Functional Improvements and Musculoskeletal Magnetic Resonance Imaging with Spectroscopy Changes following Cell Therapy in a Case of Limb Girdle Muscular Dystrophy
Alok Sharma1, AmrutaParanjape2, Ritu Varghese2*, Hemangi Sane2, Nandini Gokulchandran1, Jasbinder Kaur3 and Prerna Badhe4
1Department of Medical Services and Clinical Research, Neuro Gen Brain & Spine Institute, India
2Department of Research & Development, Neuro Gen Brain & Spine Institute, India
3Department of Neurorehabilitation, Neuro Gen Brain & Spine Institute, India
4Department of Regenerative Laboratory Services, Neuro Gen Brain & Spine Institute, India
Submission: May 22, 2017; Published: July 25, 2017
*Corresponding author: Ritu Varghese, Department of Research & Development, Neuro Gen Brain & Spine Institute, Palm Beach Road, Seawoods (W), Navi Mumbai-400706, India, Tel: 91-9920200400, +9122-25283706; Email: publications@neurogen.in
How to cite this article: Alok S, Amruta P, Ritu V, Hemangi S, Nandini G, et al. Functional Improvements and Musculoskeletal Magnetic Resonance Imaging with Spectroscopy Changes following Cell Therapy in a Case of Limb Girdle Muscular Dystrophy. Int J cell Sci & mol biol. 2017; 2(4) : 555595. 10.19080/IJCSMB.2017.02.555595
Abstract
Limb girdle muscular dystrophy is a heterogeneous group of genetic disorders characterized by progressive muscle weakness caused by autosomal dominant or recessive gene mutations. Although no curative treatment is currently available, adult bone marrow stem cell transplantation maybe a promising therapeutic approach in view of their capacity to regenerate muscle fibers. Musculoskeletal magnetic resonance imaging is a useful non- invasive tool to track disease progression and to test the efficacy of a treatment. We report a twenty six year old female patient diagnosed with limb girdle muscular dystrophy and treated with intrathecal and intramuscular injections of autologous bone marrow mononuclear cells followed by multidisciplinary rehabilitation. Repeat musculoskeletal magnetic resonance imaging 6 months post cell therapy, showed no further increase in fatty infiltration or loss of muscle volume in the musculature of bilateral upper and lower limbs. Objective improvements were seen on musculoskeletal resonance spectroscopy as less area under the curve in the flattened extramyocellular lipid peak with reduction in the intramyocellular (-CH2) lipid/creatine ratio in the tibialis anterior muscle in comparison to the pre-cell transplantation scan. Functional improvements were noted, along with strength improvements were noted in the bilateral hip abductors, adductors, left hip extensors, knee flexors, ankle dorsiflexors, plantar flexors, peronei, shoulder abductors and adductors, internal and external rotators, right wrist extensors, abdominals and back extensors on the modified Medical Research Council's manual muscle testing scale with the improvements being maintained over the 9 month follow up duration. These functional improvements and positive musculoskeletal spectroscopic changes provide evidence of the disease modifying benefits of cell therapy in limb girdle muscular dystrophy. However, since these solitary results cannot be generalized to the limb girdle muscular dystrophy population, larger clinical controlled trials are needed.
Keywords: Limb girdle muscular dystrophy; Musculoskeletal magnetic resonance imaging; Musculoskeletal resonance spectroscopy; Cell therapy; Stem cells; Autologous bone marrow mononuclear cells
Introduction
Limb girdle muscular dystrophy (LGMD) is a heterogeneous group of genetic disorders, caused by autosomal dominant or recessive gene mutations and is characterized by progressive muscle weakness [1]. In the Indian setting, LGMD is the commonest adult onset muscular dystrophy [2]. Clinically, LGMD are characterized by proximal, pelvic/scapular and/or distal muscle involvement, high serum creatine kinase levels with sparing of facial muscles. The proteins that are involved in the disease process are diverse and include sarcomeric, sarcolemmal and enzymatic proteins [3]. There are no existing definite treatments for LGMD. As no pharmacological therapies exist that can cure the disease, alternative treatments based on cell transplantation or gene therapy are being widely explored. Significant challenges continue to remain in the use of gene therapy as a treatment option [4]. Use of adult bone marrow stem cells for the treatment of muscular dystrophies have shown some promise in view of their capacity to regenerate damaged skeletal muscle fibers [5]. Musculoskeletal magnetic resonance imaging (MSK MRI) is a useful non-invasive tool to investigate the muscles that are affected and the extent of muscle involvement. MR spectroscopy (MRS) provides information about chemical composition and the metabolite levels of muscle. With disease progression MR Spectroscopy of LGMD patients show progressive increase in lipid content, which reflects the fatty infiltration in the muscle [6]. We report a case of LGMD aged 26 years, treated with serial autologous bone marrow derived mononuclear cells (BMMNC).
