In Silico screening of Putative miRNAs and their Targets from a Common Mangrove Bruguiera gymnorrhiza
Nirjhar Dasgupta, Anjan Hazra, Sabyasachi Bhattacharya and Sauren Das*
Agricultural and Ecological Research Unit, Indian Statistical Institute, India
Submission: February 21, 2017; Published: April 17, 2017
*Correspondence Address: Sauren Das, Agricultural and Ecological Research Unit, Indian Statistical Institute, 203, Barrackpore Trunk Road, Kolkata 700 108, India, Email: sauren@isical.ac.in
How to cite this article: Nirjhar D, Anjan H, Sabyasachi B, Sauren D. In Silico screening of Putative miRNAs and their Targets from a Common Mangrove Bruguiera gymnorrhiza. IInt J cell Sci & mol biol. 2017; 2(1): 555579. DOI : 10.19080/IJCSMB.2017.02.555579
Abstract
Micro RNAs, the non-coding single stranded molecules with 18-22 nucleotide sequences, have established its enormous involvement in biological processes including stress response. As the mangrove species restoration program are being conducted world-wide for their protecting and producing nature, investigation on miRNA would be a significant approach towards understanding the small RNA mediated gene regulation leading to plant adaptation. But insufficient molecular data of salt responsive genes and miRNAs have triggered to the present work which might enrich knowledge on adaptability with the rapid salinization of the habitat. The present work elucidate that the target genes for the two miRNAs bg-miR1029 and bg-miR5021 in Bruguiera gymnorrhiza are involved in major stress response characteristics. Experimental validation and characterization of these two miRNAs and their predicted target genes along the salinity gradient is yet to be validated.
Keywords: Bruguiera gymnorrhiza; miRNA; miRNA target; RNA secondary structure; Salt response
Introduction
Micro RNAs are small (˜22 nt), single-stranded, and noncoding RNA molecules. The RNA Pol II mediates the transcription of capped and polyadenylated miRNA precursors (pri-miRNA). Mature miRNAs regulate a wide array of biological processes like development, metabolism, stress response, pathogen defense etc. [1,2]. Its mode of action involves its integration into the RNA-induced silencing complex (RISC) [3], which controls gene expression by hindering translation or by corrupting coding mRNAs through complementing with the target mRNAs [4,5]. More precisely, miRNAs cleave the target genes to prevent gene expression in plants [6]. Several recent findings are in congruence with the fact that miRNAs have significant role on the plant adaptability to salt stress [7-10].
Mangrove is a specialized group of plant community, growing in the coastal estuarine environments of the tropical and subtropical world, thrive under constant abiotic extremities [11]. They provide the coastal ecology with the first line of defense against the coastal calamity like sea storms, Hurricanes, Tsunamis etc. The enormous productive and protective ecosystem that mangrove provide to both coastal environment and inhabitants, can be conservatively assessed to be worth about US$186 million per year [12]. Mangroves have to expose regularly with periodic inundation, physiological stress, high salinity, high temperature and higher UV index [13]. As a result environmental factors (increased salinity level, inundation frequency, sea level rise and global warming) and anthropogenic activities (unplanned poaching, reclamation of mangrove area and siltation of river bed leading to less fresh water discharge in the estuary), mangrove forests all over the world are declining at an alarming level [14]. During the period of 1980-1990 this loss was estimated at 2.0% per year and within 1990-2000, 0.7% per year [12]. Mangrove restoration and conservation programs have been taken up by many countries, but it could be a fruitful effort if the genetic information of the plant species could be amalgamated, which is quiet meager [15].
In the present study, putative miRNAs precursor sequences has been fished out with their potential targets in a mangrove speciesBruguiera gymnorrhiza. The genetic information of this taxa have been evaluated in many countries and hence prediction of miRNA loci can be used as a resourceful technique towards understanding its better salt adaptability [16] through molecular basis. As miRNA regulates gene expression through cleaving its targeted mRNA, the present work is pointing to the identification of miRNAs and their targets leading to understanding their possible roles in plant growth and development. The new miRNAs identified in this study would be worthwhile in understanding the complexity of miRNA- mediated genes network in various stress adaptability.
Materials and Methods
Retrieval of data
Available 8442 known plant miRNA sequences were acquired from the publicly available miRNA database, miRBase [17]. The redundant sequences were eliminated using PRINSEQ version 0.20.4 [18]. The remaining 4766 miRNA sequences were used to search their homologs in the EST database ofBruguiera gymnorrhiza. The available EST database of NCBI (National Centre for Biotechnology Information) are used for this study.
Identification of potential miRNAs in Bruguiera gymnorrhiza
The sequences of all the above mentioned 4766 miRNA sequences were subjected to BLAST (Basic Local Alignment Search Tool), available on NCBI website, for alignment against the publicly available Bruguiera gymnorrhiza EST database. The nucleotide match size between query and database was set to 15 with expectation value 0.01, match sequence less than this values are not taken into account. After checking redundancy, the selected ESTs are subjected to blastx [19] against NCBI non- redundant protein sequence collection to eliminate sequences which are protein-coding. RNAs such as tRNA, rRNA, snRNA, or snoRNA were eradicated by subjecting the ESTs to BLAST against NCBI nucleotide collection.
