In Vitro Studies on Anticancer Activity of Capsaicin a Component of Hot Chili Pepper against Human Hepatocellular Carcinoma Cells
Ahmed S Sultan1, Faten Z Mohammed2* and Al-shimaa M Abas2
1Biochemistry Department, Alexandria University, Egypt
2Biochemistry Department, Zagazig University, Egypt
Submission: April 07, 2017; Published: May 03, 2017
*Corresponding author: Faten Z Mohammed, Biochemistry Department, Faculty of Science, Zagazig University, Egypt; Email: dr_fzahran@yhoo.com
How to cite this article: Ahmed S S, Faten Z M, Al-shimaa M A.In Vitro Studies on Anticancer Activity of Capsaicin a Component of Hot Chili Pepper against Human Hepatocellular Carcinoma Cells. IInt J cell Sci & mol biol. 2017; 2(4) : 555591. DOI : 10.19080/IJCSMB.2017.02.555591
Abstract
Capsaicin is the major pungent ingredient in red peppers. It exerted antitumor activity in several tumor models. Purpose: To investigate the anti-tumor effect of capsaicin on Human HepG2 cells. Materials and Methods: The MTT assay for cell viability, cell morphology and DNA fragmentation levels andthe expression of apoptosis/cell cycle-related proteins (or genes) was examined by western blotting were as-sessed and immunocytochemistry. Results: Capsaicin was found to inhibit the growth and proliferation of HepG2 cells in a dose- and time-dependent manner. Apoptotic cell death was confirmed by observing increases in nuclear condensation, nuclear DNA fragmentation, down regulation of cyclin-Dl, increased cleaved PARP (p85) levels and increased histone release. Conclusion: Our data suggested that capsaicin, may have a role in the management of HepG2 cells.
Keywords:Capsaicin; Human HepG2 cells; PARP; Cyclin-Dl
Introduction
Fruits and vegetables are natural medicines and have been used in our daily diet. Phytochemicals present in the dietary fruits and vegetables have anticancer properties [1]. Capsaicinoids are naturally occurring phenolic compounds commonly present in the genus Capsicum [Solanaceaej and having remarkable anti mutagenic and anti tumour property [2]. Among the chilli varieties C. chinense contains the highest concentration of capsaicinoids. Capsaicin and dihydrocapsaicin are the most abundant capsaicinoids in pepper fruits [3]. In recent findings, capsaicin is seen to show anti-proliferative effects on various human cancer cell lines by apoptosis mediated cell death [4]. Diverse studies have shown that capsaicin has anti proliferative effect on several human cell lines derived from multiple myeloma, gastric cancer, pancreatic cancer, breast cancer [5] and prostate cancer [6], etc. However, the molecular mecha-nisms underlying capsaicin-induced apoptosis are cell type dependent: capsaicin induces apoptosis in sensory neurons by increasing calcium influx and does so by activating vanil-loid receptors in some transformed cells [7]. In human colon cancer cells, capsaicin triggers apoptosis through the inhibition of plasma membrane NADH-oxidoreductase activity and/or NADH: coenzyme Q oxidoreductase in the mito-chondrial electron transport system, generating reactive ox-ygen species [8]. Moreover, capsaicin was found to be as-sociated with PPARy during the regulation of cell growth and apoptotic cell death in breast or colon cancer cells. Over the past decade, there is continuous increase in Hepatocellular carcinoma [HCCj in the world as the most common malignant diseases [9].
Materials and Methods
Cell line and reagents
HepG2 Cells were obtained from American Type Culture Collection (ATCC, USA) and cultured in a 37 °C incubator with 5% CO2 according to ATCC protocols. Capsaicin was purchased from Enzo Life Science Company. Antibodies of PARP-1, Cyclin-Dl, β-actin and all secondary antibodies were purchased from Santa Cruz Bio-technology (Santa Cruz, CA, USA).