Case Presentation
A 26 year old female presented with inability to climb stairs and frequent falls while walking. Her symptoms began at the age of 8, with difficulty in getting up from the floor, difficulty in crossing obstacles, and frequent falls while walking and running. Based on the clinical picture, electromyography (EMG), muscle biopsy and raised creatine phosphokinase levels (216 IU/L), she was diagnosed as a case of LGMD. She was on regular rehabilitation since the age of 9 years. However, there was a continued progressive functional decline in the patient. By age 11, she started facing difficulty in lifting heavy objects and by the age of 15, was unable to climb stairs. On examination prior to cell therapy, she was found to be hypotonic and hypo reflexic with intact sensations. There was pseudohypertrophy of the calf, and genu recurvatum deformity of 20°, bilaterally. She walked with hyperlordosis of the lumbar spine and a wide base of support. Both, the proximal and the distal muscles were affected with greater involvement of the proximal muscles. Functionally, she was independent for most activities of daily living with some assistance required while squatting and getting up from the bed. On Functional Independence Measure (FIM) scale she scored 109. A detailed muscle testing was done using a scale devised by our experienced physiotherapists based on the modified Medical Research Council's manual muscle testing (mMRC MMT). While mMRC MMT, does not sub- classify the grades according to the range of motion (ROM), in our scale (mMRC MMT-I) all the grades were subdivided to be able to appreciate small changes in muscle strength (Appendix 1). Serological, biochemical, and hematological blood tests, chest X- ray, EMG, MSK MRI were performed a week before autologous BMMNC transplantation. EMG was suggestive of myopathic pattern. Serum creatine phosphokinase (CPK) was elevated (563 IU/L). 2D-Echocardiography and color doppler revealed trivial pulmonary regurgitations and mild tricuspid regurgitation, with an ejection fraction of 65%. MSK MRI showed extensive fatty infiltration of the pelvic girdle muscles, including gluteus maximus and minimus, iliacus, gamellus, quadratus femoris, tensor fasciae latae with relative sparing of the obturator internus and externus, and in the anterior, lateral, superficial and deep posterior group of muscles of the leg with sparing of tibialis anterior and posterior, and the popliteus muscles, marked infiltration and loss of muscle volume in the vastus medialis, semimembranosus and semitendinosus with relative sparing of the rectus femoris, sartorius and biceps femoris muscles, complete fatty infiltration and reduced muscle volume in the adductor group of muscles with relative sparing of the gracilis muscle, mild fatty infiltration of the biceps brachii, coracobrachialis, brachioradialis and the medial and lateral heads of triceps with the rest of the muscles of the arm and forearm being normal. MRS revealed raised extramyocellular and intramyocellular (both -CH2 and -CH3) lipid/creatine ratio.
