Effects of Cadmium Toxicity on Bio-distribution of Trace Elements in Normal and Protein Malnourished Rats
Syed Saleem Husain*
Faculty of Applied Medical Sciences, Jazan University, Saudi Arabia
Submission: June 15, 2016; Published: August 03, 2016
*Corresponding author: Syed Saleem Husain, Assistant Professor, Faculty of Applied Medical Sciences, Jazan University, Saudi Arabia.
How to cite this article: Syed SH. Effects of Cadmium Toxicity on Bio-distribution of Trace Elements in Normal and Protein Malnourished Rats. Int J cell Sci & mol biol. 2016; 1(1): 555553. DOI:10.19080/IJCSMB.2016.01.555553
Abstract
Cadmium is an established toxic metal with its ability to accumulate in blood, liver and kidney. Cadmium is chemically related to zinc and found wherever zinc occurs in nature. It is emitted to air and water by mines, metal foundries and industries using Cadmium in alkaline accumulators, alloys, paints and plastics. Cadmium is known to alter the tissue distribution of the essential trace metals like Zn, Cu and Fe this effect has been shown to be associated with many of the toxic effects of cadmium like anemia and anosmia. Protein malnutrition enhances the susceptibility to cadmium intoxication which affects metabolism of essential trace elements; Zn, Fe and Cu were studied, in normal and protein under-nourished rats. Estimation of metals was done routinely in samples of urine and tissue (brain, liver, kidney and blood) in rats of dietary groups, 21% and 8%, after 120 days of Cd exposure, 50 ppm, through drinking water. Significant aberrations in levels of the essential elements Zn, Fe and Cu were reported in both urine and tissues.
Keywords: Cadmium toxicity; Protein malnutrition; Essential trace elements
Introduction
The effects of a low protein diet on the body uptake and retention of cadmium, levels of essential trace elements, and cadmium-induced biochemical alterations in liver and kidneys of the rat have been investigated [1]. They found that low dietary protein disturbs cadmium induced alterations in carbohydrate metabolism, essential trace elements metabolism and offsets the hepatic and renal process of cadmium detoxification. The effects of chronic exposure to cadmium toxicity, under protein undernourished state, were studied by Syed Saleem Husain: hepatotoxicity [2], behavioral aberrations, both in Fo and F1 generations [3] and haematoxic effects [4] were reported. Cadmium can compete with some of the essential divalent elements for ligands. The displacement of essential elements may affect its transfer, storage or function at the active site of an enzyme or effect a change in the conformation of proteins or nucleic acids required for normal function [5].
Dietary deficiencies of protein, vitamins, iron, calcium and phosphorus markedly influence the absorption, retention, tissue distribution and neurotoxicity and many environmental neurotoxicants like lead, manganese and pesticides [6-9]. The effects of a low protein diet on the body uptake and retention of cadmium, levels of essential trace elements, and cadmium induced biochemical alterations in liver and kidneys of the rat have been investigated [1]. They found that low dietary protein disturbs cadmium induced alterations in carbohydrate metabolism, essential trace elements metabolism and offsets the hepatic and renal process of cadmium detoxification.
Mills and Dalgarno [10] reported that copper metabolism was markedly affected in pregnant ewes and also in their lambs when they were given cadmium levels ranging from 3.5 - 12 mg/kg of diet. Copper levels in the liver and the whole blood as well as ceruloplasmin levels were significantly reduced by cadmium treatments. However, zinc metabolism was not markedly affected. In studies with rats Campbell and Mills [11] reported that as low as 1.5 mg Cd/kg of diet decreased plasma ceruloplasmin and kidney copper levels. Increasing the cadmium levels to 18 mg/kg resulted in a progressive reduction in copper levels. How dietary cadmium may reduce the level of copper in plasma and tissues has been addressed by several researchers.