Growth inhibition
Growth inhibition was assessed via an MTT assay. Briefly, Hep G2 cells were plated at a density of 30,000 cells/well on 24- well plate. After overnight growth, the cells were treated with various concentrations of capsaicin (10-250μM) for 24h. 10μL of the 12mM MTT stock solution (Loba Chemie Pvt. Laboratory Reagents, India.) was added to each well. A negative control of 10 μL of the prepared MTT stock solution was added to 100μL of medium alone as a control. Cells were incubated at 37°C for 4 hours. 100μL of the prepared SDS-HCl solution were added to each well and mixed thoroughly using the pipette. The micro plate was incubated at 37°C for 18 hours in a humidified chamber. Each sample was mixed again using a pipette, the absorbance was read at 570nm by using a microplate ELISA reader and IC50 was determined.
Cell Morphological changes of HepG2 cell line
The morphological changes of cells were examined before and after the treatment with capsaicin drug (25-250) μM to test its effect on the morphology of HepG2 Cell line. Equal numbers of cells/well were seeded onto 12-well plates with appropriate different concentration of capsaicin drug and incubated for 48 hour. Cells were washed with PBS and fixed with 10% formalin buffer. Cells were viewed by an inverted microscope with magnification 200-X. Digital images were acquired with Codac microscopic digital camera.
DNA fragmentation
Cells were cultured and then treated with different concentration of capsaicin (50-250) μM for 3 days. Adherent cells were detached by 1ml Trypsin/EDTA. Cells were centrifuged for 5 minutes at 15000 rpm and supernatant was discarded and resuspended in (1-10)ml ice cold PBS. Cells were centrifuged for 5 minutes at 15000rpm, supernatant was discarded. Cells were re-suspended in 1 volume digestion buffer (10 mM EDTA, 50mM Tris-HCl, pH 8.0, 0.5% SDS, 0.5mg/mL proteinase K). For 3x107 cells, 0.3ml digestion buffer was used. Samples were incubated, being shacked in tightly capped tubes, 18 hours at 50°C. Samples were extracted with an equal volume of Phenol / Chloroform/ Iso-amyl alcohol, centrifuged for 10 minutes at 15000rpm. The top layer (aqueous) was transferred to a new tube. 1/2 of the volume of 7.5M ammonium acetate was added. Double of the volume of 100% ethanol was added, centrifuged for 2 minutes at 15000 rpm. Organic solvents were removed by dialysis against 100 volume TE buffer for 24 hours. Samples were washed with 70 % ethanol, left in air to dry and re-suspended in Tris-EDTA buffer at ~1mg/ml prior to electrophoresis on a 1.2 % agarose gel con-taining ethidium bromide.
Enzyme linked immunosorbent apoptosis assay
Cells were seeded at a density of 2 X 104/ well in a 96 well plate and incubated for 24 hours. Media was changed to media containing the tested drug (add your drug conc μM). Cells then incubated for extra 24 hours. An ELISA assay was performed, using Cell Death Detection ELISAPLUS kit (Roche-Applied Science, Indianapolis, USAj that measures histone release from fragmented DNA in apoptosing cells. Briefly, cells were lysed with 200-μL lysis buffer for 30 min at room temperature. The lysate was centrifuged for 10min and 20 μL of collected supernatant was incubated with anti-histone biotin and anti- DNA peroxidase at room temperature for 2h. After washing with incubation buffer three times, 100μL of substrate solution (2,2'-azino-di(3-ethylbenzthiazolin-sulphuric acid) was added well and incubated for 15-20min at room temperature. The absorbance was measured using an ELISA reader (Spectra Max Plus) at 405nm. Each assay was done in triplicate and standard deviation determined).
