Risk Factors and Treatment Outcome In NodeNegative Breast Cancer Patients With Tumors Five Cm and Larger, Impact of Pmrt
Amr Amin, Gerges Attia, Maha El Taher, Heba Eissa, Rania Fares and Samy El Badawy*
Department of Radiation Oncology, National Cancer Institute, Cairo, Egypt
Submission: November 14, 2016; Published: January 05, 2017
*Corresponding Author: Samy El Badawy, Department of Radiation Oncology, National Cancer Institute, Cairo, Egypt, Email: s_elbadawy@hotmal.com
How to cite this article: Wilson I O. Squamous Cell Carcinoma of the Breast in a Developing Community. Int J cell Sci & mol biol. 2017; 1(4): 555566. DOI:10.19080/IJCSMB.2017.01.555566
Abstract
Purpose: To assess the risk factors and treatment outcome for patients with node-negative breast cancer patients with tumor 5 cm or larger.
Methods and Materials: Between 1996 and 2006, a total of 171 patients with node-negative breast cancer and tumors 5 cm or larger were treated with mastectomy and adjuvant systemic therapies. 91% of patients also received post-mastectomy radiotherapy (PMRT) using hypo fractionation regimen, at Gharbia Cancer Society Center, Egypt. We retrospectively assessed rates and risk factors for loco regional failure free survival (LRF FS), overall survival (OS), and disease-free survival (DFS) in these patients.
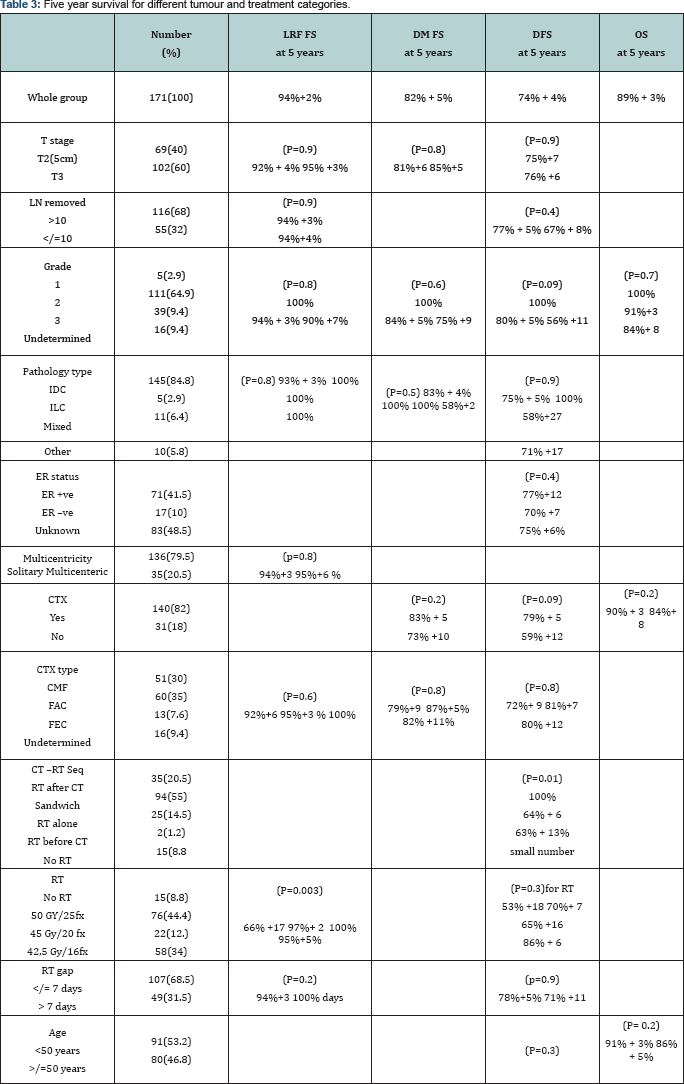
Results: With a median follow-up of 26 months (range 2-163 ms), the 5-year actuarial LRF FS at 5 years was 94%+2% for the whole series, 66% +17 for no PMRT compared to 97%+ 2 for 50 Gy/25fr, 100% for 45 Gy/20fr and 95%+5% for 42.5Gy/16fr, P= 0.003) fractionation. LRF FS was not statistically different regarding T size (T2 vs T3), multi-centricity, number of lymph node dissected (</= 10 vs >10) or grade. LRF rate in those who did not receive PMRT was 20% at chest wall and 1.7%, 1% and 0%at supraclavicular, internal mammary and axillary regions respectively compared to 0% in patients who received PMRT. The 5-year OS and DFS rates were 89% + 3% and 74% + 4% respectively. DFS at 5 years was 59% +12 for no adjuvant chemotherapy compared to 79% + 5 for adjuvant chemotherapy (P=0.09 borderline significance) and it was best when adjuvant chemotherapy given first before RT (100%) compared to RT during adjuvant chemotherapy(64% + 6) or RT alone (63% + 13) (P=0.01).
