- Research Article
- Abstract
- Introduction
- Material and Method
- Plant Extraction
- Isolation of Myricetin from the Extract
- Determination of Plant Extract Yield
- Preparation of Moieties
- Molecular Docking
- ADMET (Absorption, Distribution, Metabolism, Excretion and Toxicology)
- Result and Discussion
- Conclusion
- References
Extraction, Isolation, In-silico Studies, Synthesis & Evaluation of Semi-Synthetic Derivatives of Myricetin
Lovish Sharma, Bhartendu Sharma* and Ravnish Mishra
School of Pharmacy and Emerging Sciences, Baddi University of Emerging Sciences & Technology, India
Submission: December 12, 2023; Published: January 05, 2024
*Corresponding author: Bhartendu Sharma, School of Pharmacy and Emerging Sciences, Baddi University of Emerging Sciences & Technology, Baddi District Solan - 173205, Himachal Pradesh, India, Email: bhartendu.sharma@baddiuniv.ac.in
How to cite this article: Lovish Sharma, Bhartendu Sharma* and Ravnish Mishra. Extraction, Isolation, In-silico Studies, Synthesis & Evaluation of Semi-Synthetic Derivatives of Myricetin. Glob J Pharmaceu Sci. 2024; 11(2): 555810. DOI: 10.19080/GJPPS.2024.11.555810.
- Research Article
- Abstract
- Introduction
- Material and Method
- Plant Extraction
- Isolation of Myricetin from the Extract
- Determination of Plant Extract Yield
- Preparation of Moieties
- Molecular Docking
- ADMET (Absorption, Distribution, Metabolism, Excretion and Toxicology)
- Result and Discussion
- Conclusion
- References
Abstract
The field of drug discovery and development has seen significant advancements with the integration of computational techniques, particularly Computer-Aided Drug Design (CADD). The process of discovering and developing new drugs involves structural modifications to enhance their activity and other physical properties, often incorporating semi-synthetic methods to thoroughly explore structure-activity relationships. The rapid advancements in computational chemistry, molecular biology, combinatorial chemistry, and high-throughput screening are driving transformative changes in the pharmaceutical industry. Myrica esculenta plant has enormous numbers of chemical constituent in it Myricetin known for having anti-cancer anti- bacterial properties in it extracted and isolated from myrica esculenta leaves and synthesized theoretically on the basis of previous studies, hypothetical compounds with the base moiety as myricetin were prepared and went through in silico studies the compound with the greatest affinity with receptors, drug likeness and ADMET will enroute to be a developable compound, the receptors on which compounds were bound are divided into two one is for anti- bacterial compounds/ activity and second is for anti-cancer compounds/activity. Xanthomonas axonopodis pv. citri (Xas), Pseudomonas syringe pv. actinidiane (Pas) is for anti-bacterial and Bcap-37 = Breast cancer cell, GC- 7901 = Gastric cancer cell, MDA-MB-231 are the three receptors for Anti-cancer compounds/ activity. The compound with the highest affinity with any of the receptors will be the targeted compound for studies.
Keywords: Drug discovery and development; Molecular docking; In-silico ADMET; Myrica esculenta; Anti- cancer; Derivatives
Abbreviations: ADMET: Absorption, Distribution, Metabolism, Excretion, and Toxicological Properties; MW: Molecular Weight; HBD: Hydrogen Bond Donor; HBA: Hydrogen Bond Acceptor; TPSA: Topological Polar Surface Area; RB: Rotatable Bond count; RF: Retardation Factor
- Research Article
- Abstract
- Introduction
- Material and Method
- Plant Extraction
- Isolation of Myricetin from the Extract
- Determination of Plant Extract Yield
- Preparation of Moieties
- Molecular Docking
- ADMET (Absorption, Distribution, Metabolism, Excretion and Toxicology)
- Result and Discussion
- Conclusion
- References
Introduction
Cancer has proven to be one of the long lasting and stubborn diseases to treat, which affects millions of people around the world, and on the other side plant bacteria causes harm to the crops. As science is going ahead on daily basis new/novel compounds are prepared in the search of better efficacy and efficiency, Myricetin (3,5,7- trihydroxy-2-(3′,4′,5′- trihydroxyphenyl)- 4H-chromen-4-one) lies under the categories of flavonoids has proved to be a good base moiety for the preparation of new/novel compounds with anti-cancer, Anti- bacterial, etc. activities. Myrica esculenta, also known as Katphal, is a plant that contains myricetin and many chemical constituents. It is a high-altitude, medium-sized plant with a brown bark that is not very hard to touch. The leaf of myrica esculenta is long and always in multiples of 6-9 leaves on one branch. The fruit of myrica esculenta is very famous in hilly areas and known as katphal. It is red in colour and grows seasonally. The plant’s flowers are tiny and white in colour, and its leaves are utilized for the separation and extraction of myricetin. As quinazoline and pyridine containing moiety have been shown to be better for anti-bacterial and cancer activities, respectively, they are combined with myricetin as a base moiety [1].