Campen[12] and Starcher [13] have proposed that cadmium may inhibit copper absorption in rats and chicks by reducing the occurrence of copper binding to a low molecular weight protein in the mucosal cytosol. More recent experiments by Davies and Campbell [14] have shown that cadmium does reduce copper absorption at a molar Cd; Cu ratio as low as 4: 1, a ratio that was comparable to the one that produced a copper deficiency condition in rats [11]. Davies and Campbell [14] demonstrated that the binding of Cu to the intestinal mucosa was increased even at so low as a 1:1 Cd: Cu ratio. Their data suggested that cadmium may prevent the release of copper from mucosal cells while showing little inhibitory influence on its uptake by the mucosa.
The effects of a low protein diet on the body uptake and retention of cadmium, levels of essential trace elements, and cadmium-induced biochemical alterations in liver and kidneys of the rat have been investigated [1]. They found that low dietary protein disturbs cadmium induced alterations in carbohydrate metabolism, essential trace elements metabolism and offsets the hepatic and renal process of cadmium detoxification.
The kidney is rich in zinc dependent enzymes. The renal dysfunction caused by cadmium may be due to adverse effects of cadmium on zinc enzymes necessary for re absorption and catabolism of proteins. Simultaneous administration of zinc to cadmium-exposed animals alleviates some of the renal symptoms caused by cadmium [15]. In normal human subjects, the increase in cadmium concentration in renal cortex with age is accompanied by an equimolar increase in zinc concentrations [16,17]. This observation is valid for cadmium concentrations up to 75 μg/g wet weight. The retention of zinc in the liver and the kidney caused by cadmium excess may also cause depletion of zinc in other organs.
Cadmium and zinc appear together in nature. In animals they are biologically antagonistic to each other as they compete for binding sites on various carrier proteins. The symptoms of cadmium toxicity are quite similar to those of zinc deficiency [18]. Many of the cadmium toxicity effects, such as testicular necrosis [19,20] and anemia and weight loss [21] could be prevented or corrected by the administration of extra zinc.
Several recent reports have indicated that both copper and iron metabolism are altered in the foetus and neonate by oral administration of cadmium in drinking water (as low as 4.3 μg Cd/ml) [19,22,23]. This was reflected in markedly lower copper and iron concentrations in the serum, kidney, and liver of whole pups.
Materials and Methods
The animals were pair-fed. One group from each dietary schedule was given drinking water containing 50 ppm Cd as cadmium chloride and the other group given the normal drinking water served as the control. The animals were maintained on the above dietary and cadmium exposure schedules for 120 days.
At 120 day the animals were sacrificed by decapitation. The following tissues: liver, kidney, brain and blood were processed for metal estimation.
Metal estimation was done by the method of Donaldson et al. [24].
Urine and blood samples
First of all the volume of urine and blood samples was measured, then 5.0 ml of digestion-mixture (prepared by mixing perchloric acid (HClO4) and nitric acid in ratio of 1:6) was poured in all the samples. A blank was also run simultaneously with the samples.
The mixture was heated slowly inside a fuming hood till a clear solution or white residual powder was left over. The digest was dissolved in 0.1 N-HNO3 and the volume was made up to 10.0 ml.
Tissue sample
Tissues of liver, kidney and brain were dried between blotting papers, weighed and transferred into conical flasks. 2.0 ml of conc. HNO3 was poured over the tissue sample. The immersed samples were allowed to stand overnight. The next day, 5.0 ml of digestion-mixture was added to the sample. Rest of the procedure of digestion was similar to that as was followed in case of urine and blood samples.
Further appropriate dilutions were made for each metal, viz. Cd, Cu, Fe and Zn. The absorbance of cadmium, copper, iron and zinc was measured at wave length of 228.8, 342.7, 248.3 and 213.9 nm respectively and at slit setting 0.7 nm for Cd, Cu and Zn and 0.2 nm for Fe using the respective Hallow-Cathode- Lamps in a Perkin Elmer model 5000 Atomic Absorption Spectrophotometer. The instrument was set up for maximum sensitivity and mixture of air and acetylene was used as a fuel with oxidizing flame.
