Abstract
This study investigated the protective effects of aqueous propolis extract against aluminum chloride-induced memory and behavioral impairments in an Alzheimer’s disease model. The phytochemical composition of the extract was analyzed, focusing on total phenolic and flavonoid content using LC-MS/MS. The experimental design included two phases: initially, mice were divided into five groups: a control group, an Alzheimer’s model group, two treated groups receiving propolis at 150 mg/kg and 300 mg/kg, and a standard group receiving rivastigmine at 1.5 mg/kg for six weeks. In the second phase, all groups except the control received aluminum chloride at 100 mg/kg orally, along with D-galactose via intraperitoneal injection at 120 mg/kg daily for six weeks. Following treatment, neurological evaluations, including behavioral and memory tests, were conducted, alongside histopathological examinations of brain tissue. Phytochemical analysis revealed high levels of total phenolics (5222.1 mg GAE/kg) and flavonoids (7521.1 mg/L) in propolis. FT-IR–ATR spectroscopy confirmed the presence of key functional groups. Notably, treated Alzheimer’s mice exhibited reduced anxiety and improved learning and memory capabilities, indicating that aqueous propolis extract may serve as a promising source of neuroprotective bioactive compounds against Alzheimer’s disease.
Keywords:Alzheimer’s disease; Propolis; FT-IR–ATR Spectroscopy, Neurological tests; Histopathological study; Mice
Introduction
Nature has endowed humankind with a plethora of natural products rich in bioactive substances that play an essential role in the prevention and cure of various pathologies such as Alzheimer’s disease (AD). It is an irreversible neurodegenerative disorder characterized by a progressive decline in cognitive functions such as memory loss and learning ability [1] and physio pathologically by the presence of hyperphosphorylated protein (tau), which is the main component of neurofibrillary tangles (NFTs), and senile plaques whose main component is β-amyloid (Aβ) peptide aggregate [2]. It is recognized by the World Health Organization (WHO) as a global public health priority [3]. It is one of the main causes of dementia, accounting for 60-70% of cases [4]. No pharmacotherapy currently exists to cure Alzheimer’s disease (AD) in a curative manner [5]. Existing drug treatment is mainly based on acetylcholinesterase inhibitors (AChEIs) and N-methyl-Daspartate Receptor Antagonists (NMDRA) [6], which have only provided symptomatic relief for AD patients [7]. The efficacy of these synthetic drugs is often associated with troublesome side effects [8], which calls for a search for alternative, non-harmful, more effective natural treatments such as the beekeeping products traditionally used in “Apitherapy” [9].
Propolis is a resinous, waxy substance produced from a mixture of tree and plant secretions collected by bees [10]. It is also rich in various bioactive molecules capable of enhancing various biological activities [11], making propolis a substance with multiple therapeutic properties [12] namely, antioxidant [13], antidiabetic [14], immunomodulator [15], anti-inflammatory [16] and anticancer [17]. The present study was designed to assess the potential of aqueous propolis extract to prevent neuronal damage in Alzheimer model mice. Various chemical, neurological and histopathological parameters were also evaluated to understand the mechanisms contributing to this neurotherapeutic activity.
Results and Discussion
In vitro study The total phenolic and flavonoid contents of APE
The results show that the total phenolic contents (TPC) of the APE samples were 5222 mg GAE/Kg, while the total flavonoid contents (TFC) were 7521 mg/L. These values indicate a significant concentration of bioactive compounds; however, they are lower than those reported for ethanolic propolis extracts, as indicated by Ozdal et al. [18,19]. The observed differences between aqueous and ethanolic extracts can be attributed to the effectiveness of solvents in extracting phenolic and flavonoid compounds. Indeed, ethanolic extracts tend to contain higher levels of these compounds, which are corroborated by other studies demonstrating that ethanol is an effective solvent for extracting polyphenols and flavonoids from propolis [20]. For instance, one study revealed that extraction methods not only influence the quantity of extracted compounds but also their chemical profile, which can have implications for the antioxidant and antimicrobial properties of the extracts [21].
Furthermore, the variation in TPC and TFC can also depend on factors such as the geographical origin of the propolis, climatic conditions, and the harvest season, which influence the chemical composition of the samples [21]. These elements underscore the importance of standardizing extraction methods and analyzing the extracts in a broader context to better understand their therapeutic potential. Additionally, previous studies have highlighted that the antioxidant and antimicrobial properties of propolis extracts are often correlated with their content of polyphenols and flavonoids, thereby reinforcing the significance of these compounds in medicinal applications [22,19].
In conclusion, while the TPC and TFC values in the aqueous propolis extract are significant, they also emphasize the need for further exploration of extraction methods and the factors influencing the composition of propolis extracts to optimize their use in medicinal applications.