Materials and Methods
Selection of this patient was based on the World Medical Association Revised Declaration of Helsinki [7]. Ethical approval was obtained from the Institutional Committee for Stem Cell research and therapy (IC-SCRT). A signed informed consent for the procedure was obtained from the patient. Granulocyte colony-stimulating factor (GCSF) 300mcg was administered subcutaneously 72 hours and 24 hours prior to the mononuclear cell (MNC) transplantation to enhance the mobilization of BMMNC [8]. Motor points (the point where the innervating nerve enters the muscle belly) of muscles of functional importance were selected for intramuscular transplantation of BMMNC. On the day of transplantation, 110ml of bone marrow was aspirated from the right anterior superior iliac spine and was collected in heparinized tubes. Density gradient method was used to separate the MNC fraction. The purified MNCs were tested for total cell count, viability and CD34+ cell content by Fluorescence-activated cell sorting (FACS) analysis. The total number of cells injected was 50*106 with a viability of 98% and CD34+count of 189 cells/^l. Half of the cell fraction was injected intrathecally at the level between the fourth and fifth lumbar vertebrae. The remaining cells were diluted by the patient's own cerebrospinal fluid (CSF) owing to its properties of harboring cell growth [9]. MNCs were then injected intramuscularly, bilaterally in the respective motor points of deltoids, glutei, quadriceps, peronei, tibialis anterior, abdominals and back extensors. Methyl prednisolone 1gm in 500ml isolyte P was given intravenously to reduce the immediate inflammation. BMMNC transplantation was followed by rehabilitation during the subsequent 4 days of hospital stay. This included therapies given by a physiotherapist, an occupational therapist and a psychologist. Positive response as explained in the results provided the rationale for a second transplantation. The above-mentioned procedure was repeated with MNCs being intramuscularly injected in the respective motor points of biceps, triceps, deltoids, glutei, quadriceps, peronei, tibialis anterior, abdominals and back extensors. A detailed follow-up assessment, including, MSK MRI scan and mMRC MMT (Table 1) were repeated using the same parameters as before, prior to the second transplantation.
Results
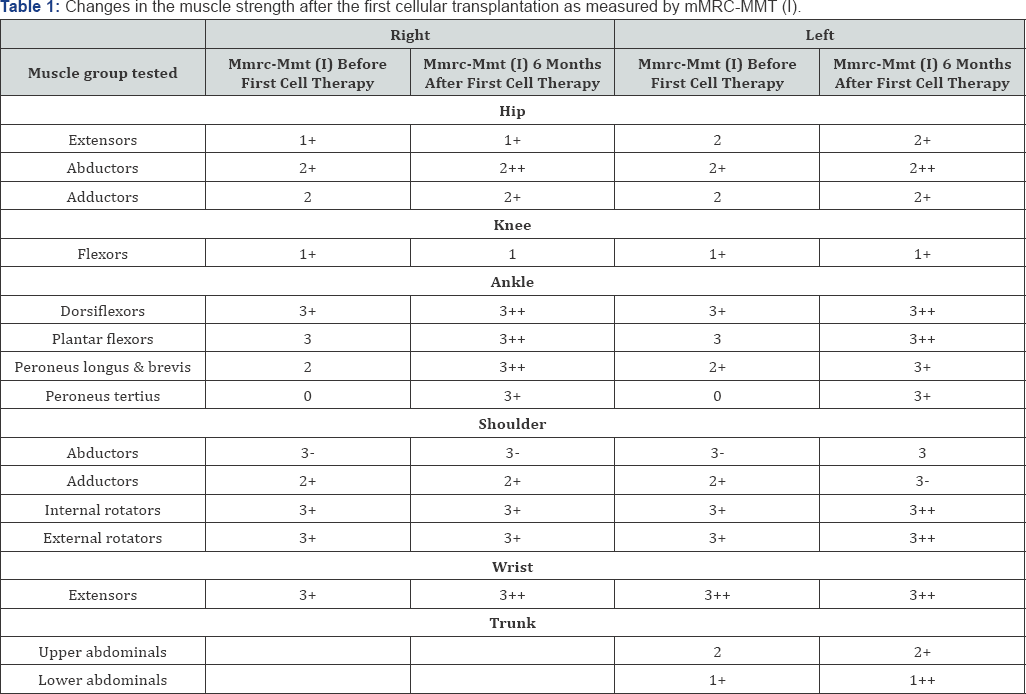
No adverse events were reported by the patient following cell therapy. At 3 months post 1st transplantation there were subjective improvements which included an improved walking balance and speed, decrease in the frequency of falls while walking. She could get up from the chair with less assistance. Her stamina improved and could exercise longer than before, without fatigue. Her pinch strength increased from 7.5 lbs to 8 lbs on the right and 7 lbs to 7.5 lbs on the left. FIM score was maintained at 109. At 6months, the patient could perform transfers with less support than before cell therapy. She could climb stairs holding on to the railing. She could balance on the balance board, which was not possible before intervention. With her being able to climb stairs, the FIM score improved from 109 to 114. As seen in Table 1, muscle power improved in bilateral hip abductors, adductors, left hip extensors, knee flexors, ankle dorsiflexors, plantar flexors, peronei, shoulder abductors and adductors, internal and external rotators; right wrist extensors; abdominals and back extensors.