Western blotting
HepG2cells were treated with (50-250) μM capsaicin for 24 hours. The cell culture dishes were placed in ice and the cells were washed with ice-cold PBS. The PBS was drained, and then lysed by the addition of 300μL of ice-cold lysis buffer (150mM Sodium chloride, 1.0%Triton X-100, 0.5% sodium deoxycholate, 0.1% SDS, 50 mM Tris, pH (8.0). Just prior to use add: 10mM beta- mercaptoethanol, 10μg/ml PMSF, 100mM sodium orthovanadate and 15μ/ml Triton X-100). Adherent cells were scraped off thedish using a cold plastic cell scraper. The supernatant from each lysate was collected into new tubes after centrifugation at 20000g for 15 minutes at 4°C. Total protein concentrations were measured using the BCATM protein assay kit (Pierce, Rock-ford, IL, USA).Proteins were separated by 10% SDS-PAGE and electrically transferred to a polyvinylidene difluoride membrane (Millipore, Billerica, MA, USA). After blocking in 5% BSA in TBS, the membranes were probed with specific primary antibodies overnight at 4°C, washed three times with TBS-Tween 20, and then incubated with HRP-conjugated secondary antibodies at room temperature for 1h. Then the membranes were washed with TBS-Tween 20 and the protein bands were visualized using 3,3',5,5' tetramethylbenzidine (TMB) is the traditional used kits. Densitometry of bands was performed using Image J analysis software (NIH).
Immunocytochemistry
HepG2 Cells were cultured and then treated with different concentration of capsaicin (150 and 200) μM for 1 day. Adherent cells were detached by 1ml Trypsin/EDTA. Cells were centrifuged for 5 minutes at 1000rpm and supernatant was discarded and washed with 1ml ice cold PBS then transferred to new eppendurf tube and centrifuged again at 1000rpm and supernatant was discarded. About 200μl collagen (Collagen I, bovine: sc-29009 supplied as 30mg in 0.012N HCl at a concentration of 2.9mg/ ml.) was added to each eppendurf tube then cooled at 1°C for 30 minutes to be solidified then transferred to fridge till to be sectioned. Then fixed in 10% buffered formalin overnight, transferred to 70% ethanol, embedded in paraffin, and sectioned at 5μm. Slides were dehydrated, put in a warm sodium citrate (10mM) in the microwave for 20 minutes then left to cool. Slides were incubated in 3% H2O2 for 15 minutes to reduce non specific background then blocked with 100-400μl blocking solution for 1 hour.
Slides were then incubated with anti-cyclin D1 antibody (1:1000 dilution) overnight and then exposed to the secondary antibody (Biotinylated Goat Anti- polyvalent secondary antibody was applied to the slides and incubated for 10 minutes at room temperature at 1:1000 dilution) from Santa Cruz. The slides were washed four times in PBS buffer. Streptavidin -HRP Peroxidase was applied to the slides and incubated for 10 minutes at room temperature and the slides were rinsed 4 times in PBS buffer. About 100μl DAB or suitable substrate was added to each section and incubated for 15 minutes. As soon as the sections were stained well, slides were immersed in dH2O. The slides were incubated into Hematoxylin for 5-10 minutes and then washed under tap water for 10 minutes and dehydrated by being put in Ethanol 70%, Ethanol 95%, Ethanol 100%, Xylene I and Xylene II for 5 minutes for each. The tissue were stabilized by mounting medium (Canda Palsm), covered by slide cover and examined by microscope.
Statistical analysis: All the statistical analysis was performed by SPSS software (version 14.0). The experiments were performed in triplicate. All the quantitative data are expressed as mean values ± standard deviation, the significant differences between two groups was assessed by a Post-Hoc comparison. A probability value of P < 0.05 was considered to represent a statistically significant difference [10].
Results
Effect of capsaicin on cell viability of HepG2 cell line
As shown in Figure 1, Capsaicin has significant (p < 0.001) inhibitory effect on HepG2 human liver cell line after treatment with 25-250μM for 24h. Collectively Capsaicin significantly decreased the percentage of viable cells in a dose-dependent manner.