Attenuated total reflectance Fourier transform infrared spectroscopy analysis of propolis (FT-IR-ATR)
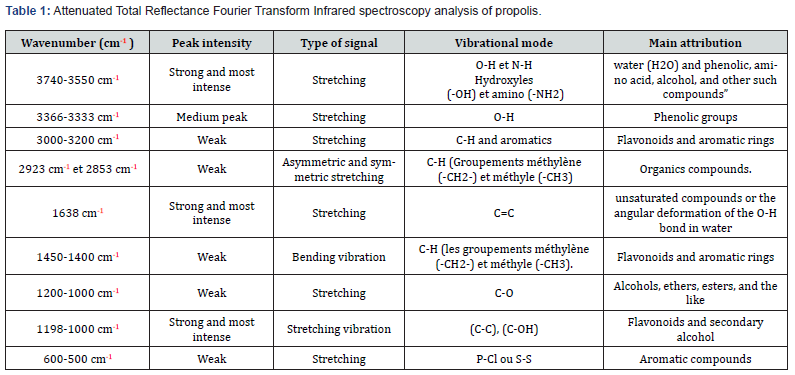
The discussion of the results obtained from FT-IR–ATR spectroscopy reveals a series of absorption bands that provide valuable insights into the chemical composition of the sample (Figure1) (Table 1). The wavenumber region from 3740 to 3550 cm-¹ exhibits a strong and intense stretching vibration, attributed to the O-H and N-H functionalities of water (H₂O) as well as various phenolic compounds, amino acids, and alcohols [23-26]. In the range of 3366 to 3333 cm-¹, a medium peak corresponds to the stretching vibration of phenolic groups. The region from 3000 to 3200 cm-¹ displays weak stretching signals associated with aromatic ring structures and C-H groups, indicating the presence of flavonoids and other aromatic compounds. The absorption bands at 2923 cm-¹ and 2853 cm-¹ are characteristic of asymmetric and symmetric stretching vibrations of methylene (-CH₂-) and methyl (-CH₃) groups, suggesting the presence of various organic compounds. The most intense peak at 1638 cm-¹ can be attributed to the stretching vibration of carbon-carbon double bonds (C=C) [27], which was also observed in extracts of red propolis [28], indicating the presence of unsaturated organic compounds or the angular deformation of the O-H bond in water. The region from 1450 to 1400 cm-¹ presents weak bending vibrations associated with methylene (-CH₂-) and methyl (-CH₃) groups, corroborating the presence of flavonoids and aromatic structures [23,29,30,26].
A series of weak absorption bands observed in the range of 1200 to 1000 cm-¹ correspond to stretching vibrations of carbonoxygen (C-O) bonds, indicating the presence of alcohols, ethers, esters, and similar oxygenated organic functionalities, which are found in phenolic acids and flavonoids [25,31,32,26], present in propolis extracts [33-37,26]. The most intense signals in the region from 1198 to 1000 cm-¹ can be attributed to the stretching vibration of (C-C) and (C-OH) bonds, as well as (C-OH) bending modes, suggesting the presence of flavonoids and secondary alcohol groups. [38,39,26].
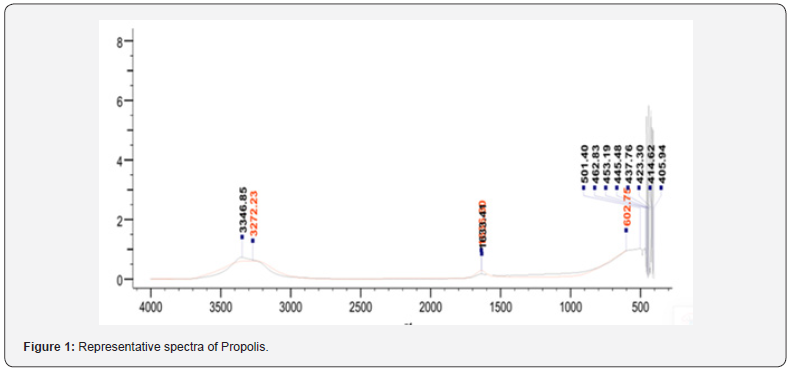
The chemical analysis of propolis (Table 2) reveals a diversity of functional groups and compound classes. The presence of phosphorus-containing P-Cl groups and sulfur-containing S-S compounds suggests that propolis may have antioxidant and detoxification properties, as these elements can participate in redox reactions and neutralize reactive species. Additionally, the identification of azines (RCH=N-N=CHR), hydrazones (CH=NNH₂), and hydroxamic compounds ((C=O) NH-OH) indicates that propolis contains bioactive nitrogen-containing compounds, which may exhibit antimicrobial, anti-inflammatory, or anticancer activities. The detection of amidines (N=CH-N) and various ketones, such as C-(C=O)-C=C-OH, C-(C=O)-Ph-βOH, R-(C=O)- C=C-NH₂, and C-(C=O)-Ph-βNH₂, further suggests the presence of compounds with potential antioxidant, anti-inflammatory, and wound-healing properties. In conclusion, the diverse chemical composition of propolis, comprising a range of phosphorus, sulfur, nitrogen, and carbonyl-containing functional groups, reflects its remarkable potential as a source of biologically active compounds with various therapeutic applications [40].
In vivo study Effect of APE on neurological tests
To investigate neurological changes in mice exposed to aluminum chloride, and the neuroprotective effect of aqueous propolis extract on ameliorating these alterations, behavioral and memory tests were carried out.
Behavioral tests
Anxiety and depression were assessed using Elevated Plus Maze (EPM) and the Forced Swim Test (FST), in addition the locomotor activity test.
i. Locomotor Activity
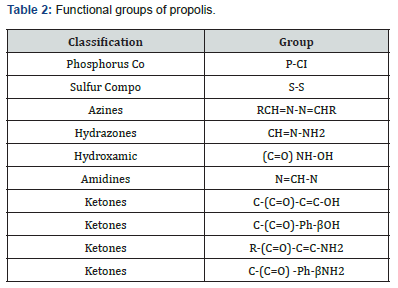
Locomotor activity of the mice was assessed by the number of squares crossed scored per 5 min for each mouse. The results of this test indicated locomotor hypoactivity in the Alzheimer model mice as opposed to the control group (Figure 2). Different experimental studies on rodents have also revealed that chloride aluminum can lead to alterations in the cholinergic system [41] and neurobehavioral changes [42]. The hypoactivity could be explained by neuronal loss in the hippocampus and disruption of cholinergic function [43]. However, administration of the studied extract and rivastigmine exerted a moderating effect on the behavior of treated Alzheimer’s model mice, which showed locomotor hyperactivity compared with the Alzheimer’s model group. These results agreed with those of [44] in which ethanolic extract of yellow propolis was evaluated for its neurobehavioral effects.