Results
Tissue and urinary metals
The tissue metal levels and their urinary excretion pattern in rats of either dietary group, after 120 days of Cd exposure are as under:
Brain: A significant increase of Cd (<0.001, in both) Zn and Cu, and a decrease in the Fe levels were observed in the Cdexposed animals but the magnitude of changes were more or less equal in both the dietary groups (Table 1).
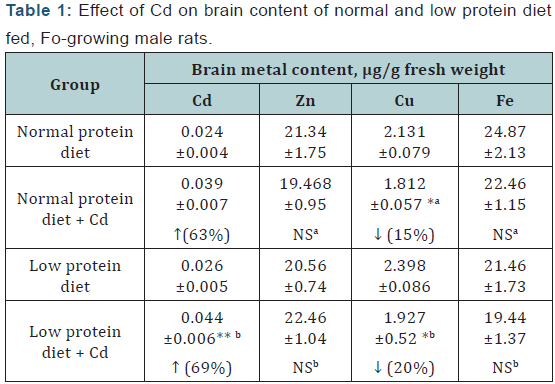
Values represent mean ± SE of six rats; Statistical evaluation by one– way ANOVA followed by LSD comparison; a= Compared to normal protein diet control, b= Compared to low protein diet control; p **
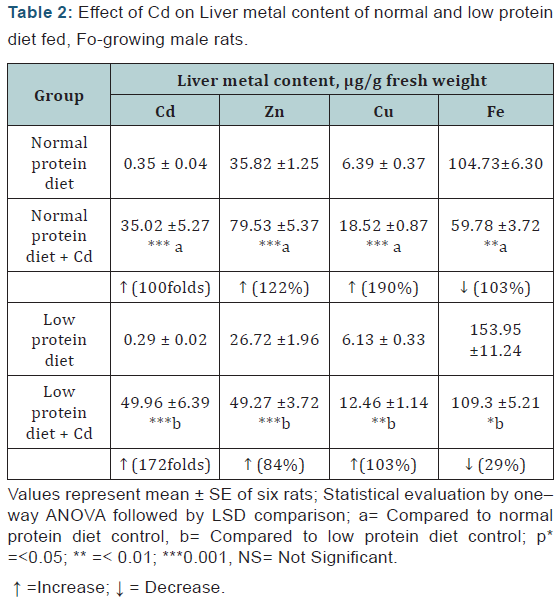
Liver: A marked increase of Cd (<0.001, in both), Zn and Cu, and a decrease in the Fe levels were observed in the Cd-exposed animals of both the dietary groups. These changes were more marked in the case of Cd and less marked in the case of Zn, Cu and Fe in the protein malnourished animals (Table 2).
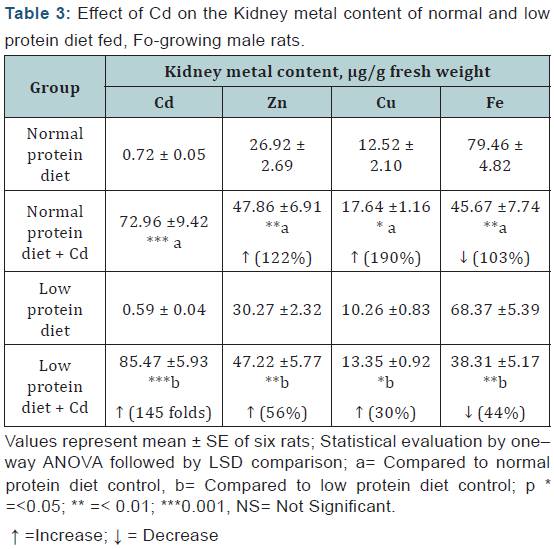
Kidney: Cd exposure resulted in a significant increase in the renal Cd, Zn and Cu concentrations and a decrease in the Fe level, which were more or less, of equal magnitudes in both the dietary groups (Table 3).