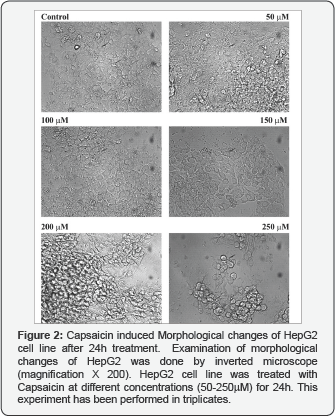
Capsaicin induced morphological changes of HepG2 cell line
Microscopic examination of the treated cells showed that the treatment with 150μM, 200μM and 250μM Capsaicin resulted in dramatic morphological changes in HepG2 cell line after 24h. We observed morphological changes of cells from epithelial- like shape to spindle and rounded shape. The monolayer cells become rounded up, lost contact with neighboring cells and detached from culture plates. Our data suggested that the morphological changes of HepG2 cell line started after treatment with 150μM. All the treated cells showed cytoplasmic condensation, shrinkage, tendency to float in the medium and reduction in size after Capsaicin treatment for 24h compared to mock. Taken together Capsaicin induced morphological changes in a dose dependent manner (Figure 2).
DNA fragmentation induction in HepG2 cell line
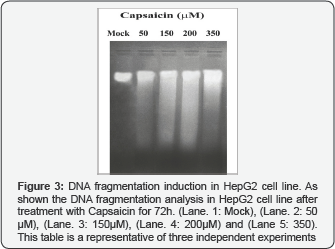
One of the biochemical features of apoptosis is the fragmentation ofgenomic DNA. For analysis ofDNA fragmentation by agarose gel electrophoresis, total DNA was extracted after treatment of the cells with 50 ~ 350 μM of Capsaicin for 72h. Capsaicin was found to induce inter-nucleosomal degradation of DNA in all treatment concentrations, resulting in ladder-shaped nucleosomal DNA fragments compared to mock as shown in Figure 3.
Capsaicin induced apoptosis in HepG2cells by increasing caspase-3 activity and histone release
We selected this time point to determine whether induction of apoptosis in HepG2cells treated with Capsaicin was associated with the activation of caspase-3 and histone release. After the HepG2 cells had been treated with or without various concentrations of Capsaicin for 24h, the cells were harvested and caspase-3 activity was analyzed by ELISA. The data indicated that Capsaicin induced caspase-3 activity in a dose-dependent manner (Table 1) (Figure 4). Our results revealed that caspase-3 activity and histone release, as a mark of apoptosis induction, were significantly increased after HepG2 cells were treated with 50, 100, 150, 200 and 350 μM Capsaicin as compared to the control group (P< 0.001.
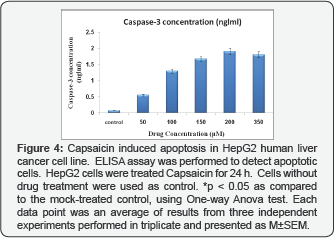
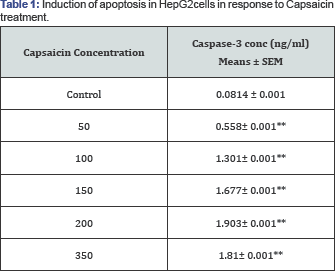
*P< 0.05, **P< 0.001 as compared to control group.
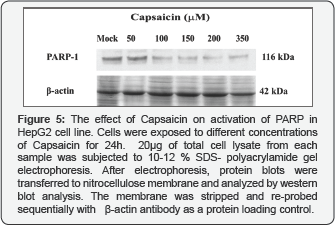
Capsaicin induced PARP-1 cleavage and cell cycle arrest by down-regulation of cyclin D1 inHepG2 cell line
Next, we studied capsaicin’s activity on cell cycle and apoptosis. Our data showed that PARP-1 protein expression underwent cleavage due to Capsaicin treatment. PARP-1 significantly decreased in dose dependant manner, moreover, the cleavage of PARP-1 is considered to be an important marker for detection of apoptosis (Figure 5). Also, we examined the protein expression level of cyclin D1, our data revealed that Capsaicin was found to down-regulate expression of cyclin D1 in a dose- dependent manner [Figure 6.