Conclusion: This series demonstrates a high LRF rate of 20% among breast cancer patients with node-negative breast cancer with tumours 5 cm and larger after mastectomy, with or without adjuvant systemic treatment and no PMRT compared to 0% for those who received PMRT. However this result cannot be conclusive due to small number of patients who did not receive PMRT and the inherent drawbacks of retrospective studies. No statistically significant risk factors for LRF were identified. The very low LRF rates without PMRT at the supraclavicular, internal mammary and axillary areas in our series support with-holding RT in these areas.
Introduction
The use of post mastectomy radiotherapy (PMRT) varied considerably over time. In the 1950's PMRT was a general consensus based on the prevailing view of the natural history of the disease during this time (being local disease and spread in a sequential manner through the lymphatics) [1].
Later there was a change in view of the natural history of the disease from localized in some patients to systemic in all patients [2]. Several meta-analysis by Stjernsward in the 1970s [3-5] and Cuzick et al. [6] in the 1980s and the Early Breast Cancer Trialists' Collaborative Group (EBCTCG) [7] in the 1990s suggested decreased survival in patients randomized to PMRT
Updates of these meta-analyses in 1994 and 2000 questioned this conclusion based on cause-specific analyses [8,9] Two large Danish randomized trials (1997) examined the role of PMRT in high risk women with breast cancer treated by mastectomy and adjuvant chemotherapy [10-12]. They showed a significant decrease in local recurrence, disease specific mortality, and overall mortality at 10 and 15 years with PMRT thus proving that loco regional disease control is critical to improving survival.
In these studies, high risk factors included four or more positive axillary lymph nodes and/or large tumors more than 5 cm, high risk node-negative patients had a reduction in loco regional failure (LRF) for both premenopausal and postmenopausal patients, and a survival benefit was seen for the premenopausal group. [11,13].
The estimates of positive axillary lymph nodes in pT2 and pT3 tumors is as high as 50% and 67%, respectively [14], thus large, node-negative tumors are relatively uncommon. Management often involves primary mastectomy, neoadjuvant or adjuvant systemic therapy, and radiation therapy.
Although radiation oncologists differ greatly within and among institutions in their use of PMRT and also varies from country to country [15], a recent survey of practicing radiation oncologists indicates that 88% of 1137 respondents from North America and Europe would offer PMRT to the chest wall in the setting of tumors greater than 5 cm with negative axillary lymph nodes, and 48% of these respondents would treat both chest wall and supraclavicular fields [16].
The risk of LRF in node-negative patients treated without PMRT was assessed in many retrospective studies [17-20], in some [17-19], patients with large T2(5 cm) T3 N0 treated without PMRT demonstrated low LRF risk thus suggesting omitting PMRT in those patients in the absence of other risk factors, on the other hand, Troung et al. [20] analyzed 1505 patients with T1 T2 N0 disease treated without PMRT and demonstrated high LRF thus suggesting offering PMRT to those patients according to other risk factors like grade and lympho-vascular invasion LVI
To determine rates and risk factors for local failure, distant failure, and overall survival in this subset of patients, we have reviewed our data at Gharbia Cancer Society, Egypt between the years 1996 and 2006 .
Methods and Materials
Between Jan 1996 and Dec 2006, the charts of two hundred patients diagnosed with Large pT2 (5cm) N0M0 and T3N0M0 primary breast cancer, referred to Gharbia Cancer Society Center , Egypt were retrospectively reviewed. Large T2 (5 cm) patients were included in this study since it was the radiation oncology team practice at that time to offer PMRT to those patients and since they constituted 40% of the study sample, meaningful statistical analysis regarding effect of tumor size is possible. Mastectomy was performed in 177 patients (88.5%) and breast- conserving surgery BCS was performed in 23 patients (11.5%). Because the objective was to evaluate the LRF risk and survival after PMRT, patients with BCS were excluded. Six other patients received neoadjuvant chemotherapy were also excluded. The remaining 171 patients with large T2 (5cm)/ T3- N0M0 breast cancer with complete pathologic information treated with mastectomy with clear surgical margins formed the study cohort for this analysis.