ii. Elevated Plus Maze (EPM)
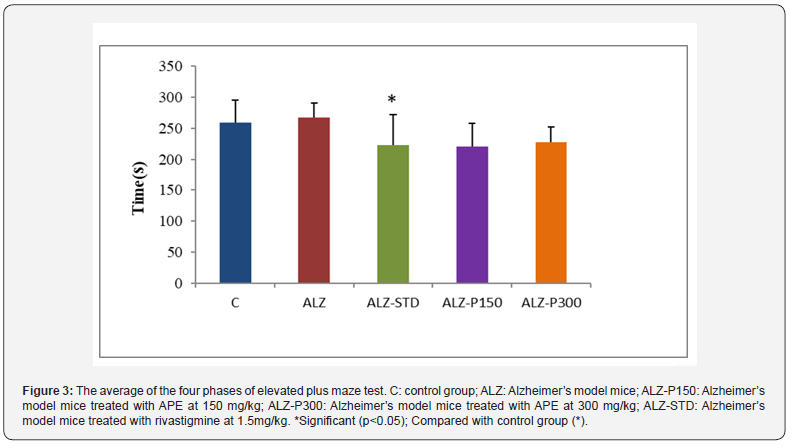
To test whether APE can affect the anxiety behavior of mice, EPM assay was performed. Results show the mice treated with propolis at 150 (ALZ-P150) and 300 mg/kg (ALZ-P300) and rivastigmine 1.5 mg/kg (ALZ-STD) spend less time in the protected arms than the Alzheimer’s model group (ALZ). The same observation was made between the Alzheimer’s model group and the control group, but with only a slight difference (Figure 3). These results indicate that the consumption of APE induced no anxiolytic effect in mice. Very similar findings were observed by [45]. In addition [46], who worked with propolis essential oil.
iii. Forced Swim Test (FST)
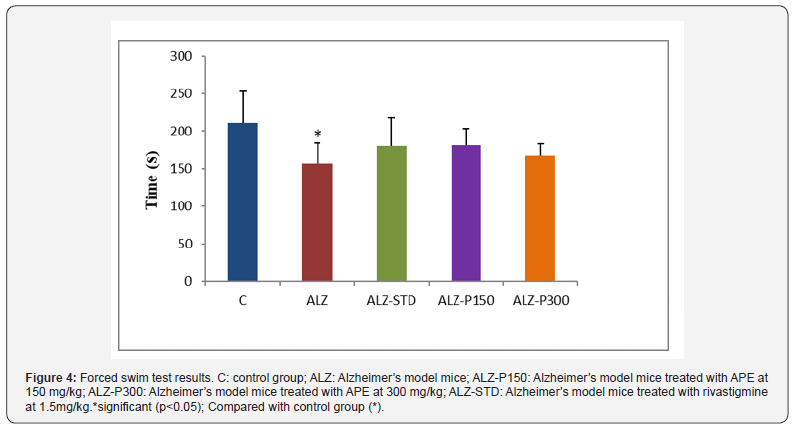
During the FST, the immobility time recorded in the Alzheimer’s group was less than that recorded in the control and treated groups with propolis at 150 and 300mg/kg (ALZ-P150), (ALZ-P300) respectively and rivastigmine at 1.5mg/kg (ALZ-STD) (Figure 4). These data were consistent with the observations of [47]. Aluminum chloride significantly reduced the immobility time, which was reflected in the increase in depressive state [48]. The absence of significant difference between the model control group and APE-treated groups on immobility time, locomotion and anxious behavior is a consistent indication that they had no motor or motivational deficits, these findings could be explained by the effect exerted by APE on central nervous system (CNS).
iv. Memory tests
The spatial learning and memory were assessed using the Radial arm-maze (RAM) and the Morris water maze (MWM).
The Arm Radial Maze (ARM) i. Spatial Working Memory (SWM)
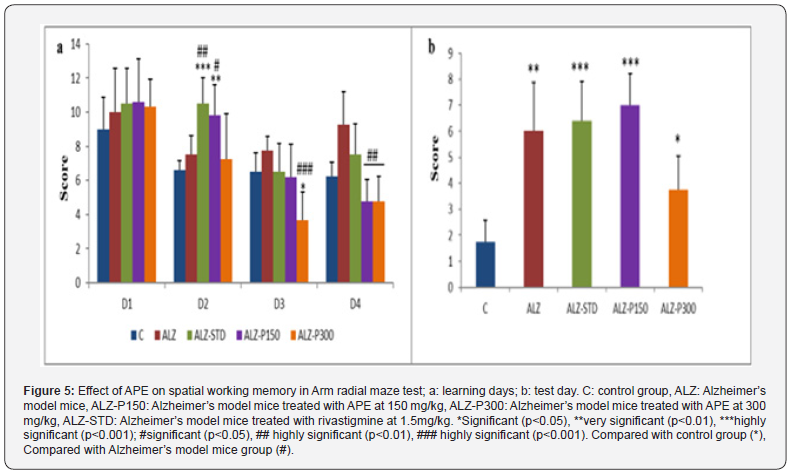
Recorded scores show that repeated arms visiting by Alzheimer’s model mice (ALZ) is significantly higher compared with the control group. Remarkable difference between the mice treated with the aqueous propolis extract at 300mg/kg (ALZ-P300) and Alzheimer’s model mice (Figure 5). Our results showed that Alcl3 induced cognitive impairment characterized by impaired working memory and spatial learning performance, leading to a spatial memory retention deficit. These results are in line with various studies on memory disorders showing that Alcl3 induces cognitive dysfunction and negatively affects spatial learning and memory capacity in mice by [2]. The same results were obtained in male and female rats treated with different doses of aluminum 0.125; 0.25; 0.5 and 1 mg/kg/day for 8 weeks by [48].
