme is composed of only two protein subunits UreA and UreB with ratio of 1: 1, unlike other ureolytic bacteria which consists of another UreC subunit. It is to be noted that bacterial ureases are activated by supplementary proteins UreD, UreE, UreF, UreG,and UreH through a complex process [7,8]. Targeting these formulation of drug aimed towards deregulation in colonization proteins as well as their respective genes can lead to an ideal of H. pylori (Figure 1).
Current Therapeutics against H. Pylori
H. pylori is usually treated with triple therapy consisting of clarithromycin, amoxicillin and a proton pump inhibitor. Due to hike in antimicrobial resistance sequential, quadruple regimes had been introduced recently. However, newer quadruple combinations still result in a high frequency of side-effects and some studies report low cure rates [10]. The antibiotics administered, usually act on the bacterium, on the other hand inactivation of urease enzyme is not yet totally exploited. At present Palmatine, Bis (N-methylaminomethyl) phosphinic acid, and some derivatives of 3-Arylpropionylhydroxamic acid, pyrogallol and catechol were observed to suppress urease by acting on active site [11-14]. Acetohydroxamic acid (AHA), which is used to treating H. pylori by inhibiting urease enzyme, also exhibits severe side effects [15].
Urease: The Ultimate Target for H. Pylori
It is the enzyme 'urease' and its activity by which H. pylori colonize in the host. Therefore if the urease can be arrested, the bacteria would not have the advantage of inhibiting the acidic condition of the stomach. Though the available drugs work on various factors of urease as a whole, attention on its proteins UreA and UreB as well as UreD, UreE, UreF, UreG, and UreH, hence their respective genes could be focused on. Poor patient compliance with the available eradication therapy due to the development of side-effects may be associated with higher treatment failure rates and may favor the development of antibiotic-resistant strains of H. pylori. Therefore, H. pylori treatment still remains a challenge due to antibiotics resistance, side-effect and cost, mainly in developing countries [16-18].
Thus, developing alternative therapeutics with higher efficacy and lower side effects is a burning necessity. In this scenario, phytochemicals could be an effective alternative [1922]. Till date, very few studies have been conducted on plant derived compounds for the identification of novel therapeutics against H. pylori [11,14]. If urease is widely explored to inhibit/ deactivate/ distort the enzyme through bioactive compounds from plant source(s), it can lead to a new hope to "un-root" H. pylori and prevent further infection.
Conflict of Interest
Authors declare no conflict of interest.
References
- Chiba T, Marusawa H, Seno H, Watanabe N (2008) Mechanism for gastric cancer development by Helicobacter pylori infection. J Gastroenterol Hepatol 23(8):1175-1181.
- Wroblewski LE, Peek RM, Wilson KT (2010) Helicobacter pylori and gastric cancer: factors that modulate disease risk. Clin Microbiol Rev 23(4): 713-739.
- Dunn BE, Cohen H, Blaser MJ (1997) Helicobacter pylori. Clin Microbiol Rev 10(4): 720-741.
- Zhang RG, Duan GC, Fan QT, Chen SY (2016) Role of Helicobacter pylori infection in pathogenesis of gastric carcinoma. World J Gastrointest Pathophysiol 7(1): 97-107.
- Parkin DM, Bray F, Ferlay J, Pisani P (2002) Global cancer statistics. CA Cancer J Clin 49(1): 33-64.
- Uemura N, Okamoto S, Yamamoto S, Matsumura N, Yamaguchi S, et al. (2001) Helicobacter pylori Infection and the Development of Gastric Cancer. N Engl J Med 345(11): 784-789.
- Rutherford JC (2014) The emerging role of urease as a general microbial virulence factor. PLoS Pathog 10(5): e1004062.
- Konieczna I, Zarnowiec P, Kwinkowski M, Kolesinska B, Fraczyk J, et al. (2012) Bacterial urease and its role in long-lasting human diseases. Curr Protein Pept Sci 13(8):789-806.
- Kuwahara H, Miyamoto Y, Akaike T, Kubota T, Sawa T, et al. (2000) Helicobacter pylori urease suppresses bactericidal activity of peroxynitrite via carbon dioxide production. Infect Immun 68(8): 4378-4383.
- Thung I, Aramin H, Vavinskaya V, Gupta S, Park JY, et al. (2016) Review article: the global emergence of Helicobacter pylori antibiotic resistance. Aliment Pharmacol Ther 43(4): 514-533.
- Zhou JT, Li CL, Tan LH, Xu YF, Liu YH, et al. (2017) Inhibition of Helicobacter pylori and Its Associated Urease by Palmatine: Investigation on the Potential Mechanism. PLoS One 12(1): e0168944.
- Macegoniuk K, Grela E, Biernat M, Psurski M, Gosciniak G, et al. (2017) Aminophosphinates against Helicobacter pylori ureolysis— Biochemical and whole-cell inhibition characteristics. PLoS One 12(8): e0182437.
- Shi WK, Deng RC, Wang PF, Yue QQ, Liu Q, et al. (2016) 3-Arylpropionylhydroxamic acid derivatives as Helicobacter pylori urease inhibitors: Synthesis, molecular docking and biological evaluation. Bioorg Med Chem 24(19): 4519-4527.
- Xiao ZP, Ma TW, Fu WC, Peng XC, Zhang AH, et al. (2010) The synthesis, structure and activity evaluation of pyrogallol and catechol derivatives as Helicobacter pylori urease inhibitors. Eur J Med Chem 45(11): 50645070.
- Kosikowska P, Berlicki L, Berlicki t (2011) Urease inhibitors as potential drugs for gastric and urinary tract infections: a patent review. Expert Opin Ther Pat 21(6): 945-957.
- Zhang M (2015) High antibiotic resistance rate: A difficult issue for Helicobacter pylori eradication treatment. World J Gastroenterol 21(48): 13432-13437.
- Safavi M, Sabourian R, Foroumadi A (2016) Treatment of Helicobacter pylori infection: Current and future insights. World J Clin cases 4(1): 5-19.
- Kim SY (2015) Antibiotic treatment for Helicobacter pylori: Is the end coming? World J Gastrointest Pharmacol Ther 6(4): 183-198.
- Schmidt B, Ribnicky DM, Poulev A, Logendra S, Cefalu WT, et al. (2008) A natural history of botanical therapeutics. Metabolism 57(7 Suppl 1): S3-S9.
- Sharma SB, Gupta R (2015) Drug development from natural resource: a systematic approach. Mini Rev Med Chem 15(1): 52-57.
- Hossain MU, Khan MA, Rakib-Uz-Zaman SM, Ali MT, Islam MS, et al. (2016) Treating Diabetes Mellitus: Pharmacophore Based Designing of Potential Drugs from Gymnema sylvestre against Insulin Receptor Protein. Biomed Res Int 2016: 3187647.
- Hossain MU, Hashem A, Keya CA, Salimullah M (2016) Therapeutics Insight with Inclusive Immunopharmacology Explication of Human Rotavirus A for the Treatment of Diarrhea. Front Pharmacol 7: 153.






