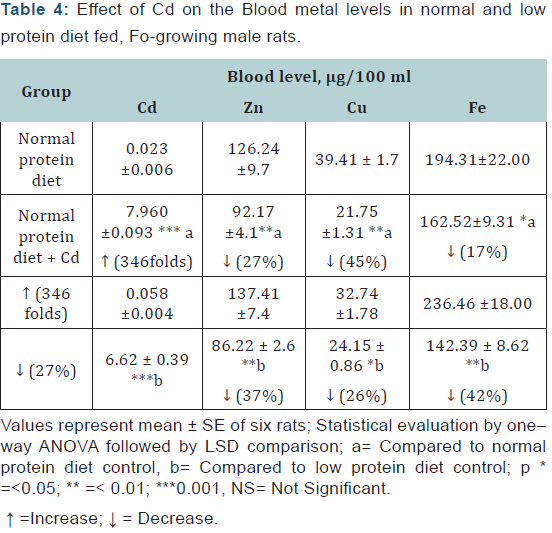
Blood: The blood Cd levels were significantly elevated in the Cd exposed animals in both dietary groups. The Zn, Cu and Fe levels were decreased in the Cd-exposed animals of both the dietary groups but the effects on Zn and Fe were more pronounced in the malnourished animals whereas the effect on Cd and Cu level was more marked in the normal protein diet-fed group (Table 4).
Urine
Cd: The urinary Cd levels in the controls of both the dietary groups were below detectable limits throughout the experimental period. The Cd excretion in the Cd-exposed animals of both the dietary groups increased steadily from day 30 onwards and the effect was more marked in the protein malnourished group especially on days 60 and 90 of Cd exposure (Table 5).
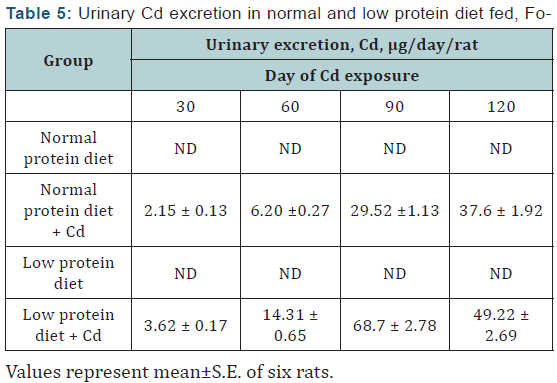
Statistical evaluations by one–way ANOVA, followed by LSD comparison.
a= Compared to normal protein diet content; b= Compared to low protein control.
Zn: The urinary excretion of Zn was enhanced in the Cdexposed animals from day 30 onwards and the effect was more or less of equal magnitude in either diet group (Table 6).
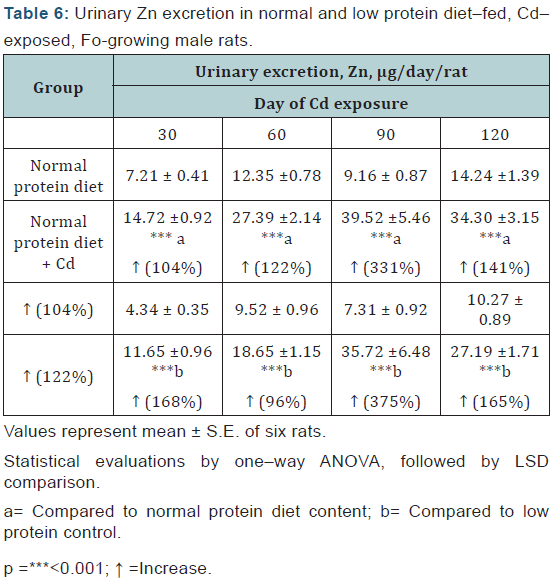
Statistical evaluations by one–way ANOVA, followed by LSD comparison.
a= Compared to normal protein diet content; b= Compared to low protein control.
Cu: The urinary excretion of Cu was increased in the Cdexposed animals from day 30 onwards in both the diet groups and the excretion was more marked in the malnourished animals (Table 7).