ii. Position Distinction test
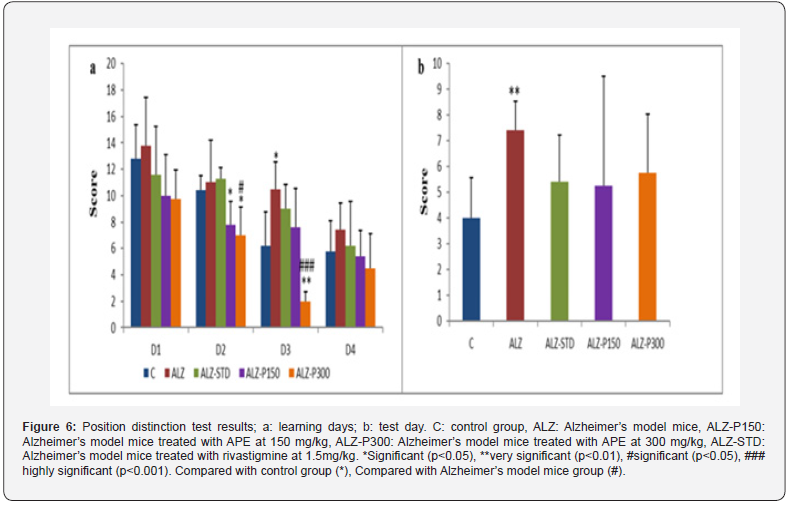
The results obtained from this test during the learning days reveal that the control group and Alzheimer model mice treated with propolis at 300mg/kg showed a significant (P<0.050) and highly significant (P<0.010) difference respectively compared with the Alzheimer’s model mice. On the test day, the mice in the control group and those treated with propolis, and the synthetic treatment (ALZ-STD) scored lower on visits to the baited arms than mice in the Alzheimer’s model (ALZ) (Figure 6). Impaired learning memory observed is the consequence of neurodegeneration and alterations to the cerebral cortex, cerebellum and hippocampus caused by exposure to aluminum. These results agreed with those of [49].
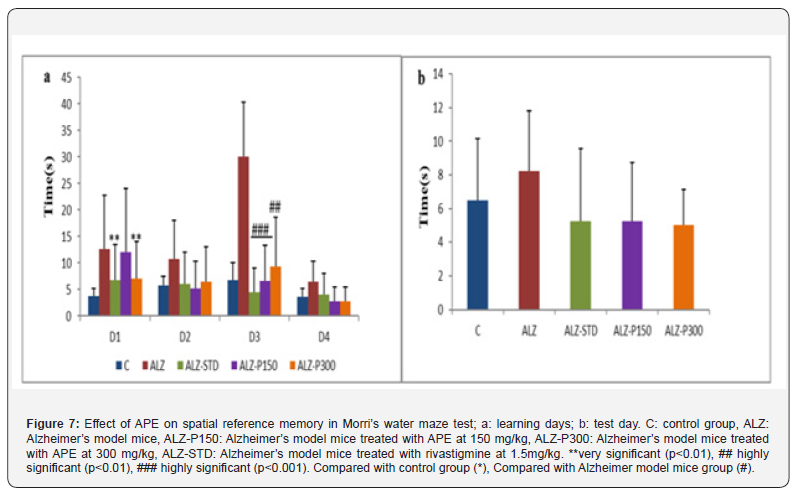
Morris Water Maze i. Spatial Reference Memory
The results of spatial reference memory in the Morris water maze show a significantly longer time for Alzheimer’s model mice to reach the invisible platform compared with treated Alzheimer’s models (Figure 7). This is in line with the findings of [50], which revealed a significant attenuation of scopolamine-induced amnesia in the group treated with 100mg/kg of aqueous propolis extract. 3.3 Histopathological study.
Histological examination of brain sections revealed the toxic effect of AlCl3 combined with D-galactose by detecting brain tissue damage and neuronal degeneration (Figure 8). These results are in line with those of [51,52].
Aluminum chloride readily enters the central nervous system through the blood-brain barrier (BBB), forms a stable complex with L-glutamic acid and accumulates in various regions, particularly in the hippocampus which is the key mediator of spatial learning and memory and in cerebral cortex, causing neuronal damage [53-56].
Histopathological evaluation showed that the hippocampus of Alzheimer’s model mice presents histopathological changes induced certainly by exposure to aluminum chloride, represented by the deposition of amyloid plaques (AP), neurofibrillary tangles and neuronal loss, unlike the control group that had a normal dentate gyrus (DG) structure. The hippocampus region of groups treated with propolis extract at 150 and 300 mg/kg appeared normal, with high cell density (Figure 8). These results are consistent with those of [57].
Neuronal alterations were also detected in the cerebral cortex of the group treated with the aluminum chloride, with the presence of amyloid plaques and hemorrhage. In the treated groups the aqueous propolis extract exerted a protective action, while rivastigmine maintained the structure of the cerebral cortex. Histological sections of the cerebellum in the Alzheimer’s model mice treated with propolis at 300mg/kg (ALZ-P300) and rivastigmine (ALZ-STD) show good positioning of the normally apparent Purkinje cell layers. They are almost similar to the control group. In contrast, the Alzheimer’s group, which shows necrose of Purkinje cells, with the presence of hemorrhages (Figure 8). These results are in line with the work of [58] who noted almost the same remarks.