Prediction of RNA secondary structures
The selected candidates were then assessed for secondary structure using the mfold Web Server [20]. The default parameters were used for this study. Potential miRNAs were identified based on the following criteria - a) the position of the miRNA is on hairpin, b) minimum number of residues in miRNA should be 15, c) the maximum number of unpaired residues should be 6, d) the maximum number of G-U pairs in miRNA should be 5, e) the maximum size for a bulge in miRNA sequence should be 5nt, f) the negative minimal folding free energy (MFE) should be low, and g) the minimal folding free energy index (MFEI) should be high. Minimal folding free energy index (MFEI = [(MFE/length of the RNA sequence) *100] / (G+C) %).
Prediction of potential miRNA targets
Potential targets for the predicted miRNAs from Bruguiera gymnorrhiza were analyzed using psRNATarget web server 2017 version [21]. Since no miRNAs have been reported from Bruguiera gymnorrhiza till now, potential target was searched against the database of Arabidopsis thaliana.
Result and Discussion
Previously reported 8442 mature miRNA sequences were used in this study to find their homologs in Bruguiera gymnorrhiza, a mangrove species. Since their discovery, miRNAs have emerged as principal regulators in plant growth and development. miRNAs role in plant stress responses have arisen after the discovery that miR398 target genes have known role in stress responses [22]. In that context we have predicted the miRNAs utilizing Bruguiera gymnorrhiza expressed sequence tag database.
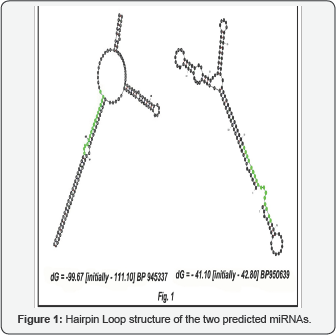
The redundancy check decreased the total number of miRNA sequences to 4766. These sequences were used as query to find out their homologs present in the Bruguiera gymnorrhiza EST database. BLAST result produced a total of 272 hits. These were the ESTs which aligned with the query miRNA sequences (Table 1). These ESTs were further subjected to BLAST to eliminate the protein coding sequences and non-coding RNAs. We finally got 14 sequences potential to be miRNA. RNA secondary structure predicting software mfold was used for this study. In the predicted structure the nucleotides that were homologous to the miRNAs were selected and inspected for the above mentioned 7 criterions. The criterion got matched for two ESTs. They were named as bg-miR1029 and bg-miR5021. Both of the sequences were 17 nucleotides long (Table 2). The software generated secondary structure (Figure 1) show -99.67 Kcal/mol minimal folding free energy (MFE) and -13.96 minimal folding free energy index (MFEI). Second one (Figure 1) show -41.1Kcal/mol minimal folding free energy (MFE) and - 8.34 minimal folding free energy index (MFEI).

Web server psRNA Target was used to predict the target genes for these two miRNAs. The cDNA library of Arabidopsis thaliana was used for this study, as cDNA library of Bruguiera gymnorrhiza was not available; and being a genetically well studied model organism Arabidopsis thaliana can give the most accurate prediction. Default parameters were set for this study. The specificity of target binding for these two miRNAs might provide some useful information towards understanding the stress response. bg-miR5021 showed a total of 146 hits (Table 1). Target genes for this miRNA include NAD kinase1, fatA acyl- ACP thioesterase, Calmodulin S, Topoisomerase, Circadian clock associated 1, Pectin-lyase like siperfamily protein, NADPH dehydrogenase B, Ferrodoxin - like superfamily proteinamong others. All of these genes have some role in growth and development of plants. The binding of the miRNAs to these genes results in despaired activity. On the other hand some of these genes have plant stress responsive activities. Ca+2-dependent protein kinases (CDPKs) and sucrose non-fermentation 1 (SNF1)-related kinases (SnRKs) regulate stress responsive gene expression which include ABA-responsive transcription factors and LEA genes [23]. Several protein kinases involved in stress tolerance are stimulated by ABA, which have a immense role in stress response [24]. Calmodulin (CaM) is a major Ca2+- sensing protein, involved in transduction of Ca2+ signals. CaM undergoes conformational change after interacting with Ca2+ and stimulates the activities of a diverse range of CaM-binding proteins. Most important role played by CaM is in adaptation to adverse environmental conditions [25]. bg-miR1029 showed a total of 12 hits, which includes GDSL-like Lipase/Acylhydrolase superfamily protein among others.
The salinity is increasing all over the globe, this rapid salinization may pose serious threat to the growth and development of the plants [26]. Genes and miRNAs involved in salinity stress have been identified to some extent [27]. But the molecular data of salt responsive genes and miRNAs available currently is very limited. Genome wide molecular level study on the halophytes and mangroves could provide clear insight for coping with the rapid salinization all along the globe. Hence Hence the present work predicted that the target genes for the two miRNAs bg-miR1029 and bg-miR5021, include some genes very important for plant growth and development, as well as plant stress responses. Experimental validation and characterization of these two miRNAs expression and their predicted target genes along the salinity gradient would give us more clear understanding of adaptability within saline atmosphere for the species Bruguiera gymnorrhiza.