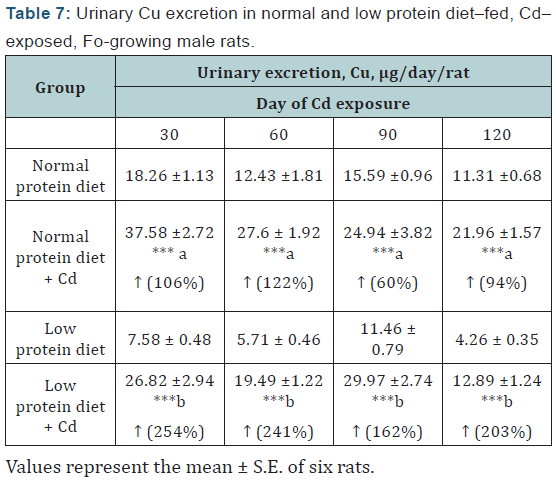
Statistical evaluation by one–way ANOVA, followed by LSD comparison.
a= Compared to normal protein diet content; b= Compared to low protein control.
p =***<0.001; ↑ =Increase
Fe: A significant increase in the urinary Fe excretion was observed from day 30 of Cd exposure onwards in both the protein malnourished and normal protein diet-fed animals and this effect was more marked in the malnourished animals (Table 8).
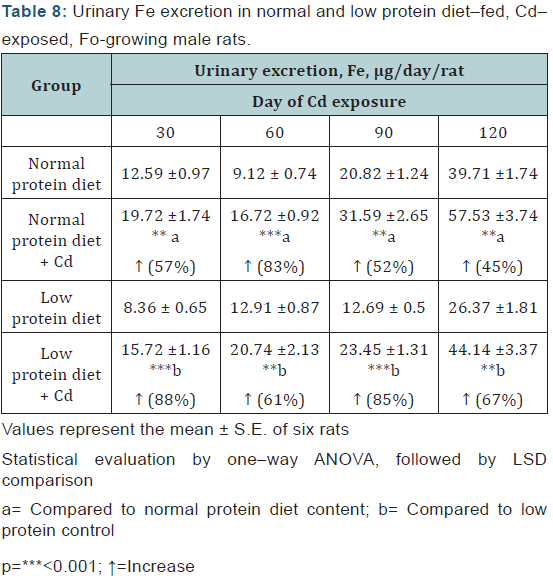
Statistical evaluation by one–way ANOVA, followed by LSD comparison.
a= Compared to normal protein diet content; b= Compared to low protein control.
Discussion
Interactions between Cd and essential trace metals in biological systems have been widely reported and have been reviewed by Bremner [25]. Such inter-relationships may influence not only the disposition, but also the homeostasis, of the essential trace metals. In present investigation, a significant increase in the Cd concentration and a decrease in the Cu levels were observed in brains of the Cd-exposed animals. The protein malnutrition did not cause any significant change in the traceelements levels. Hence, the changes were due to Cd-toxicity alone. Brain-Cd levels have been reported to increase in Cdexposed animals [26]. Davis and Campbell [14] have shown that Cd dose reduce Cu absorption at a molar Cd: Cu ratio as low as 4:1, a ratio that was comparable to the one that produced a Cu deficiency condition in rats [11] . The decrease in blood-Cu and increase in urine-Cu levels indicate less absorption of Cu and consequently decreased brain-Cu levels.
Liver and Kidney showed a marked increase in levels of Cu. Tewari et al. [1] have reported no change in renal and hepatic levels of Cu in Cd-exposed rats, while there was a significant increase in Cd and Zn levels and a decrease in Fe levels. Bernardet al. [27] have also reported increased concentrations of Cd in kidney and liver. In the present study a marked increase of Cd and Zn was observed in liver and kidney of both the dietary animals. The blood-Cd levels were significantly elevated in the Cd-exposed animals in both dietary groups. The Zn and Fe levels were decreased in blood of either dietary animals but the effects on Zn and Fe were more pronounced in the malnourished animals.
These results show liver and kidney to be the primary sites of Cd and Zn accumulation. Our results are similar to those of Buhler et al. [28]. The increased levels of these metals in the tissue indicate increased levels of metallothionein [28]. Levels of Fe decreased significantly in liver, kidney and blood and this decrease might have resulted due to the less intestinal absorption of Fe in presence of Cd, Cd competes with Fe for one or more steps in Fe transfer [29].