At 6 months post first transplantation, a second transplantation was repeated to enhance and/or maintain the effects achieved by the first intervention. Repeat MSK MRI was done and it showed no further increase in fatty infiltration or loss of muscle volume in bilateral upper and lower limb musculature compared to the pre-cell transplantation scan. MRS showed less area under the curve in the flattened extramyocellular lipid peak with reduction in the intramyocellular (-CH2) lipid/creatine ratio in the tibialis anterior muscle in comparison to the precell transplantation scan. At 3 months follow up after the second transplantation and 9 months after the first transplantation, it was seen that all the above-mentioned improvements gained were maintained including the FIM score.
Discussion
Due to the heterogeneous nature of LGMD it is difficult to define a common pathway of muscle damage. Possible mechanisms include membrane instability, errors in formation of a functional dystroglycan complex, and defects in muscle repair mechanisms. It is likely that in most LGMD subtypes, membrane instability leads to muscle fiber damage. Damage of muscle fibers leads to the release of inflammatory cytokines and consecutively neutrophils and macrophages dispatched to degrade cellular debris. Muscle satellite cells, the undifferentiated muscle fiber progenitor cells, replace the damaged or necrotic tissue. Eventually, satellite cell populations are either exhausted or lose their reparative ability and deposition of fibrotic and adipose tissue occurs [10]. Management to prolong survival and improve quality of life includes physical therapy to promote mobility and to prevent contractures, use of mechanical aids to help ambulation and mobility, use of respiratory aids when indicated, monitoring for cardiomyopathy in LGMD subtypes with cardiac involvement [11]. These treatment methods however, do not target the underlying pathology. Bone marrow-derived stem cells with their paracrine activity have shown all the necessary attributes for tissue regeneration including, angiogenesis, inhibition of apoptosis, anti-inflammation, homing of endogenous cells, and regulation of specific metabolic pathways [12]. With their capacity to regenerate muscle fiber and renew cell pool, treatment measures using bone marrow cells show some promise [13]. Further, exosomes which are small-membrane vesicles and are responsible for intercellular communication are generated by stem cells. Stem cell derived exosomes promote muscle regeneration by enhancing myogenesis and angiogenesis [14]. Several pre-clinical studies have shown cell therapy as a possible therapeutic approach for muscular dystrophy [3,5,15,16]. In our previously published data we studied the effect of autologous bone marrow mononuclear cells in 65 patients with LGMD. FIM and MMT showed statistically significant improvement, post-cell therapy [17]. However, MSK MRI was not used as an outcome measure in the study. As seen in table 1, this patient showed improvements in strength after cell therapy in the bilateral hip abductors, adductors, ankle dorsiflexors, plantar flexors, peronei, right hip extensors, right knee flexors, right shoulder abductors and adductors, right internal rotators, external rotators, left wrist extensors, abdominals and back extensors on mMRC MMT-I which was maintained through the 9 month follow up duration. The rest of the muscles showed maintained muscle strength.