The patients factors analyzed were age, menopausal status. Tumor factors analyzed were T stage (large T2, T3), tumor site, size and laterality and, number of axillary nodes removed, histologic features (ductal, lobular, other), histologic grade (1, 2, 3), ER PR status (positive, negative) and, multicentricity. Treatment factors analyzed were adjuvant systemic treatment: chemotherapy (used or not), type (cyclophosphamide, methotrexate, fluorouracil CMF or cyclophosphamide, fluorouracil, and doxorubicin FAC or cyclophosphamide, fluorouracil, and epirubicin FEC), number of cycles and hormonal treatment (used or not) and type (predominantly Tamoxifen), or both and radiation therapy (used or not), fractionation regimen (50 Gy/25 fractions/5 weeks or 45 Gy/20 fracions/4 weeks or 42,5Gy/16 fractions/3 weeks and1 day), supraclavicular and internal mammary fields (used or not) radiotherapy gaps and chemotherapy/radiotherapy sequence (before, after, sandwich) and surgery-radiotherapy interval. Outcome factors analyzed were time, site and management of relapse and time of, and patients' status at, last follow up.
Statistical Analysis
Descriptive analysis for the entire cohort regarding: patients, tumor and, treatment factors were performed. The clinical characteristics of those with and without failure were compared using chi-square tests. For loco regional control or distant control, first site of local or distant relapse was the only event considered. Recurrence in the chest wall or regional lymph nodes, including the axilla and supraclavicular region, was considered loco regional failure (LRF). For overall survival (OS), loss to follow-up was censored, and death for any reason was considered an event. For disease-free survival (DFS), disease recurrence or death from disease was scored as an event. Death for reasons other than disease was not scored as an event. Time periods were calculated from the date of surgery. The 5-year Kaplan-Meier OS, DFS, distant metastasis free survival DMFS and LRF free survival and the associated standard errors (SEs) were computed for the entire cohort and for subgroups of patient, tumor, and treatment characteristics. Log-rank tests of the equality of survival curves for the subgroups were performed. All p-values were two-tailed and were performed using the Statistical Package for Social Sciences, version 11.0 (SPSS, Chicago, IL), and values less than 0.05 were considered statistically significant in this analysis. Cox proportional hazards and competing risks models were used to estimate the significance of the prognostic and treatment factors as independent risk factors for local control and DFS [21].
Results
Patient and tumor characteristics at diagnosis are detailed in Table 1.
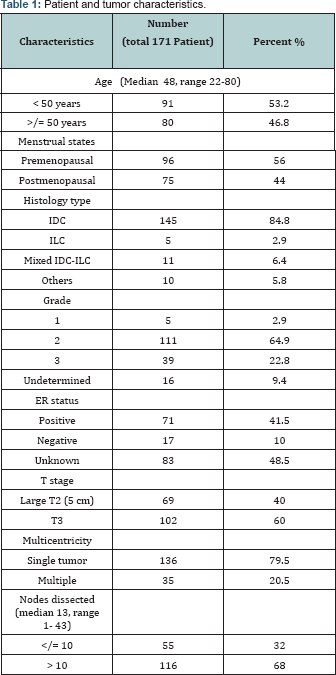
Median age for all patients was 48 years and those below 50 years constituted 53.2% of cases. Duration of symptoms ranged from 1 to 60 months with a median of 6 months with painless breast mass being the presenting symptom in 92% of cases and in 58.5% of cases, the left breast was the seat of the disease. Upper outer quadrant involvement at presentation represents 36% of cases with undetermined site in 30%.
Histopathological diagnosis of invasive duct carcinoma was encountered in 83.6% of cases and grade 2 was documented in 64.9% of cases. ER positive patients constituted 41.5% of cases. Unfortunately data on lymphovascular invasion were deficient in the pathology reports.
