Gene Cascades in Early Drosophila Development
Paromita Das1, Manika Pal-Bhadra1* and Utpal Bhadra2*
1Centre for Chemical Biology, Indian Institute of Chemical Technology, India
2Functional Genomics and Gene Silencing Group, Centre For Cellular and Molecular Biology, India
Submission: March 01, 2017; Published: May 02, 2017
*Correspondence Address: Utpal Bhadra, CSIR - Centre for Cellular and Molecular Biology, Uppal Road, Hyderabad-500 007, India,Tel: +91 40 27192513; Fax: +91 40 27160591; Email: utpal@ccmb.res.in Manika Pal Bhadra, Centre for Chemical Biology, CSIR- Indian Institute of Chemical Technology, Uppal Road, Hyderabad-500007, India, Tel: +91 40 27193236; Email: manikapb@gmail.com
How to cite this article: Paromita D, Manika P-B, Utpal B. Gene Cascades in Early Drosophila Development. IInt J cell Sci & mol biol. 2017; 2(3) : 555587.10.19080/IJCSMB.2017.02.555587
Summary
The segmented body plan in Drosophila embryos is an outcome of the cascade of zone and stage-specific transcription factors operating in unison. The initial players in this cascade are the maternal genes having localization in the pole cells of the embryo. Their gene products create long-range information that subsequently relay the activation of the next cadre of genes, the gap genes, the foremost expressing zygotic genes. These genes having the topmost position in hierarchy are expressed in discrete domains along the longitudinal axis of the preblastoderm. The gradients established by these proteins act as spatial cues to generate repeated pattern of subordinate pair-rule gene in the form of stripes. Henceforth, the developmental fate of the blastoderm cells is pre-destined, in accordance to their position within the anterior- posterior axis and thus, the embryo gets sub-divided into increasingly smaller units that can be later visualised in the larva.
Introduction
The embryo marks its initial steps of development through intricate processes like fertilisation, completion of meiosis and the very first cell divisions. The earliest embryonic development input is by the virtue of the maternal mRNAs and proteins already deposited in the egg ever since the oogenesis. There is an absence of de novo transcription in such a stage and the embryo is in transcriptional quiescence [1,2]. This period of quiescence is followed by the commencement of zygotic transcription, with the subsequent decline of the maternal control of development [3]. The cell cycle shows some very significant changes in such a stage of transfer of control from maternal to zygotic stage.
Cycle of Development in Drosophila
Drosophila, a holometabolous organism, has three distinct stages of their post-embryonic life cycle, having variable body plan, beginning from larva, pupa and finally, to adult. The machinery that ensures the smooth transition between phases develops during embryogenesis. The larval develops post -hatching into the first larval instar with rudimentary cellular structures, called imaginal disc, that destine the presumptive adult structures, as imaginal discs. After the pupal stage, these imaginal discs grow and produce the adult body organs. The egg hatches after 24 hours of fertilization, into a larva, which transits through three molts, encompassing about 5.5 to 6 days, to form a pupa. This pupa then metamorphoses into an adult fly, in about 3.5 to 4.5 days. Thus, the entire growth process from egg to adult fly comprises about 10 to 12 days at 25°C [Russell, Peter J. iGenetics. p.564]. The oocytes have pre-destined anterior-posterior and dorsal-ventral axes, already defined by the maternal activities.
Drosophila presents the most unique pattern of embryogenesis such that cleavage occurs in a multinucleate syncytium, when about 256 nuclei migrate to the perimeter of the egg, forming the syncytial blastoderm. The pole cell formation at the posterior end of the embryo, demarcates the germ line from the somatic cells at the posterior end of the embryo. After thirteen mitotic divisions, about 4 hours after fertilization, about 6,000 nuclei accumulate in the undivided cytoplasm of the oocyte before migration to the surface, enclosed by plasma membranes to form a cellular blastoderm. Gastrulation forms three germ layers: the endoderm, mesoderm, and ectoderm, where the mesoderm invaginates from the ventral furrow, as the ectoderm to form the midget. The pole cells undergo internalization, with germ band elongation caused by rearrangements of cells into the three distinct germ bands. The posterior region expands and extends towards the anterior pole along the dorsal side of the embryo, with the subsequent surfacing of the segments with stripes showing the anterior-posterior axis. The para-segmental furrows form showing earliest inputs of segmentation. Germ band retract thrusting the hindgut to the dorsal side of the posterior pole and coinciding with segmentation. The remaining stages include the nervous system internalization with the formation of internal organs.