The improved muscle strength in the plantar flexors correlated with improvements in performing transfer functions and standing from sitting position that were reported by the patient. The improved strength in the back extensors on mMRC MMT-I correlated to an improved standing balance [18]. Muscle biopsy used to be the gold standard for diagnosis of muscular dystrophy, but muscle imaging is becoming more acceptable as it is noninvasive [19,20]. Also, studies have shown that it provides reliable information on disease progression and is a useful tool for assessing therapeutic efficacy [20-22]. MRS efficiently assesses intramyocellular and extramyocellular lipid content helping in measuring the degree of fatty infiltration of muscles. It also serves as a useful tool to detect biochemical deviations in muscle by evaluating creatine metabolism [23]. Lipid and creatine serve as sensitive MR biomarkers for studying disease progression in muscular dystrophy using MRS [24,25]. Studies have shown correlation of functional assessments and MRS studies on follow up in muscular dystrophy patients [26]. It has also shown good reproducibility of quantitative assessment of intramuscular lipid content across different centers and at different points of time in patients with Duchenne muscular dystrophy [27]. Quantitative MRI techniques applied to patients with LGMD has revealed increase in fatty infiltration with disease progression [21]. In this case, six months post intervention MSK-MRI didn't show any fresh individual or muscle group involvement as compared to the previous MRI scan. On MRS, less area under the curve in the flattened extramocellular lipid peak with reduction in the intramyocellular lipid/creatine ratio in the tibialis anterior muscle in comparison to the pre-intervention scan was noted. These are indicative of positive alteration in the disease process. This was also the muscle that was injected with stem cells. Over the duration of 16 years from the time of diagnosis, there was functional deterioration in this patient, indicating progression of disease. But after cell therapy, improvements in muscle strength and functional improvements, over the 13 month follow up period, along with objective improvements seen on MSK MRI, can be considered as positive outcomes of cell therapy. Lack of genetic testing for diagnosing the subtype of LGMD is a limitation of the study. Also, being a single case study, the results cannot be generalized.
- Case Report
- Abstract
- Introduction
- Case Presentation
- Materials and Methods
- Results
- Discussion
- Conclusion
- References
Conclusion
The objective improvements in MSK MRI along with MRS and muscle strength in this case report demonstrates that cell therapy may positively alter the disease process in LGMD. However, these results cannot be generalized and larger clinical controlled trials are needed to establish the therapeutic benefits of cellular therapy in LGMD. MSK MRI together with MRS serves as a reliable objective outcome measure in assessing the efficacy of a treatment and for monitoring disease progression.
References
- Mitsuhashi S, Kang PB (2012) Update on the genetics of limb girdle muscular dystrophy. Semin Pediatr Neurol 19(4): 211-218.
- Khadilkar SV, Singh RK (2008) Limb girdle muscular dystrophies in India. Neurol India 56(3): 281-288.
- Daniele N, Richard I, Bartoli M (2007) Ins and outs of therapy in limb girdle muscular dystrophies. Int J Biochem Cell Biol 39(9): 1608-1624.
- Mendell JR, Clark KR (2006) Challenges for gene therapy for muscular dystrophy. Curr Neurol Neurosci Rep 6(1): 47-56.
- Corbel SY, Lee A, Yi L, Duenas J, Brazelton TR, et al. (2003) Contribution of hematopoietic stem cells to skeletal muscle. Nat Med 9(12): 15281532.
- Kim HK, Lindquist DM, Serai SD, Mariappan YK, Wang LL, et al. (2013) Magnetic resonance imaging of pediatric muscular disorders: recent advances and clinical applications. Radiol Clin North Am 51(4): 721742.
- Carlson RV, Boyd KM, Webb DJ (2004) The revision of the Declaration of Helsinki: past, present and future. Br J Clin Pharmacol 57(6): 695713.
- Haas R, Murea S (1995) The role of granulocyte colony-stimulating factor in mobilization and transplantation of peripheral blood progenitor and stem cells. Cytokines Mol Ther 1(4): 249-270.
- Miyan JA, Zendah M, Mashayekhi F, Owen-Lynch PJ (2006) Cerebrospinal fluid supports viability and proliferation of cortical cells in vitro, mirroring in vivo development. Cerebrospinal Fluid Res 3: 2.
- Murphy AP, Straub V (2015) The classification, natural history and treatment of the limb girdle muscular dystrophies. J Neuromuscul Dis 2(S2): S7-S19.