Changes in the urinary excretion of Cd, Zn and Cu with repeated subcutaneous injection of Cd were reported in detail together with the chemical forms of the three metals in urine [30] and it was related to the change in the tissue concentrations of Cd [31]. There was a steady increase in urinary Cd concentrations from day 30 of exposure onwards and the effect was more marked in the protein malnourished group especially on days 60 and 90 of Cd exposure. Accompanied to Cd were also increased levels of Zn, Cu and Fe. Excretion of Cu and Fe was more marked in protein malnourished group than normal group. Enhanced excretion of Cd into the urine along with Zn and Cu irrespective of doses administered during repeated injections of Cd has been reported [30-34].
The comparatively large increase of urinary Cd in Cdexposed, low protein diet rats has been reported by Suzuki et al. [35]. The above mentioned finding fit with studies on mice [36] showing that the urinary excretion of Cd on a group basis was correlated with the total body burden. When during exposure, proteinuria is present; there is always an increase in the excretion of Cd, which is also in accordance with the results from animal experiments.
Acknowledgement
The author acknowledges with thanks the financial grant by Indian Council of Medical Research, New Delhi, India and cooperation of Dr. M. M. Ali, Assistant Director, Indian Institute of Toxicology Research, Lucknow, India.
References
- GOTewari PC, Jain VK, Aashquin M, Tandon SK (1986) Influence of protein deficiency on cadmium toxicity in rats. Archives of Env. Contamination and Toxicology 15(4): 409- 415.
- Husain SS (2013) Studies on hepato and renal toxicity of cadmium on normal and protein malnourished rats. J Material Sci Eng 2: 129.
- Husain SS (2014) Behavioral toxicity of cadmium in normal and protein malnourished rats (Two-Generation Study). Biochem Physiol 3: 124.
- Husain SS (2015) Hemotoxic Effects of Cadmium in Normal and Protein Malnourished Rats. Toxicol. Environ. Health Sci Vol 7(2): 129-135.
- Fox (1976) M.R.S In: Trace Elements in Human Health and Diseases. Prasad AS (Ed.), Academic Press, New York Vol II: 401-416.
- Six KM, Goyer RA (1972) The influence of iron deficiency on tissue content and toxicity of ingested lead in the rat. J Lab Clin Med 79(1): 128-136.
- Ali MM, Murthy RC, Saxena DK, Srivastava RS, Chandra SV (1983) Effect of low protein diet on manganese neurotoxicity: II. Brain GABA and seizure susceptibility. Neurobehav Toxicol Teratol 5(3): 377-383.
- Ali MM, Murthy RC, Saxena DK, Srivastava RS, Chandra SV (1983) Effect of low protein diet on manganese neurotoxicity: I. Developmental and biochemical changes. Neurobehav Toxicol Teratol 5(3): 377-383.
- Ali MM, Murthy RC, Chandra SV (1985) Effect of low protein diet on manganese neurotoxicity: III. Brain neurotransmitter levels. Neurobehav Toxicol Teratol 7(5): 427-431.
- Mills CF, Dalgarno AC (1972) Copper and Zinc Status of Ewes and Lambs receiving Increased Dietary Concentrations of Cadmium. Nature 239( 5368): 171-173.
- Campbell JK, Mills CF (1974) Effects of dietary cadmium and zinc on rats maintained on diets low in copper. Proceedings of Nutrition Society 33 (1): 15A-16A.
- Campen DR (1966) Effects of zinc, cadmium, silver and mercury on the absorption and distribution of copper-64 in rats. J Nutr 88(1): 125- 130.
- Starcher BC (1969) Studies on the mechanism of copper absorption in the chick. J Nutr 97: 321.
- Davis NT, Campbell JK (1977) The effect of cadmium on intestinal copper absorption and binding in the rat. Life Sciences 20(6): 955-960.
- Vigiliani EC (1969) The biopathology of cadmium. American Industrial Hygiene Association J 30(4): 329-340.