References
- Mallory AC, Vaucheret H (2006) Functions of microRNAs and related small RNAs in plants. Nature Genet 38: S31-S36.
- Bushati N, Cohen SM (2007) microRNA functions. Annu Rev Cell Dev Biol 23: 175-205.
- Bartel DP (2004) MicroRNAs: genomics, biogenesis, mechanism, and function. Cell 116(2): 281-297.
- Carrington JC, Ambros V (2003) Role of microRNAs in plant and animal development. Science 301(5631): 336-338.
- Djuranovic S, Nahvi A, Green R (2011) A parsimonious model for gene regulation by miRNAs. Science 331(6017): 550-553.
- Vaucheret H (2006) Post-transcriptional small RNA pathways in plants: mechanisms and regulations. Genes Dev 20(7): 759-771.
- Bottino MC, Rosario S, Grativol C, Thiebaut F, Rojas CA, Farrineli L, et al. (2013) High-throughput sequencing of small RNA transcriptome reveals salt stress regulated microRNAs in sugarcane. PloS one 8(3): e59423.
- Ren Y, Chen L, Zhang Y, Kang X, Zhang Z, et al. (2013) Identification and characterization of salt-responsive microRNAs in Populus tomentosa by high-throughput sequencing. Biochimie 95(4): 743-750.
- Zhu J, Li W, Yang W, Qi L, Han S (2013) Identification of microRNAs in Caragana intermedia by high-throughput sequencing and expression analysis of 12 microRNAs and their targets under salt stress. Plant cell Rep 32(9): 1339-1349.
- Mondal TK, Ganie SA (2014) Identification and characterization of salt responsive miRNA-SSR markers in rice (Oryza sativa). Gene 535(2): 204-209.
- Tomlinson PB (1986) The botany of mangroves. Cambridge University Press, pp. 413.
- FAO (2007) The World's Mangroves 1980-2005. FAO Forestry Paper, Rome, Italy, p. 153.
- Hogarth P (2007) The biology of mangroves and sea grasses. In: Hogarth P (Ed.), (2nd edn), Oxford University Press, New York, USA.
- Giri C, Ochieng E, Tieszen LL, Zhu Z, Singh A, et al. (2011) Status and distribution of mangrove forests of the world using earth observation satellite data. Global Ecology and Biogeography 20(1): 154-159.
- Dasgupta N, Nandy P, Sengupta C, Das S (2105) RAPD and ISSR marker mediated genetic polymorphism of two mangroves Bruguiera gymnorrhiza and Heritierafomes from Indian Sundarbans in relation to their sustainability. Physiology and Molecular Biology of Plants 21(3): 375-384.
- Dasgupta N, Sengupta C, Das S (2014) Role of Secondary Metabolites and Radical Scavenging Aptitude for Better Adaptability of Mangroves in Varying Salinity of Sundarbans, India. Annals of Tropical Research 36(2): 1-22.
- Griffiths-Jones S, Saini HK, van Dongen S, Enright AJ (2008) miRBase: tools for microRNA genomics. Nucleic acids research 36(suppl 1): D154-D8.
- Schmieder R, Edwards R (2011) Quality control and preprocessing of metagenomic datasets. Bioinformatics 27(6): 863-864.
- Altschul SF, Gish W, Miller W, Myers EW, Lipman DJ (1990) Basic local alignment search tool. Journal of molecular biology 215(3): 403-410.
- Zuker M (2003) Mfold web server for nucleic acid folding and hybridization prediction. Nucleic Acids Res 31(13): 3406-3415.
- Dai X, Zhao PX (2011) psRNATarget: a plant small RNA target analysis server. Nucleic Acids res 39(suppl 2): W155- 159.
- Sunkar R, Chinnusamy V, Zhu J, Zhu JK (2007) Small RNAs as big players in plant abiotic stress responses and nutrient deprivation. Trends in plant science 12(7): 301-309.
- Saijo Y, Hata S, Kyozuka J, Shimamoto K, Izui K (2000) Over-expression of a single Ca2+-dependent protein kinase confers both cold and salt/ drought tolerance on rice plants. Plant J23(3): 319-327.
- Wasilewska A, Vlad F, Sirichandra C, Redko Y, Jammes F, et al. (2008) An update on abscisic acid signaling in plants and more.... Mol plant 1(2): 198-217.
- Virdi AS, Singh S, Singh P (2015) Abiotic stress responses in plants: roles of calmodulin-regulated proteins. Front plant sci 6: 809.
- Hasegawa PM, Bressan RA, Zhu J-K, Bohnert HJ (2000) Plant cellular and molecular responses to high salinity. Annu Rev Plant Physiol Plant Mol Biol 51(1): 463-499.
- Seki M, Narusaka M, Ishida J, Nanjo T, Fujita M, et al. (2002) Monitoring the expression profiles of 7000 Arabidopsis genes under drought, cold and high-salinity stresses using a full-length cDNA microarray. Plant J 31(3): 279-292.






