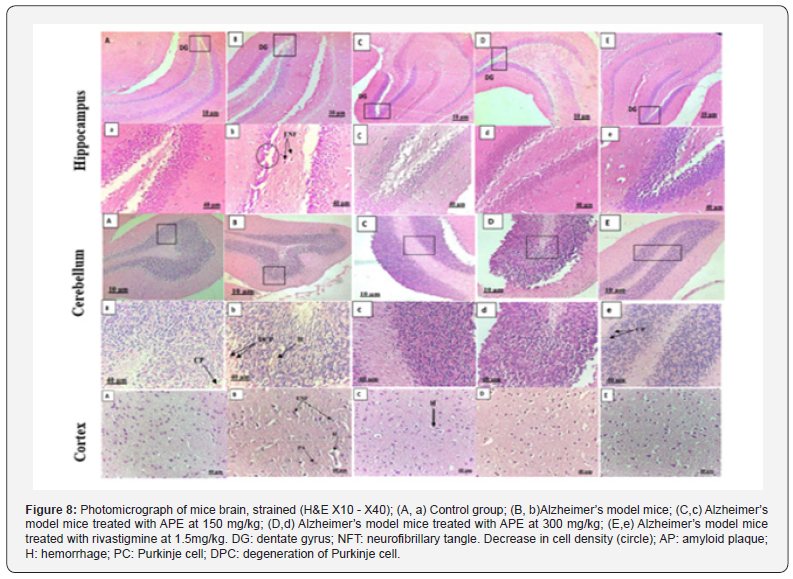
According to [59], propolis improves the antioxidant defense system by reducing malondialdehyde in the brain, which is a major factor in Alzheimer’s disease, inhibits acetylcholinesterase (Ache) activity and stimulates Brain-Derived Neurotrophic Factor (BDNF) potential, which plays an important role in neuronal survival and growth and serves as a neurotransmitter modulator
The results of the present study demonstrate that the aqueous propolis extract (APE) exerted a neuroprotective effect against aluminum chloride-induced memory and behavior disorders, thanks to its rich chemical composition and its broad-spectrum biological capabilities, which helps to reduce brain tissue toxicity. It is worth noting that the administration chronic of propolis extract at respected doses exerted a neuroprotective action, giving positive and more effective results against neurodegeneration. This could open up new avenues of research into the treatment of neurological diseases, including Alzheimer’s disease.
Materials and Methods
Propolis
In the current study, the aqueous propolis extract (6.67%) was obtained from the Balparmak bee products research and quality control laboratory (APILAB), Turkey. The propolis used was harvested in 2019.
Chemicals and reagents
Aluminum chloride, D-galactose, Folin-ciocalteu reagent, gallic acid, formalin, ethanol, acetone, xylem, Harris hematoxylin, eosin, hydrochloric acid, lithium bicarbonate and eukit were purchased from Sigma-Aldrich. Rivastigmine (Exelon®).
In vitro study Total phenolic content
Total phenolic content in APE was determined according to the colorimetric method using the Folin-Ciocalteu reagent and gallic acid as standard [60]. APE (0.5 mL) was mixed with 0.5 mL of the Folin-Ciocalteu reagent and 0.5 mL of 10% Na2CO3, and the absorbance was measured at 760 nm after 1h of incubation at room temperature. APE samples were evaluated at the final concentration of 20 μg/mL. Total phenolic content was expressed as milligram gallic acid equivalents per kilogram (mg/GAE/kg).
Total flavonoid content
Total flavonoid in APE were determined according to the method of [61].
Attenuated Total Reflectance Fourier Transform Infrared spectroscopy analysis of propoli
The FT-IR–ATR Spectroscopy is a surface analysis technique that is based on the phenomenon of attenuated total reflection. The propolis sample is simply placed in contact with a crystal made of a high refractive index material, such as diamond. An infrared beam is then directed into the crystal, creating an evanescent wave that interacts with the sample and undergoes an attenuation characteristic of the present chemical bonds. A sample reading of 32 scans was performed at a resolution of 2 cm-1. The analysis was carried out from 4000 to 400 cm-1.
In Vivo Study Animals
25 female Albinos weighing 30± 5 were used in this study; the mice were obtained from Pasteur institute of Algeria. They were evaluated in the animal house of pharmacognosy and Apiphytotherapy laboratory Mostaganem University. Algeria. These mice were allowed to access food (Standard diet) and water ad libitum. They were kept under controlled laboratory condition with favorable temperature, relative humidity and light / dark cycles 12h during the experimental protocol. This study is strictly followed as recommended by the institutional Animal Ethics Committee (1205/c/08/CPCSEA .21.04.08).
Study design
After two weeks of acclimatization, the mice were divided into
5 groups (n = 5)
a. Group C: control group, received distilled water orally
and normal saline given intraperitoneal (IP).
b. Group ALZ: Alzheimer’s model group was injected with
120 mg/kg of D-galactose and received the AlCl3 at 100 mg/kg
orally.
c. Group ALZ-STD: The mice were given rivastigmine
at 1.5 mg/kg intragastrical daily for 6 weeks, followed by coadministration
of AlCl3 (100 mg/kg / orally) and D-gal (120 mg/
kg -IP) for the rest of 12 weeks.
d. Group ALZ-P150: The mice were received the aqueous
propolis extract at 150mg/kg intragastrical daily for 6 weeks,
followed by co-administration of AlCl3(100 mg/kg / orally) and
D-gal (120 mg/kg -IP) for the rest of 12 weeks.
e. Group ALZ-P300: The mice received the aqueous
propolis extract at 150mg/kg intragastrical daily for 6 weeks,
followed by co-administration of AlCl3 (100 mg/kg / orally) and
D-gal (120 mg/kg -IP) for the rest of 12 weeks.