Pathologic tumor sizes for patients in the study group ranged from 5-14 cm in largest dimension with a median tumor size of 6 cm. Tumors 5 cm in size represent 40% of cases, whereas 49% had tumors more than 5 and less than 8 cm. Patients with very large tumors (10 cm or greater) comprised 6.4% of the total number of patients. All patients were pathologically node negative, with a median of 13 nodes dissected. Patients with more than 10 lymph nodes dissected constituted 68% of the study group and those with 7 or less nodes dissected constituted only 9%.
Treatment characteristics and outcome are detailed in Table 2.
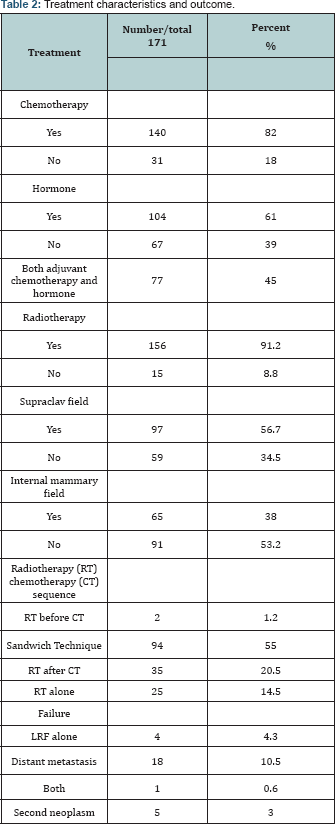
Adjuvant chemotherapy was given in 82% of cases (30% CMF, 35% FAC and 7.6% FEC and undetermined in 9.4% of cases). Adjuvant hormonal treatment was given in 61% of cases (Nolvadex 60% and Aromatase inhibitors 1% only). Seventy seven patients (45%) received sequential adjuvant chemotherapy and hormonal treatment while only 4 patients (2.3%) didn't receive any form of systemic treatment. PMRT was used in 91.2% of cases (50Gy/25 fractions in 44.4% and 42.5Gy/16 fractions in 34% and 45 Gy/ 20 fractions in 12.8%). Radiotherapy (RT) gap ranged from 0 to 35 days with median 4 days. Supra-clavicular (SC) field was used in 56.7% of cases and internal Mammary (IM) field in 38% of cases. RT was used in between adjuvant chemotherapy cycles (Sandwich technique) in 55% of cases and after adjuvant chemotherapy in 20.5% of cases. Median follow-up time for all patients was 26 months (range, 2-163 months). Five patients (2.9%) had LRF (3 in chest wall, 1 in supraclavicular nodes and 1 in IM nodes, none in the axilla), all were recorded within the first 4 years. One of them had distant metastasis at the same time. Of the 4 patients with LRF as first site of failure, none were recorded as subsequently developing distant metastases. All five patients were still alive at time of last follow-up, which ranged from 9 to 116 months.
Interestingly, none of the five patients with LRF had RT to the site of failure (thus LRF rate after RT was 0%). 3 out of 15 patients who did not receive chest wall PMRT failed (LRF rate 20%), and 1 out of 92 who did not receive internal mammary RT failed (LRF rate 1%) and 1 out of 59 who did not receive supraclavicular RT failed (LRF rate 1.7%) the latter received only chest wall RT
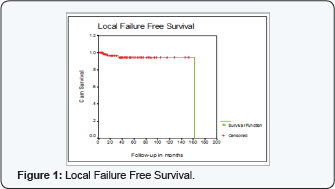
LRF Free Survival (LRF FS) at 5 years was 94%+2% for the whole series (Figure 1).
LRF FS at 5 years was 66% +17 for no RT compared to 97%+ 2 for 50 Gy/25fr, 100% for 45 Gy/20fr and 95%+5% for 42.5Gy/16fr, P= 0.003).
However due to small number of patients who did not receive PMRT, meaningful analysis is not possible.
LRF FS at 5 years was not statistically different for T stage, multi-centricity, number of nodes removed, pathology type or grade. (92% + 4% for large T2 and 95% +3% for T3 , P=0.9), (94%+3 for solitary tumor and 95%+6 % for multicenteric disease, p=0.8) (94%+4% for </= 10 nodes removed compared to 94% +3% for > 10 nodes removed, P=0.9), (IDC 93% + 3% 100% for ILC 100% for mixed and 100% for others P=0.8) (grade 1, 100% grade 2 ,94% + 3% grade 3 ,90% +7%, P=0.8).