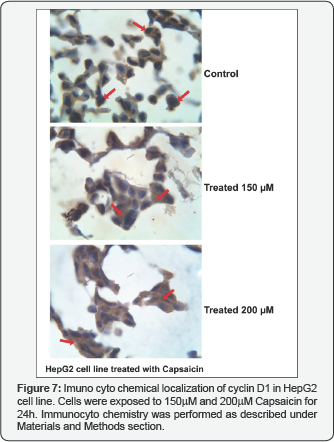
To confirm the down-regulated of tested cyclin D1 expression after Capsaicin treatment, we determined cyclin D1 localization and expression in nuclei of HepG2 cell line by immunocytochemistry assay (ICC), using specific anti- cyclin D1 monoclonal antibody. Nuclear expression of cyclin D1 was decreased after treatment with 150 ~200μM Capsaicin for 24h and cyclin D1 expression was increased in the cytoplasm of treated cells compared to mock (compare the brown staing of cyclin D1 with the counter staining blue color of the nuclei) as shown in Figure 7.
Discussion
Capsaicin acts as a carcinogen,co-carcinogen, or anticarcinogen is controversial. Nonetheless, accumulating findings indicate that capsaicin possesses anti-cancer properties in various cancer celllines [11]. The goal of our study was to explore the mechanism underlying capsaicin-induced cell death inhuman HepG2 cancer cells. Here, we report that treatment of HepG2 cells with capsaicin inhibits their proliferation/ viability, halts cell cycle progression, and induces apoptosis through increasing PARP-1 cleavage and induce cell cycle arrest by decreasing the expression of cyclin D1. HepG2 liver cancer cell line is widely used due to its relatively high steady-state functioning of the free radical production and antioxidant defenses; therefore, variations of responses at different conditions are more easily detected [12]. To investigate the effect of capsaicin on cell viability of HepG2 cells. Cells were treated with or without different concentrations of capsaicin. Our data found that capsaicin significantly decreased in cell viability of treated HepG2 cells using MTT assay. The proliferation of HepG2 cells showed strong inhibition by capsaicin at 150μM, 200μM and 250 μM, indicating anti-proliferation activity of capsaicin on HepG2 cell line and raising the possibility that capsaicin might be a potential chemo- preventive or therapeutic agent. Those effective doses showed no cytotoxic effects on different cell lines [4]. Our results are in agreement with who stated that treatment of human colon cancer cells HCT116 with capsaicin showed that at low concentration (0-40μM), capsaicin had shown little effect on the growth inhibition, but at high concentration (80-160μM), long-term (48-72h) treatment with capsaicin substantially inhibited cell proliferation [13]. Amruthra et al. revealed that HepG2 cells showed reduction in the cell viability through MMT assay due to capsaicin exposure in a dose-dependent manner [14].
Apoptosis is an important feature of epithelial tissues with a high turnover and serves to balance the rate of new cell production. Death by apoptosis eliminates specific cells without extensive tissue damage, playing a pivotal role during regeneration and elimination of tumor cells [15]. Tumor cells are known to be resistant to apoptotic stimuli by several mechanisms. Tumors overexpress anti-apoptotic proteins such Bcl-2 and have decreased expression of pro-apoptotic proteins such as Bax or BH3, have impaired signals from death receptors, or activated nuclear factor NF-kB which prevents activation of caspase-mediated cleavage [16]. Large numbers of previous experiments have consolidated capsaicin's activity in cancer prevention, and moreover, it was shown that capsaicin induced apoptosis played an important role in its antitumor activity in a variety of cancer cell lines. The capacity of capsaicin to suppress the growth of cancer cells is primarily mediated through induction of apoptosis. Additionally, the activities associated with capsaicin-induced anti-cancer effects include the arrest of cell cycle progression, regulation of transcription factor expression, and suppression of growth signal transduction pathways [17]. Our study reported that capsaicin induced apoptosis in human HepG2 cells, demonstrated by DNA fragmentation induction and morphological changes of apoptotic cell death characteristics.