- Pegoraro E, Hoffman EP (2012) Limb-girdle muscular dystrophy overview.?
- Burdon TJ, Paul A, Noiseux N, Prakash S, Shum-Tim D (2011) Bone marrow stem cell derived paracrine factors for regenerative medicine: current perspectives and therapeutic potential. Bone Marrow Res 2011: 207326.
- Bittner RE, Schofer C, Weipoltshammer K, Ivanova S, Streubel B, et al. (1999) Recruitment of bone-marrow-derived cells by skeletal and cardiac muscle in adult dystrophic mdx mice. Anat Embryol 199(5): 391-396.
- Nakamura Y, Miyaki S, Ishitobi H, Matsuyama S, Nakasa T, et al. (2015) Mesenchymal-stem-cell-derived exosomes accelerate skeletal muscle regeneration. FEBS Lett 589(11): 1257-1265.
- Salani S, Donadoni C, Rizzo F, Bresolin N, Comi GP, et al. (2012) Generation of skeletal muscle cells from embryonic and induced pluripotent stem cells as an in vitro model and for therapy of muscular dystrophies. J Cell Mol Med 16(7): 1353-1364.
- Cossu G, Sampaolesi M (2004) New therapies for muscular dystrophy: cautious optimism. Trends Mol Med 10(10): 516-520.
- Sharma A, Sane H, Gokulchandran N, Gandhi S, Bhovad P, et al. (2015) The role of cell transplantation in modifying the course of limb girdle muscular dystrophy: a longitudinal 5-year study. Degenerative Neurological and Neuromuscular Disease 5: 93-102.
- Caruthers EJ, Thompson JA, Schmitt LC, Best TM, Chaudhari AMW, et al. (2014) Individual Muscle Forces during Sit to Stand Transfer.
- Fan Zheng Jane (2015) Overview of Current Treatments for Muscular Dystrophy. Muscular Dystrophy Springer International Publishing 6571.
- Kumbhare, Dinesh A (2015) Advanced skeletal muscle MR imaging approaches in the assessment of muscular dystrophies. International Journal of Physical Medicine & Rehabilitation
- Willis TA, Hollingsworth KG, Coombs A, Sveen ML, Andersen S, et al. (2014) Quantitative magnetic resonance imaging in limb-girdle muscular dystrophy 2I: a multinational cross-sectional study. PLoS one 9(2): e90377.
- Willis TA, Hollingsworth KG, Coombs A, Sveen ML, Andersen S, et al. (2013) Quantitative muscle MRI as an assessment tool for monitoring disease progression in LGMD2I: a multicentre longitudinal study. PloS one 8(8): e70993.
- Deshmukh S, Subhawong T, Carrino JA, Fayad L (2014) "Role of MR spectroscopy in musculoskeletal imaging. Indian J Radiol Imaging 24(3): 210-216.
- Willcocks RJ, Rooney WD, Triplett WT, Forbes SC, Lott DJ, et al. (2016) Multicenter prospective longitudinal study of magnetic resonance biomarkers in a large duchenne muscular dystrophy cohort. Ann Neurol 79(4): 535-547.
- McIntosh L, Granberg KE, Briere KM, Anderson JE (1998) Nuclear magnetic resonance spectroscopy study of muscle growth, mdx dystrophy and glucocorticoid treatments: correlation with repair. NMR in Biomedicine 11(1): 1-10.
- Hogrel JY, Wary C, Moraux A, Azzabou N, Decostre V, et al. (2016) Longitudinal functional and NMR assessment of upper limbs in Duchenne muscular dystrophy. Neurology 86(11): 1022-1030.
- Forbes SC, Walter GA, Rooney WD, Wang DJ, DeVos S, et al. (2013) Skeletal muscles of ambulant children with Duchenne muscular dystrophy: validation of multicenter study of evaluation with MR imaging and MR spectroscopy. Radiology 269(1): 198-207.






