LRF FS was also not statistically different for adjuvant chemotherapy type or RT gap (CMF 92%+6, FAC 95%+3 % FEC 100%, P=0.6) (no gap 94%+3 and 100% for gap > 7 days (49 patients), P=0.2) Table 3.
Distant metastasis occurred in 19 patients (11%) during the study period (mainly in bone and brain followed by liver and lung). One had simultaneous LRF and none of the remaining 18 patients was recorded to have any subsequent local failure.
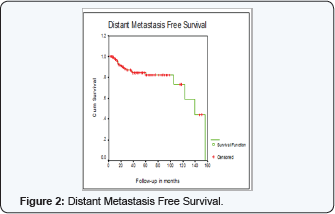
Distant Metastasis Free Survival was 82% + 5% at 5 years
DM FS was not statistically different for T stage, pathology type or grade (81%+6 for large T2 (5 cm) compared to 85%+5 for T3, P=0.8) (IDC 83% + 4%, 100% for ILC, 100% for mixed and 58%+25 for others, P=0.5) (grade 1, 100%, grade 2, 84% + 5%, grade 3, 75% +9, P=0.6) DM FS was also not statistically different for adjuvant chemotherapy or its type (73% +10 for no adjuvant chemotherapy compared to 83% + 5 for adjuvant chemotherapy, P=0.2) (CMF 79%+9, FAC 87%+5%, FEC 82% +11%, P=0.8)
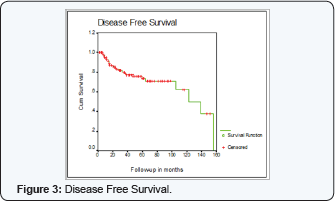
Disease free Survival (DFS) at 5 years was 74% + 4% at 5 years
DFS at 5 years was not statistically different for age, T stage, number of nodes removed, pathology, grade or ER status. ( < 50 years 73% + 4% compared to 74% + 5% for > 50 years, P=0.3) (75%+7 for T2 (5cm) compared to 76% +6 for T3, P=0.9) (77% + 5% if number of nodes removed > 10 compared to 67% + 8% if > 10 nodes removed, P=0.4) (IDC 75% + 5%, 100% for ILC, 58%+27 for mixed and 71% +17 for others, P=0.9) (grade 1, 100%, grade 2, 80% + 5%, grade 3, 56% +11 P=0.09) (ER -ve 77%+12, ER +ve 70% +7, ER unknown 75% +6% P=0.4)
DFS at 5 years was 59% +12 for no adjuvant chemotherapy compared to 79% + 5 for chemotherapy, (P=0.09 borderline significance) and best when chemotherapy given first before RT (100%) compared to RT during chemotherapy (64% + 6) or RT alone (63% + 13) (P=0.01) only 2 patients received RT before chemotherapy.
DFS at 5 years was not significant for chemotherapy type, PMRT, or RT gap. (72%+ 9 for CMF, 81%+7 for FEC, 80% +12 for fec, P=0.8) (53% +18 no RT, 70%+ 7 for 200cgy/fr, 65% +16 for 225 cgy/fr, 86% + 6 for 267/fraction, P value for fraction size 0.3) (78%+5% gap < 7 days and 71% +11 if > 7 days, p=0.9)
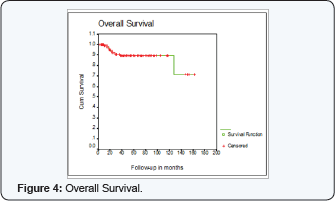
Overall Survival (OS) at 5 years was 89% + 3%
OS was not statistically significant for age, T stage, Grade or adjuvant chemotherapy. (< 50 years 91% + 3% compared to 86% + 5% for > 50 years, p= 0.2) (large T2 (5 cm) 88% +4% compared to 90% +4 for T3, P=0.7) (grade 1, 100%, grade 2, 91%+3, grade 3, 84%+ 8, P=0.7) (90% + 3 with chemotherapy compared to 84%+ 8 without chemotherapy, P=0.2)
Grade 3 acute skin reactions (moist desquamation) occurred in 15 patients, (13 of them received chest wall and supraclavicular and internal mammary fields). Of these 15 patients 12 (out of 76 patients 16%) were treated under 50 Gy/25 fr. regimen, and 3 (out of 22 patients 14%) were treated under 45 Gy/20 fr regimen and in none of 58 patients who used 42,5 Gy/16 fr. regimen. None of the patients developed grade 4 acute reactions (Figure 2).