Our data revealed that capsaicin induce cell death in a dose- dependent manner in HepG2 cell line. In addition, microscopic examination of the treated cells showed that treatment with capsaicin for 24h resulted in dramatic morphological changes with the following features: monolayer cells become rounded up, shrinkage, with cytoplasmic condensation, lost contact with neighboring cells, nuclear condensation, and tendency to float in the medium. This was consistent with partially DNA fragmentation data.
The fragments were in oligonucleosome size, which could be approve of apoptotic induction by capsaicin treatment in HepG2 cells. Our results are in agreement with Cho et al. [5] stated that MG63 human osteosarcoma cells were cultured for 24h with different concentrations of capsaicin, the cells exhibited the morphological features of apoptosis when treated with 150|iM capsaicin for 24h. These morphological changes of the cells represent the apoptotic cell death [18]. Also, Kim stated that A375 human melanoma cells treated with capsaicin induced DNA ladder formation [19]. PARP-1 is a nuclear enzyme, the critical role of PARP1 in DNA repair, PARP1 is involved in the repair of modified bases, ssDB and dsDB, blocking the ADP-ribosylation activity with small molecules, can achieve synthetic lethality with DNA damaging agents in the treatment of cancer [20]. Besides DNA repair, the importance of PARP1 as a transcriptional regulator is also well established. As an enzyme, PARP1 acts on chromatin remodeling complexes to control DNA accessibility for RNA polymerase. PARP1 also functions as a transcription factor by binding an octamer motif in promoter elements to regulate gene expression [21]. During apoptosis, cleavage of PARP-1 in fragments of 89 and 24 kDa has become a useful hallmark of this type of cell death. This cleavage is well studied and is generated by the caspases 3 and 7, proteases activated during apoptosis [22]. Here, we investigated the possibility to PARP-1 expression by capsaicin treatment. Protein extracts were prepared from treated cells and analyzed by immuno blotting, using an antibody specific for PARP-1.
Our data showed that expression level of PARP-1 significantly decreased after treatment of HepG2 cell line with different concentrations of capsaicin for 24h compared to mock cells, indicating that PARP-1 underwent cleavage, thereby confirming apoptosis. The appearance of cleaved PARP fragment indicates that the DNA repair system in the cell has been damaged. Our results are in line with who found that treatment of KB cancer cells with capsaicin induced disruption of the mitochondrial membrane potential as well as activation of caspase 9, 3 and poly- (ADP-ribose)polymerase in KB cells [13]. Also, Jin et al. reported that capsaicin had a profound anti-proliferative effect on human colon cancer cells via inducing cell cycle G0/G1 phase arrest and apoptosis, which was associated with an increase of p21, Bax and cleaved PARP [14]. Zhang et al. indicated that capsaicin induced apoptosis in human pancreatic cancer cells is initiated by the generation of ROS, which was followed by increased protein expression of Bax, disruption of the mitochondrial membrane potential and release of cytochrome c and AIF into the cytosol leading to the activation of caspase 9/3cascade. Mitochondria play a pivotal role in the signal transduction of apoptosis. Disruption of mitochondrial membrane potential results in the modulation of Bcl-2family of pro- and anti-apoptotic proteins and release of cytochrome c and/or apoptosis inducing factor (AIFJ from the mitochondria into the cytosol. Once cytochrome cis released into the cytosol, it forms an apoptosome withApaf-1 and procaspase-9. This causes the activation ofcaspase-9, which further activates caspase-3 and PARP as evident by their cleavage. Results suggest the activation of intrinsic mitochondrial death pathway by capsaicin [23].