- Piscator M, Lind B (1972) Cadmium, zinc, copper, and lead in human renal cortex. Environ Health Perspect 24: 426-431.
- Hammer DI, Calocci AV, Hasselblad V, Williams ME, Pinkerson (1973) Cadmium and lead in autopsy tissues. J Occup Med 15(12): 956-963.
- Powell GW, Miller WJ, Morton JD, Clifton CM (1964) Influence of dietary cadmium level and supplemental zinc and cadmium toxicity in the bovine. J Nutr 84: 205-214.
- Gunn SA, Goulde TC, Anderson WAD (1961) Zinc protection against cadmium injury to rat testis. Archives of Pathology 71: 274-281.
- Parizek J (1957) The destructive effect of cadmium ion on testicular tissue and its prevention by zinc. J Endocrinol 15(1): 56-63.
- Petering HG, Johnson MA, Stemmer KL (1971) Studies on zinc metabolism in the rat. 1: Dose- response effects of cadmium. Arch Environ Health 23(2): 93-101.
- Petering HG, Murthy L, Cerklewski FL (1977) Role of Nutrition in Heavy Metal Toxicity. In: Biochemical Effects of Environmental Pollutants, p. 365. Edited by S.D. Lee, Ann. Arbor Science, Ann. Arbor, MI.
- Petering HG, Choudhary H, Stemmer KL (1979) Some effects of oral ingestion of cadmium on zinc, copper, and iron metabolism. Environ Health Perspect 28: 97-106.
- Donaldson J, Cloutier T, Minnich JL, Barbeau A (1974) Trace metals and biogenic amines in rat brain. Adv Neurol 5: 245-252.
- Bremner I (1974) Heavy metal toxicities. Quarterly Reviews of Biophysics 7(1): 76.
- Chandra SV, Kalia K, Hussain T (1985) Biogenic amines and some metals in brain of cadmium-exposed diabetic rats. J Appl Toxicol 5(6): 378-381.
- Bernard A, Goret A, Buchet JP, Roels H, Lauwerys R (1980) Significance of cadmium levels in blood and urine during long‐term exposure of rats to cadmium. Journal of Toxicology and Environmental Health 6(1): 175-184.
- Buhler DR, Wright DC, Smith KL, Tinsley IJ (1981) Cadmium absorption and tissue distribution in rats provided low concentrations of cadmium in food or drinking water. J Toxicol Environ Health 8(1-2): 185-197.
- Hamilton DL, Valberg LS (1974) Relationship between cadmium and iron absorption. Am J Physiol 227(5): 1033-1037.
- Suzuki Y, Yoshikawa H (1981) Cadmium, copper and zinc excretion and their binding to metallothionein in urine of cadmium exposed rats. J Toxicol Environ Health 8(3): 479-487.
- Suzuki Y (1980) Cadmium metabolism and toxicity in rats after long term subcutaneous administration. J Toxicol Environ Health 6(3): 469- 482.
- Ashby SL, King LJ, Parke DV (1981) The effect of cadmium administration on the biliary excretion of copper and zinc and tissue disposition of these metals. Environ Res 26(1): 95-104.
- Bonner FW, King LJ, Parke DV (1979) The tissue disposition and urinary excretion of cadmium, zinc, copper and iron, following repeated parenteral administration of cadmium to rats. Chem Biol Interact 27(2-3): 343-351.
- Suzuki KT, Yaguchi K, Ohnuki R, Nishikawa M, Yamada YK (1983) Extent of cadmium accumulation and its effect on essential metals in liver, kidney, and body fluids. J Toxicol Environ Health 11(4-6): 713- 726.
- Suzuki KT, Miyamoto E, Tanaka Y, Kawamura R, Yamamura M (1984) Effect of diet on urinary and fecal excretion of cadmium, copper and zinc, from rats pre accumulated heavily with cadmium. Arch Environ Contam Toxicol 13(5): 621-626.
- Nordberg GF (1972) Cadmium metabolism and toxicity. Environmental Physiology and Biochemistry 2: 7.