Late lung toxicity (clinical radiation pneumonitis) was documented in 3 patients (2%). (2 out of the 76 patients who received 50 Gy/25 Fx regimen 2.6%, and 1 out of 22 patient who received 45 Gy/20 fr. regimen, 4.5%). Clinical progression was not documented for any of them. Late cardiac toxicity was not documented in any of the studied patients.
Late skin reactions occurred in 5 patients, 4 out of 58 who were treated under 42,5 Gy/16 fr. (7%) and 1 out of 76 who were treated under 50 Gy/25 fr. regimen (1.3%). Five patients (3%) developed second primary in our series, first patient had urinary bladder squamous cell carcinoma, second had non Hodgkin lymphoma diffuse large b-cell type right inguinal stage I, third had renal cell carcinoma and the fourth and fifth patients had contra lateral breast carcinoma. Second primary occurred in the first year of follow up in the first 2 patients and the fifth patient, and in 4th and 6th years of follow up in second and fourth patients respectively.
Discussion
The impact of PMRT was well established in the randomized clinical trials reported by Overgaard et al. [11,13]. In these studies, 135 premenopausal [11] and 132 postmenopausal [13] patients were having T3N0 tumors and were randomly assigned to adjuvant cyclophosphamide, methotrexate, and fluorouracil chemotherapy with and without PMRT. In these studies PMRT decreased the LRF rate from 17% to 3% in premenopausal patients and from 23% to 6% in postmenopausal patients. Moreover, PMRT significantly increased the OS rate from 70% to 82% in premenopausal patients but this increase was not significant in postmenopausal patients.
However this impact of PMRT in T3N0 tumours was questioned in a number of retrospective studies. [17-19] The first trial [17] included 101 patients with T3N0 and found only 12% incidence of LRF. Taghian et al. [18] and Floyd et al. [19] analyzed charts of 313 and 70 patients with tumors 5 cm or larger, node negative respectively. The actuarial cumulative LRF was 7.1% at 10 years in the former and 7.6% at 5 years in the latter. The authors concluded that the risk of LRF in these patients is low and does not warrant the routine use of PMRT They failed to identify significant prognostic factors for LRF apart from LVI in multivariate analysis in Floyd study.
Overgaard trials [11,13] were criticised for inadequate axillary LN dissection because an average of only seven LNs were removed with the possibility that some of the T3 N0 patients may have been found to be node positive if additional axillary LNs had been removed at surgery. Interestingly in Taghian et al. [18] study, contrary to this postulation, patients with 6 to 9 LN removed had a lower LRF compared to those with more than or equal to 10 LN removed, 2.6% and 7.3% respectively. Only patients with 1 to 5 LN removed had high incidence of LRF (16.7%) however with 11.4% standard error and they constituted 3.8% only of the study group. These difference in LRF by number of LN removed were not statistically significant (Figure 3).
Tumor size was not significant prognostic factor for LRF in Taghian et al. (18), and Floyd et al. [19] studies with low LRF rates, however in their retrospective study on 1505 patients with T1-T2 node negative breast cancer patients treated with mastectomy and without PMRT, Truong et al. [20] found approximately 20 % incidence of LRF in T1-2N0 patients in the presence of grade 3 disease with LVI or grade 3 disease, T2 tumours and no systemic therapy. So in their study, T stage was found significant prognostic factor predicting LRF in addition to grade, LVI and systemic therapy use.
In our study LRF FS at 5 years was not statistically different for T stage, multi-centricity, number of nodes removed, pathology type or grade. Unfortunately data for LVI were missing in the charts. The detrimental effect of these factors on LRF, if any, was masked by PMRT and thus cannot be assessed in our study since the majority of patients (91.2%) received PMRT.
In our study five patients had LRF (3 in chest wall, 1 in supraclavicular nodes and 1 in IM nodes, none in the axilla) chest wall recurrence constituted (60%) of recurrences compared to (86% and 80%) in Taghian et al. [18], and Floyd et al. [19] studies respectively, the difference probably attributed to the differential effect of PMRT with higher percentage of chest wall compared to regional irradiation thus decreasing chest wall recurrences.