We additionally examined the effect of capsaicin on cell cycle progression; we examined cyclic D1 expression by capsaicin treatment. Protein extracts were prepared from treated cells and analyzed by immunoblotting, using an antibody specific for cyclin D1. Our data showed that expression level of cyclin D1significantly decreased after treatment of HepG2 cell line with different concentrations of capsaicin for 24h compared to mock cells, indicating that cyclin D1 down-regulated due to capsaicin treatment. The cyclin D1 is required for the activity of cyclin-dependent kinase 4, which phosphorylates Rb, thus releasing E2F to mediate the G1to S transition, inturn leading to DNA synthesis and cell cycle progression [24]. Our results are in aggrement with Le et al. [25] revealed that capsaicin suppressed the cell cycle progression at the G1/S phase in FaDu cells by decreasing the expression of the regulators of cyclin B1 and D1, as well as cyclin-dependent protein kinases cdk-1, cdk-2 and cdk-4 [25]. Results revealed that antiproliferative effect of capsaicin correlateswith G1arrest of endothelial cells. It is possible that this effect is mediated through either inhibition of cyclin D1 expression or induction of a cyclin-dependent kinase inhibitor, p21. Our results of western blotting are confirmed by cyclin D1 localization in the nucleus of HepG2 cell line. Immunocytochemistry assay (ICC) was performed using ant- cyclin D1 antibody. Results revealed nuclear expression of cyclin D1 showed weak expression after treatment with 150μM and 200μM capsaicin for 24h.
Conclusion
It is suggested that capsaicin inhibit the growth and proliferation of HepG2 cells by increasing nuclear condensation, nuclear DNA fragmentation, down regulation of cyclin-D1, increased cleaved PARP (p85) levels and increased histone release. Capsaicin could be developed as a promising chemopreventive natural supplement for liver cancer.
References
- Chung SY, Sung MK, Kim NH, Jang JO, Go EJ, et al. (2005) Inhibition of P-glycoprotein by natural products in human breast cancer cells. Arch Pharm Res 28(7): 823-828.
- George FA, Terry B, Robert LJ (2009) Pungency in Capsicum chinense: Variation among countries of origin. Journal of Environmental Science and Health Part B 44(2): 179-184.
- Reyes-Escogido Mde L, Gonzalez-Mondragon EG, Vazquez-Tzompantzi E (2011J Chemical and Pharmacological Aspects of Capsaicin. Molecules 16(2): 1253-1270.
- Lin CH, Lu WC, Wang CW, Chan YC, Chen MK (2013) Capsaicin induces cell cycle arrest and apoptosisin human KB cancer cells. BMC Complementary and Alternative Medicine 13: 46.
- Chou CC, Wu YC, Wang YF, Chou MJ, Kuo SJ, et al. (2009) Capsaicin induced apoptosis in human breast cancer MCF-7 cells through caspase-independent pathway. Oncol Rep 21(3): 665-671
- Mori A, Lehmann S, O'Kelly J, Kumagai T, Desmond JC, et al. (2006) Capsaicin, a component of red peppers, inhibits the growth of androgen-independent, p53 mutant prostate cancer cells. Cancer Res 66(6): 3222-3229.
- Amantini C, Mosca M, Nabissi M, Lucciarini R, Caprodossi S, et al. Capsaicin-induced apoptosis of glioma cells is mediated by TRPV1 vanilloid receptor and requires p38 MAPK activation. J Neurochem 102(3): 977-990.
- Kim MY, Trudel LJ, Wogan GN (2009) Apoptosis induced by capsaicin and resveratrol in colon carcinoma cells requires nitric oxide production and caspase activation. Anticancer Res 29(10): 3733-3740.
- Bruix J, Sherman M (2005) Management of Hepatocellular Carcinoma. Hepatology 42:1208-1236.