Neurological study
At the end of the experiment, the mice were evaluated by a
Behavioral Tests i. Locomotor Activity or Open Field Test (OFT)
The animal was placed in a closed space measuring 32X32 cm2 and divided into 16 identical squares, numbered 1 to 16. The mouse’s behavior in a new environment was evaluated for 20 minutes, divided into four consecutive phases of 5 min each. Each movement was considered a score [62].
ii. Elevated Plus Maze (EPM)
A test for measuring developed anxiety-type behavior. It consists of a 50 cm high cross with two opposing lanes, one open (unprotected) and the other closed (protected), linked by a central platform. This test makes it possible to assess the anxiety of the mouse by comparing the percentage of time spent in open arms versus closed arms [62].
iii. Forced-Swim Test (FST)
The Forced-Swim Test is a behavioral immobility model that can be used to predict the efficacy of antidepressant treatment [63]. The principle of this test is to force mice to swim in a situation from which they cannot escape, in a bath of lukewarm water at 25°C. After an initial period of vigorous activity, it eventually stops moving at all, making only the movements necessary to keep its head above water, adopting a behavior of desperation. This behavioral immobility is the sign of a state of despair in which the mouse has learned that escape is impossible and is resigned to the experimental conditions. The immobility time of each mouse was measured.
Memory tests Arm Radial Maze test (ARM)
i. Spatial Working Memory (SWM)
This test was developed by [64]. In this test, food is deposited at one end of a corridor of eight. The mouse is then placed on the central platform with free access to all corridors for five minutes. The mouse must search for food at the end of each corridor, and each error is recorded if the mouse visits the same corridor twice. The number of visits for each mouse is counted over 4 learning trials for 4 days, with the 5th day representing the test.
ii. Position Distinction Test
The mouse is placed on the central platform and the six arms are opened one after the other, three with food (baited arms) and the others without food (unbaited arms). The test is performed by opening the arms in pairs of baited and unbaited arms (3 pairs in total). The score is recorded each time by connecting the number of baited arms chosen by each mouse measured over 4 learning trials for 4 days, with the 5th day representing the test.
Morris Water Maze test (MWM) i. Spatial reference memory (SRM)
The Morris pool is one of the most widely used tests, designed by Morris, to assess the mouse’s ability to memorize and manage spatial information in an unpleasant situation. The animal must escape an aversive situation by taking refuge on a platform. To do so, it must form and use a representation of its environment based on available spatial cues. The water in the container is colored with a non-toxic dye to make the platform invisible. The time it takes the mouse to find the platform is calculated over 4 learning trials over 4 days, with the 5th day representing the 5-minute test.
Histopathological study
At the end of the experiment, the mice were sacrificed, and the mice brains were removed, rinsed in ice cold saline and fixed in 10% formalin solution for Hematoxylin-Eosin (HE) staining according to observed histopathological changes.
Statistical analysis
Statistical analysis of the experimental data obtained during the tests was carried out using XLstat software. Results were expressed as mean plus or minus standard deviation. Significant (P<0.05); very significant (P<0.01); highly significant (P<0.001).
Acknowledgement
This study was fully supported by Balparmak bee products research and quality control laboratory (APILAB) of Turkey.
References
- Xing Z, He Z, Wang S, Yan Y, Zhu H, et al. (2018) Ameliorative effects and possible molecular mechanisms of action of fibrauretine from fibraure are cisapierre on D-galactose/ALCL3 mediated Alzheimer’s disease.Rsc Adv. 10 8(55) :31646-31657.
- Uzuegbunam BC, Librizzi D and Yousefi BH (2020) PET Radiopharmaceuticals for Alzheimer ’s disease and Parkinson’s Disease Diagnosis, the Current and Future Landscape. Molecules 25(4): 977.
- Lane CA, Hardy J, Schott JM (2018) Alzheimer’s disease. National Library of Medicine 25(1).
- (2023) World Health Organization (WHO).
- Lawn T, Aman Y, Rukavina, Sideris-Lampretsas G, Howard M et al. (2021) Pain in the neurodegenerating brain: insights into pharmacotherapy for Alzheimer disease and Parkinson disease. Pain 162(4): 999-1006.
- Breijyeh Z, Karaman R (2020) Comprehensive Review of Alzheimer’s Disease: Causes and Treatment., Breakthroughs in Medicinal Chemistry 25(24): 5789.
- Colovic MB, Krstic DZ, Lazarevic-Pasti TD, Bondzic AM, Vasic VM, et al. (2013) Acetylcholinesterase inhibitors: pharmacology and toxicology. Curr. Neuropharmacology 11(3): 315-335.
- Huang LK, Chao SP, Hu CJ (2020) Clinical trials of new drugs for Alzheimer disease. Journal of Biomedical science 27: 18.
- Mohammed AA, Kunugi H (2020) Apitherapy for age-related skeletal muscle dysfunction: A review on the effects of royal jelly, propolis and bee pollen. Foods 9(10): 1362.
- Seibert JB, Jessica PB, TatianeRoquete A, Alicia P, Pauline P, et al. (2019) Development of propolis nanoemulsion with antioxidant and antimicrobial activity for use as a potential natural preservative. Food Chemistry 287: 61-670.
- Ouahab A, Grara N, Menaiaia K, Khaldi K, Bensouici Cet, al. (2023) Phytochemical Analysis, Antioxidant, and Acetylcholinesterase Inhibitory Activity of Propolis from Northeastern Algeria. Phytothérapie 21(2-3): 119-21.
- Boufadi YM, Soubhye J, Nève J, Antwerpen PV, Riazi A, et al. (2016) Antimicrobial effects of six Algerian propolisextracts. Iteratioaoljoural of food science techology 51(12): 2613-2620.
- Soltani EK, Zaim K, Mokhnache K, Haichour N, Mezaache-Aichour S, et al. (2021) Polyphenol Contents, Antioxidant and Antibacterial Activities of Aqueous Algerian Propolis Extracts. Phytothérapie 19: 408-415.
- Karagecili H, Yılmaz MA, Ertürk A, Kiziltas H, Güven L et al. (2023) Comprehensive Metabolite Profiling of BerdavPropolis Using LC-MS/MS: Determination of Antioxidant, Anticholinergic, Antiglaucoma, and Antidiabetic Effects. Extraction, Separation and Identification of Compounds from Natural Sources 28(4): 1739.