Three out of the 15 patients who did not receive chest wall PMRT failed in chest wall (local failure rate 20%), and 1 out of 92 who did not receive internal mammary field failed in this region (regional failure rate 1%) and 1 out of 59 who did not receive supraclavicular field failed in this region (regional failure rate 1.7%) the latter received only chest wall RT. None of the five patients with LRF had RT to the site of failure (thus LRF rate after RT was 0%). However due to small number of patients who did not receive PMRT and small number of events, meaningful analysis regarding LRF is not possible. Moreover as inherent drawback of retrospective studies, the omission of PMRT for the patients was determined by patient or physician choice thus may have affected the results.
The 20% chest wall failure rate and the low failure rates at supraclavicular, internal mammary, and axillary areas without regional radiotherapy in our study (1.7%, 1%, and 0% respectively) justifies irradiating chest wall only without regional nodal areas and agree with The National Comprehensive Cancer Network (NCCN) v.1.2010 guidelines of PMRT to chest wall only in this subset of patients. This will thereby minimize the adverse effects of PMRT.
Overall Survival (OS) and Disease Free Survival (DFS) at 5 years in our study group were 89% +/- 3% and 74% +/- 4% respectively, compared to 83% and 86% respectively in Floyd et al. group. The lower DFS in our group with notable lower LRF after PMRT is explained by higher distant metastasis rate 11% compared to 8.6% in Floyd's stud. On comparing different tumor characteristics in the two groups, it seems that patients with LN removed less than 10 is higher in our study group compared to Floyd’s, 32% and 17 % respectively (Figure 4).
The higher 5 year OS in our group 89% compared to Floyd and Overgaard groups 83% and 82% respectively can be explained by the higher percentage of patients receiving adjuvant systemic treatment (82% received adjuvant chemotherapy and 61% received adjuvant hormonal treatment).
When comparing the 3 fractionation regimens used in this study group, all were associated with 0% LRF in irradiated areas. Grade 3 early skin reactions (moist desquamation) were 0% in 42,5 Gy/16 fr. regimen compared to 16% and 14% in 50 Gy/ 25 fr. and 45 Gy/20 fr. regimens respectively. This compares favourably with the rate of 10 to 15 % in Recht et al. study [22]. However late skin reactions were higher (7%) in 42,5 Gy/16 fr. regimen compared to 1.3% and 0% in 50 Gy/ 25 fr. and 45 Gy/20 fr. regimens respectively. Late lung toxicity was documented in 3 patients (2%) which matches well with incidence reported in Overmoyer et al. study [23], specially with the majority of patients receiving adjuvant chemotherapy or Nolvadex. Late cardiac toxicity was not documented in any of the studied patients, since high precision RT was not used in the treatment of these patients and IM field was used in 38% of patients, this more likely related to under estimation or poor documentation.
Conclusion
This series demonstrates a high LRF rate of 20% among breast cancer patients with node-negative breast cancer with tumours 5 cm and larger after mastectomy, with or without adjuvant systemic treatment and no PMRT compared to 0% for those who received PMRT. However this result cannot be conclusive due to small number of patients who did not receive PMRT and the inherent drawbacks of retrospective studies. No statistically significant risk factors for LRF were identified. The very low LRF rates without PMRT in the supraclavicular, internal mammary and axillary areas in our series support with-holding RT in these areas.
References
- Halsted WS (1907) The results of radical operations for the cure of carcinoma of the breast. Ann Surg 46: 1-27.
- Fisher B (1980) Laboratory and clinical research in breast cancer, a personal adventure: The David A. Karnofsky Memorial Lecture. Cancer Res 40: 3863-3874.
- Paterson R, Russell MH (1959) Clinical trials in malignant disease. III: Breast cancer: Evaluation of post-operative radiotherapy. Clin Radiol 10(4): 175-180.
- Paterson R (1962) Breast cancer: A report of two clinical trials. J R Coll Surg Edinb 7: 243-254.
- Stjernsward J (1974) Decreased survival related to irradiation postoperatively in early operable breast cancer. Lancet 2(7892): 12851286.
- Cuzick J, Stewart H, Peto R, Baum M, Fisher B, et al. (1987) Overview of randomized trials of postoperative adjuvant radiotherapy in breast cancer.Cancer Treat Rep 71: 15-29.