The Maternal to Zygotic Transition
The Drosophila embryo undergoes 13 nuclear divisions, devoid of cell division, to form a syncytium. The cell cycle lengthens. At cycle 14, there is a prolongation of cell cycle with the occurrence of cellularisation that causes zygotic transcription to ensue in bulk proportions [4]. The zygotic transcription initiates in embryo, with the maternal factor, Zelda or Vitelfaltig, which prepares the particular genes to be activated first during the MZT [5-7] (Figure 1). Recent studies have cited that embryos lacking Zelda cause disruption in the initiation of zygotic transcription and cellularisation. Thus, it proves that the transcription factors are in scant quantities in the early embryo and the post-transcriptional regulation of maternal mRNAs, particularly the deadenylation, provide the correct input for zygotic transcriptional initiation.
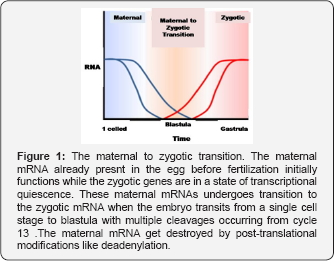
The studies made by workers like Wieschaus, O'Farrell and Grotehans have recently shown that the cell cycle mechanism is regulated by a transcriptional control of a gene subset that cause Cdc25 phosphatase, Twine to destroy, loss of Twine allow the Cdkl to phosphorylate and cause mitosis to occur [8-10]. According to some, the nucleus to cytoplasmic ratio causes the onset of transcription of genes for twine destruction [8-9]. While a parallel group of workers suggest that Twine gets destroyed independently, irrespective of the nucleo-cytoplasmic ratio [10]. Of late, it has been clarified that the gross zygotic transcription is initiated not due to variations of the nucleo-cytoplasmic ratio, but due to temporal changes of events, post-fertilization [11].
Anterior-Posterior Axis Patterning in Drosophila
The antero-posterior axis is patterned via the contribution of three fundamental classes of genes, namely the maternal effect genes, segmentation genes, and homeotic genes (Figure 2). There are 4 sets of maternal effect genes that establish the embryonic axes. The anterior system comprises Exuperantia, Swallow, Staufen, Bicoid, the posterior system comprising Nanos, Pumilio and caudal and the terminal system including the Torsolike and Trunk.

The initial cues of development are already patterned, right from oogenesis, prior to the fertilization and deposition of egg. The maternal effect genes decide the polarity of the egg and of the embryo via the differential localization of the mRNA molecules. The genes that code for these maternal effect genes, encode proteins that after fertilization, form protein gradients to establish concentration gradients across the egg. Bicoid and hunchback constitute the most crucial maternal effect genes for patterning of anterior regions, comprising the head and thorax of the embryo. Likewise, the posterior abdominal segements are dictated by the genes, Nanos and Caudal [12]. The mRNA molecules get localized to specific region based on the attachment to the microtubules. The bicoid mRNA thus attach to the anterior end of the oocyte. As per some reports the transcript is strictly localized to the anterior tip of the unfertilised egg while the transcript transverse to the about 20 to 40 percent of the anterior domain of the egg. Nanos mRNA gets attached to the microtubules and concentrated to the posterior. There is an even distribution of Hunchback and Caudal mRNA all throughout the egg.