- Chan GCF, Cheung KW, Sze DMY (2013) The Immunomodulatory and Anticancer Properties of Propolis. Clinic Rev AllergImmunol 44: 262-273.
- Balderas-Cordero D, Canales-Alvarez O, Sánchez-Sánchez R, Cabrera-Wrooman A, Canales-Martinez MM, et al. (2023) Anti-Inflammatory and Histological Analysis of Skin Wound Healing through Topical Application of Mexican Propolis. Int J Mol Sci 24(14): 11831.
- Altabbal S, Athamnah K, Rahma A, Wali AF, Eid AH, et al. (2023) Propolis: A Detailed Insight of Its Anticancer Molecular Mechanisms. Pharmaceuticals 16(3): 450.
- Altuntaş Ü, Güzel İ, Özçelik B (2023) Phenolic constituents, antioxidant and antimicrobial activity and clustering analysis of propolis samples based on PCA from different regions of anatolia. Molecules 28(3): 1121.
- Paula VB, Estevinho LM, Cardoso SM, Dias LG (2023) Comparative Methods to Evaluate the Antioxidant Capacity of Propolis: An Attempt to Explain the Differences. Molecules 28(12): 4847.
- Hossain R, Quispe C, Khan R A, Saikat A S M, Ray P et al. (2022) Propolis: An update on its chemistry and pharmacological applications. Chinese medicine 17(1): 100.
- Touzani S, Imtara H, Katekhaye S, Mechchate H, Ouassou H, et al. (2021) Determination of phenolic compounds in various propolis samples collected from an African and an Asian region and their impact on antioxidant and antibacterial activities. Molecules 26(15): 4589.
- Ferreira, LMDMC, Souza, PDQD, Pereira et al. (2024) Étude préliminaire sur les propriétés chimiques et biologiques de l'extrait de propolis d'abeilles sans dard de la région nord du Brésil. Processus 12(4): 700.
- Svečnjak L, Marijanović Z, Okińczyc P, Marek Kuś P, Jerković I (2020) Mediterranean propolis from the adriatic sea islands as a source of natural antioxidants: Comprehensive chemical biodiversity determined by GC-MS, FTIR-ATR, UHPLC-DAD- QqTOF-MS, DPPH and FRAP assay. Antioxidants 9(4): 337.
- Erdoğan Ü (2023) Extraction de propolis assistée par ultrasons : caractérisation par ATR- FTIR et détermination de sa capacité antioxydante totale et de sa capacité à piéger les radicaux. Journal international du métabolite secondaire 10 (2): 231-239.
- Prđun S, Svečnjak L, Valentić M, Marijanović Z, Jerković I (2021) Characterization of bee pollen: Physico-chemical properties, headspace composition and FTIR spectral profiles. Foods 10(9): 2103.
- Pavia DL, Lampman GM, Kritz GS (2010) Introduction to Spectroscopy. 4th edn, Brooks Cole, Washington.
- Soares SE (2002) Ácidos fenólicos como antioxidantes. Revista de nutrição 15: 71-81.
- de Cardoso EO, Conti BJ, Santiago KB, Conte FL, Oliveira LPG, et al. (2017) Phenolic compounds alone or in combination may be involved in propolis effects on human monocytes. J Pharm Pharm Pharmacol 69(1): 99-108.
- Cao J, Peng LQ, Du LJ, Zhang QD, Xu JJ (2017) Ultrasound-assisted ionic liquid-based micellar extraction combined with microcrystal line cellulose as sorbent in dispersive microextraction for the determination of phenolic compounds in propolis. Anal Chim Acta 963: 24–32.
- Soltani EK, Cerezuela R, Charef Netal (2017) Algerian propolis extracts: chemical composition, bactericidal activity and in vitro effects on gilthead seabream innate immune responses. Fish Shellfish Immunol 62: 57-67.
- Bankova V (2009) Chemical diversity of propolis makes it a valuable source of new biologically active compounds. J ApiProduct ApiMedical Sci 1(2): 23-28.
- Menezes H (2005) Própolis : Uma Revisão Dos Recentes Estudos. Arq Inst Biol 72: 405-411.
- Banskota AH, Tezuka Y, Kadota S (2001) Recent progress in pharmaco logical research of propolis. Phyther Res 15(7): 561-571.
- Chang-Bravo L, López-Córdoba A, Martino M (2014) Biopolymeric matrices made of carrageenan and corn starch for the antioxidant extracts delivery of Cuban red propolis and yerba mate. React Funct Polym 85 :11-19.
- Toutou Z, Fathi S, Chibani N, Benichou L, Skia M et al. (2022) Optimisation, caractérisation et effet biologique de la propolis algérienne. Acta Skia et zootechnique : ISSN 1336-9245 25(4).
- da Silva C, Prasniewski A, Calegari MA, de Lima VA, Oldoni TL (2018) Determination of total phenolic compounds and antioxidant activity of ethanolic extracts of propolis using ATR–FT-IR spectroscopy and chemometrics. Food Analytical Methods 11: 2013-2021.
- Saleh S, Salama A, Ali AM, Saleh AK, Elhady BA, et al. (2023) Egyptian propolis extract for functionalization of cellulose nanofiber/poly (vinyl alcohol) porous hydrogel along with characterization and biological applications. Scientific Reports 13(1): 7739.
- Yellamma K, Saraswathamma S, Kumari BN (2010) Cholinergic System under Aluminium Toxicity in Rat Brain. Toxicology International 17: 106-112.
- Kumar V, Gill KD (2009) Aluminium Neurotoxicity: Neurobehavioural and Oxidative Aspects. Archives of Toxicology 83(11): 965-978.
- Traissard N, Herbeaux K, Cosquer B Jentsch, H Ferry B, Galani R, et al. (2007) Combined Damage to Entorhinal Cortex and Cholinergic Basal Forebrain Neurons, Two Early Neurodegenerative Features Accompanying Alzheimer's disease: Effects on Locomotor Activity and Memory Functions in Rats. Neuropsychopharmacol 32(4): 851-871.