- Levesque R (2007) SPSS Programming and Data Management: Guide for SPSS and SAS Users, Fourth Edition, SPSS Inc., Chicago, IL 606066412, USA.
- Ip SW, Lan SH, Huang, AC, Yang JS, Chen YY, et al. (2012) Capsaicin induces apoptosis in SCC-4human tongue cancer cells through mitochondria-dependent and independent pathways. Environ Toxicol 27(6): 332-341.
- Alia M, Mateos R, Ramos S, Lecumberri E, Bravo L, et al. (2006) Influence of quercetin and rutin on growth and antioxidant defense system of a human hepatoma cell line (HepG2). Eur J Nutr 45(1): 1928.
- Jin J, Lin G, Huang H, Xu D, Yu H, et al. (2014) Capsaicin Mediates Cell Cycle Arrest and Apoptosis in Human Colon Cancer Cells via Stabilizing and Activating p53. Int J Biol Sci 10(3): 285-295.
- Amruthra NJ, Preetamraj JP, Saravanan S, Lebel LA (2014) In vitro studies on anticancer activity of capsaicinoids from capsicum Chinensea against human hepatocellular carcinoma cells. Int J Pharm Pharm Sci 6(4): 254-558.
- Yang WY, Liu CH, Chang CJ, Lee CC, Chang KJ, et al. (2006) Proliferative activity, apoptosis and expression of oestrogen receptorand BCl-2 oncoprotein in canine mammary gland tumors. J Comp Path 134: 7483.
- Teijido O, Dejean L (2010) Upregulation of Bcl2 inhibits apoptosis- driven BAX insertion but favors BAX relocalization in mitochondria. FEBS Lett 584(15): 3305-3310.
- Pramanik KC, Boreddy SR, Srivastava SK (2011) Role of mitochondrial electrontransport chain complexes in capsaicin mediated oxidative stressleading to apoptosis in pancreatic cancer cells. PLoS One 6: e20151.
- Cho WH, Lee HJ, Choi YJ, Oh JH, Kim HS (2013) Capsaicin induces apoptosis in MG63 human osteosarcoma cells via the caspase cascade and the antioxidant enzyme system. Molecular Medicine Reports 8(6): 1655-1662.
- Kim MY (2011) Nitric oxide triggers apoptosis in A375 human melanoma cells treated with capsaicin and resveratrol. Molecular Medicine Reportes 5(2): 585-591.
- Krishnakumar R, Kraus WL (2010) The PARP side of the nucleus: Molecular actions, physiological outcomes, and clinical targets. Mol Cell 39(1): 8-24.
- Ko HL, Ren EC (2011) Novel poly (ADP-ribose) polymerase 1 binding motif in hepatitis B virus core promoter impairs DNA damage repair. Hepatology 54(4): 1190-1198.
- Ame JC, Spenlehauer C, de Murcia G (2004) The PARP superfamily. Bioessays 26(8): 882-893.
- Zhang R, Humphreys I, Sahu RP, Shi Y, Srivastava SK (2008) In vitro and in vivo induction of apoptosis by capsaicin in pancreatic cancer cells is mediated through ROS generation and mitochondrial death pathway. Apoptosis 13(12): 1465-1478.
- Mukhopadhyay A, Banerjee S, Stafford L J, Xia C, Liu M, et al. (2002) Curcumin-induced suppression of cell proliferation correlates with down-regulation of cyclin D1 expression and CDK4-mediated retinoblastoma protein phosphorylation. Oncogene 21(57): 88528861.
- Le TD, Jin DC, Rho SR, Kim MS, Yu R, et al. (2012) Capsaicin-Induced Apoptosis of FaDu Human Pharyngeal Squamous Carcinoma Cells. Yonsei Med J 53(4): 834-841.






