- Early Breast Cancer Trialists' Collaborative Group (1995) Effects of radiotherapy and surgery in early breast cancer. N Engl J Med 333(22): 1444-1455.
- Cuzick J, Stewart H, Rutqvist L, Houghton J, Edwards R, et al. (1994) Cause specific mortality in long-term survivors of breast cancer who participated in trials of radiotherapy. J Clin Oncol 12(3): 447-453.
- Early Breast Cancer Trialists' Collaborative Group. Favourable and unfavorable effects on long-term survival of radiotherapy for early breast cancer: An overview of the randomized trials. Lancet 355(9217): 1757-1770.
- Overgaard M, Christensen JJ, Johansen H, A Nybo-Rasmussen, H Brincker, et al. (1988) Post mastectomy irradiation in high-risk breast cancer patients. Acta Oncol 27(6): 707-714.
- Overgaard M, Hansen PS, Overgaard J, Rose C, Andersson M, et al. (1997) Postoperative radiotherapy in high-risk premenopausal women with breast cancer who receive adjuvant chemotherapy. N Engl J Med 337(14): 349-355.
- Ragaz J, Jackson SM, Le N, Plenderleith IH, Spinelli JJ, et al. (1997) Adjuvant radiotherapy and chemotherapy in node-positive premenopausal women with breast cancer. N Engl J Med 337(14): 956-962.
- Overgaard M, Jensen MB, Overgaard J, Hansen PS, Rose C, et al. (1999) Postoperative radiotherapy in high-risk postmenopausal breast-cancer patients given adjuvant tamoxifen: Danish Breast Cancer Cooperative Group DBCG 82c randomised trial. Lancet 353(9165): 1641-1648.
- Viale G, Zurrida S, Maiorano E, Mazzarol G, Pruneri G, et al. (2005) Predicting the status of axillary sentinel lymph nodes in 4351 patients with invasive breast carcinoma treated in a single institution. Cancer 103(3): 492-500.
- Foroudi F, Tyldesley S, Walker H, Mackillop WJ (2001) An evidence- based estimate of appropriate radiotherapy utilization rate for breast cancer. Int J Rad Onco Biol Phys 53(5): 1240-1253.
- Ceilley E, Jagsi R, Goldberg S, Grignon L, Kachnic L, et al. (2005) Radiotherapy for invasive breast cancer in North America and Europe: Results of a survey. Int J Radiat Oncol Biol Phys 61(2): 365-373.
- Mignano J, Gage I, Piantatosi S, Ye X, Henderson G, et al. (1996) Local recurrence after mastectomy in patients with T3N0 breast carcinoma treated without postoperative irradiation. Breast Cancer Res Treat 41: 255.
- Taghian AG, Jeong JH, Mamounas EP, Parda DS, Deutsch M, et al. (2006) Low locoregional recurrence rate among node-negative breast cancer patients with tumors 5 cm or larger treated by mastectomy with or without adjuvant systemic therapy and without radiotherapy: results from five hational surgical adjuvant breast and bowel project randomized clinical trials. J Clin Oncol 24(4): 3927-3932.
- Scott R Floyd, Thomas A, Bruce G, Saveli Goldberg, Andrzej Niemierko, et al. (2006) Low local recurrence rate without postmastectomy radiation n node-negative breast cancer patients with tumors 5 cm and larger. Int J Radiat Oncol Biol Phys 66(2): 358-364.
- Pauline T Truong , Lesperance M Culhaci A, Kader HA, Speers CH, Olivotto IA (2005) Patients subsets with T1-T2, node-negative breast cancer at high locoregional recurrence risk after mastectomy. Int J Radiat Oncol Biol Phys 62(1): 175-182.
- Kalbfleisch JD, Prentice RL (2002) The statistical analysis of failure time data.
- Recht A, Come SE, Henderson IC, Gelman RS, Silver B, et al. (1996) the sequencing of chemotherapy and radiation therapy after conservative surgery for early stage breast cancer. N Engl J Med 334(21): 13561361.
- Overmoyer B, Fowble B (1992) The long term results of conservative surgery and radiation with concurrent chemotherapy for early stage breast cancer. Proc Am Soc Clin Oncol 11: 90.






