- da Silveira CC, Fernandes LM, Silva ML, Luz DA, Gomes AR, et al. (2016) Neurobehavioral and Antioxidant Effects of Ethanolic Extract of Yellow Propolis. Oxid Med CellLongev 2016: 2906953.
- Bhalla P, Garg ML, Dhawan DK (2010) Protective role of lithium during aluminium induced neurotoxicity. Neuro Inter 56(2): 256-262.
- Li YJ, Xuan HZ, Shou QY, Zhan ZG, Lu X, et al. (2012) Therapeutic effects of propolis essential oil on anxiety of restraint-stressed mice. Human&ExperimentalToxicology 31(2): 157-165.
- Silva T, Paulo MEF, da Silva JRM, Alves A, Britto LR, et al. (2020) Oral treatment with royal jelly improves memory and presents neuroprotective effects on icv-STZ rat model of sporadic Alzheimer's disease. HeliyonHeliyon 6(2): e03281.
- Zghari O, Rezqaoui A, Ouakki S, Lamtai M, Chaibat J et al. (2018) Effect of Chronic Aluminum Administration on Affective and Cognitive Behavior in Male and Female Rats. Journal of Behavioral and Brain Science 8: 179-196.
- Li H, Ip S, Zheng G, Xian Y, Lin Z (2018) Isorhynchophylline alleviates learning and memory impairments induced by aluminum chloride in mice. Chinese Medicine 13: 29.
- Chen J, Long Y, Han M, Wang T, Chen Q, et al. (2008) Water-soluble derivative of propolis mitigates scopolamine-induced learning and memory impairment in mice. Pharmacology Biochemistry and Behavior 90(3): 441-446.
- Kawahara M, Kato Negishi M (2011) Link between aluminum and the pathogenesis of Alzheimer’s disease: The integration of the aluminum and amyloid cascade hypotheses. Int J Alzheimer’s Dis 2011: 276393.
- Mahdi O, Chiroma SM, Hidayat Baharuldin MT, MohdNor NH, Mat Taib CN, et al. (2021) WIN55, 212-2 attenuates cognitive impairments in AlCl3+ d-Galactose-Induced Alzheimer’s disease rats by enhancing neurogenesis and reversing oxidative stress. Biomedicines 9(9): 1270.
- Ojo SA, Hambolu JO, Adebisi SS (2011) Effects of Aluminium Chloride on Anxiety-Related Behaviour. Department of Human Anatomy, Department of Veterinary Anatomy, Faculty of Veterinary Medicine,Ahmadu Bello University, Zaria 2(2): 65-69.
- Gurel A Coskun O, Armutcu F Kanter M, AslanOzen O (2005) Vitamin E against oxidative damage caused by formaldehyde in frontal cortex and hippocampus: Biochemical and histological studies Journal of Chemical Neuroanatomy 29(3): 173-178.
- Thirunavukkarasu S, Upadhayay L, Venkataraman S (2012) Effect of manasamitravatakam, an ayurvedic formulation, on Aluminium-induced neurotoxicity in rats. Journal of Pharmaceutical Research 11(1): 75-83.
- Khalil H, Salama H, Al Mokaddem A, Aljuaydi S, Edris A (2020) Edible dairy formula fortified with coconut oil for neuroprotection against aluminium chloride-induced Alzheimer's disease in rats. Journal of FunctionalFoods 75: 104296.
- Nanaware S, Shelar M, Sinnathambi A, Mahadik KR, Lohidasan S (2017) Neuroprotective effect of Indian propolis in β-amyloid induced memory deficit: Impact on behavioral and biochemical parameters in rats. Biomedicine&Pharmacotherapy. 93: 543-553.
- Singleton VL, Orthofer R, Lamuela-Raventos RM (1999) Analysis of total phenols and other oxidation substrates and antioxidants by means of Folin-Ciocalteu reagent. MethodsEnzymol 299: 152-178.
- Ahn MR, Kumazawa S, Usui Y, Nakamura J, Matsuka M, et al. (2007) Antioxidant activity and constituents of propolis collected in various areas of China. Food Chemistry 101(4): 1383-1392.
- Carola V, D’Olimpio F, Brunamonti E, Mangia F, Renzi P (2002) Evaluation of the elevated plus-maze and open-field tests for the assessment of anxiety-related behaviour in inbred mice. Behavioural Brain Research 134(1-2): 49-57.
- Ullrich M, Weber M, Post MA, Popp S, Grein J, et al. (2018) OCD-like behavior is caused by dysfunction of thalamo-amygdala circuits and upregulatedTrkB/ERK-MAPK signaling as a result of SPRED2 deficiency. Mol Psychiatry 23(2): 444-458.
- Persolt RD, Jalve M (1997) Poison M a new model rensitivve to anti-depressant treatment. Naturepharmacodynamy 266(5604): 730-732.
- Oltan D, Collison CH, Werz MA (1997) Spatial memory and radial arm maze performance of rats, Learning and Motivation 8(3): 298-314.
- Santini L, Save E, Poucet B (2001) Evidence for a relationship between place-cell spatial firing and spatial memory performance. Hippocampus 11(4): 377-390.
- Morris R (1984) Developments of a water-maze procedure for studying spatial learning in the rat. National Library of medicine 11(1): 47-60.
- Vander Linden M (2014) L’évaluation neuropsychologique dans la démence: un changement d’approche. Science Techniques 575-598.
- Marck V (2010) Anatomy and pathological cytology. In: Marck V (Ed.), Manual of anatomo‐cytopathology techniques. Masson SAS. Elsevier, pp. 35-132.






