The ds RNA binding protein Staufen guides the proteins Bicoid, Nanos and other proteins forming the anterior-posterior axis and creates apt region-wise protein gradients. The maternal effect gene transcribed mRNAs translate to protein gradients that form at the posterior end. BICOID protein inhibits the expression of the Caudal mRNA such that caudal protein gets a lower concentration at the anterior end. This caudal protein activates genes to form the posterior structures during the segmentation. The Nanos protein creates a posterior-to-anterior gradient and contributes to abdomen formation. Nanos protein, along with Pumilio protein, binds to the hunchback mRNA to blocks its translation in the posterior end. The levels of Hunchback protein early embryo get augmented by the transcription of the new hunchback gene and the translation of the zygotic mRNA. Gene expression in the syncitium nuclei is done by the Bicoid, hunchback, and Caudal proteins. Their expression patterns in the early embryo are determined by the maternal effect gene products and shown in the diagrams on the right side of this page. The gap genes, which are a part of the segmentation genes that establish the segmented body plan of the embryo along the anterior-posterior axis. These genes code for 14 para segments that relate to the final anatomical segments [13-15].
The pair-rule genes express in stripes of seven bands perpendicular to the anterior-posterior axis within the syncytial blastoderm. Post cell membrane formation, cellular blastoderm forms. The engrailed gets expressed in one row of cells at the edge of each para segment and gets initiated by the pair rule genes. The wingless acts on the cell rows by acting through the receptor Frizzled. The engrailed-expressing cells show expression after the cellular blastoderm forms. Hedgehog and Wingless, show reciprocal signalling by the patched and naked proteins that determine the boundary of each segment. The order of the gene expression in the anterior-posterior axis is based on the order in which the gene is expressed.
The homeotic selector genes labial, antennapedia, sex combs reduced, deformed, and proboscipedia constitutes the antennapedia. Labial and Deformed proteins express in head segments while sex-combs-reduced and Antennapedia specify thoracic segments. The bithorax group genes control the specializations of the third thoracic segment and the abdominal segments with mutations that could be lethal.
Dorsal-Ventral Axis Patterning
The dorsal-ventral axis formation is dependent on the concentration of a maternally synthesized Dorsal at ventral regions of the nucleus. The movement of the oocyte nucleus along microtubules, from posterior to anterior-dorsal oocyte margin lead to the determination of the dorsal side of the embryo. The nuclear protein, Gurken activates follicle cells in the dorsal region by interacting locally with the Torpedo receptor which inhibits the production of PIPE protein. The follicular cells that express PIPE are on the ventral side. PIPE leads to the activation of an extracellular protease cascade in the perivitelline space between the follicle cells and the egg. This leads to the cleavage of the Toll-ligand, Spatzle and activates the Toll signalling cascade, ventrally. Even though the dorsal protein is present all throughout the embryonic cytoplasm, it is never translocated to the nucleus because it is bound to Cactus. According to Wolpert and Lewis, the signalling cascade of the Toll receptors cause cactus to be degraded so that Dorsal can enter the nuclei of the blastoderm ventrally.
The overall variation in the localization of the oocyte creates a state for signalling the neighbouring follicular cells to relay the signal to the nuclei of the blastoderm. The nuclear localization of Dorsal results in the concentration-dependent activation of different genes and lead to the differential expression of the genes, dorsally and ventrally. The blastoderm nuclei at the ventral end gets exposed to colossal concentrations of Dorsal protein in the embryo that cause twist and snail to get induced, with the simultaneous repression of zerknullt and decapentaplegic, which results in the mesoderm formation. Laterally, there are low concentrations of the nuclear concentrations. In the lateral regions of the embryo, low nuclear concentrations of Dorsal cause rhomboid to express which lead to the presumptive neuroectoderm. Rhomboid repressed by the Dpp signalling, leads to the confinement in the lateral blastoderm causes specification of non-neural ectoderm. At the ventral part, TGF-p family signaling protein DPP is maintained by the expression of Dpp-agonist Sog (the neuroectoderm. SOG after binding to DPP, prevents it from diffusing to the ventral side of the embryo and and cleaving SOG by Tolloid it restores the dorsal gradient to steepness on the dorsal side. In this way, the Dorsal-ventral axis of Drosophila, is a culmination of the interaction between the ventrally concentrated Dorsal and a dorsally concentrated DPP.
Conclusion
The Drosophila embryogenesis is hence, a time oriented and stage oriented phenomenon operating via the unification ofa large number of transcriptional factors. The normal functioning of the embryogenesis is dependent on the time-wise functioning of all these factors. Anomaly in functioning of any of the gene, leads to mutation and defects in embryogenesis. Drosophila, as a model organism has over the years shed abundant evidences regarding the genes functioning in embryogenesis. It is expected that in the years to come, we shall come cross even more interesting and unknown mechanisms underlying embryogenesis. If all the factors have been discovered, it can be extrapolated from Drosophila to higher vertebrates and subsequently, to humans. In this way, understanding of the Drosophila embryogenesis can remediate diseases pertaining to development in humans.
References
- Di Talia S, She R, Blythe SA, Lu X, Zhang QF, et al. (2013) Posttranslational control of Cdc25 degradation terminates Drosophila's early cell-cycle program. Current Biology 23(2): 127-132.
- Dominguez M, Brunner M (1996) Sending and receiving the hedgehog signal: control by the Drosophila Gli protein Cubitus interruptus. science 272(5268): 1621.
- Edgar BA, Schubiger G (1986) Parameters controlling transcriptional activation during early Drosophila development. Cell 44(6): 871-877.
- Farrell JA, O'Farrell PH (2013) Mechanism and regulation of Cdc25/ Twine protein destruction in embryonic cell-cycle remodeling. Current Biology 23(2): 118-126.
- Giraldez AJ, Mishima Y, Rihel J, Grocock RJ, Van Dongen S, et al. (2006) Zebrafish MiR-430 promotes deadenylation and clearance of maternal mRNAs. science 312(5770): 75-79.
- Harrison MM, Li X-Y, Kaplan T, Botchan MR, Eisen MB, (2011) Zelda binding in the early Drosophila melanogaster embryo marks regions subsequently activated at the maternal-to-zygotic transition. PLoS Genet 7(10): e1002266.
- Johnson RL, Rothman AL (1996) Human homolog of patched, a candidate gene for the basal cell nevus syndrome. science 272(5268): 1668.
- Liang H-L, Nien C-Y, Liu H-Y, Metzstein MM, Kirov N, Rushlow C (2008) The zinc-finger protein Zelda is a key activator of the early zygotic genome in Drosophila. Nature 456(7220): 400-403.
- Lu X, Li JM, Elemento O, Tavazoie S, Wieschaus EF (2009) Coupling of zygotic transcription to mitotic control at the Drosophila mid-blastula transition. Development 136(12): 2101-2110.
- Marlow FL (2010) Maternal control of development in vertebrates, Colloquium Series on Developmental Biology. Morgan & Claypool Life Sciences, pp. 1-196.
- Nien C-Y, Liang H-L, Butcher S, Sun Y, Fu S, et al. (2011) Temporal coordination of gene networks by Zelda in the early Drosophila embryo. PLoS Genet 7(10): e1002339.
- Pennisi E (1996) Gene linked to commonest cancer. science 272(5268): 1583-1585.
- Rivera-Pomar R, Jackle H (1996) From gradients to stripes in Drosophila embryogenesis: filling in the gaps. Trends in Genetics 12(11): 478-483.
- Sung H-w, Spangenberg S, Vogt N, GroRhans J (2013) Number of nuclear divisions in the Drosophila blastoderm controlled by onset of zygotic transcription. Current Biology 23(2): 133-138.
- Tadros W, Lipshitz HD (2009) The maternal-to-zygotic transition: a play in two acts. Development 136(18): 3033-3042.






