- Research Article
- Abstract
- Introduction
- Material and Method
- Plant Extraction
- Isolation of Myricetin from the Extract
- Determination of Plant Extract Yield
- Preparation of Moieties
- Molecular Docking
- ADMET (Absorption, Distribution, Metabolism, Excretion and Toxicology)
- Result and Discussion
- Conclusion
- References
Material and Method
The research was started by collection of leaves, Myrica esculenta leaves were collected from South Shimla’s vicinity on 1st of August 2022, Once collected the leaves were sent for authentication/identification to Raw Material Herbarium and Museum, Delhi (RHMD) on 30/08/2022. (Authentication No: NIScPR/RHMD/Consult/2022/4189-90).
- Research Article
- Abstract
- Introduction
- Material and Method
- Plant Extraction
- Isolation of Myricetin from the Extract
- Determination of Plant Extract Yield
- Preparation of Moieties
- Molecular Docking
- ADMET (Absorption, Distribution, Metabolism, Excretion and Toxicology)
- Result and Discussion
- Conclusion
- References
Plant Extraction
Collected leaves were cleansed under running water several times for the purpose of removing accumulated dirt and dust. After being washed, the leaves were air dried before being placed in a room with a normal temperature to dry in the shade. Leaves were shadow dried for two to three weeks away from direct sunlight in the Pharmaceutics laboratory of Advance Research Block at Baddi University of Emerging Sciences and Technology. After shadow drying for about 2-3 weeks, leaves had become hard & moisture free as there no water present in them anymore. A mechanical grinder was used to grind the dried leaves.150 gm of dried leaves was grinded to collect a coarse powder. After the leaves were powdered, the powder was sieved to ensure that it was of a uniform size for the next process. Maceration as a extraction technique was chosen for the extraction of the plant, finely coarse plant leave (250gms) were weighed on a weighing machine and kept in a 1000ml beaker and Acetone (800ml) was poured in the beaker, after stirring the beaker was covered with aluminum foil to ensure no air leak, after that the beaker was kept for 3 days with periodic stirring, once the time period for the maceration was completed i.e., 3 Days, the product was suction filtered, solvent collected after the filtration was evaporated in Rota evaporator [2].
- Research Article
- Abstract
- Introduction
- Material and Method
- Plant Extraction
- Isolation of Myricetin from the Extract
- Determination of Plant Extract Yield
- Preparation of Moieties
- Molecular Docking
- ADMET (Absorption, Distribution, Metabolism, Excretion and Toxicology)
- Result and Discussion
- Conclusion
- References
Isolation of Myricetin from the Extract
To extract myricetin, 10g of acetone leaf extract underwent column chromatography. Silica gel (100-200 mesh) was employed as the matrix. The mixture, when applied to the column, was eluted first with 100% hexane. Subsequently, the polarity was increased using a combination of chloroform, ethanol, and methanol in ratios of 90:10, 80:20, 70:30, and 50:50. The collected fractions underwent TLC analysis, where those with similar Rf values were combined. TLC development occurred in a twin chamber with various solvent systems like chloroform and methanol in a 1:1 ratio. These fractions were then run on a silica gel 60 F254 pre-coated aluminum plate, 0.2 mm thick., the calculation of Retardation factor (Rf) was performed with the help of the formula, Rf = Distance moved by the solute/Distance moved by the solvent [3].
- Research Article
- Abstract
- Introduction
- Material and Method
- Plant Extraction
- Isolation of Myricetin from the Extract
- Determination of Plant Extract Yield
- Preparation of Moieties
- Molecular Docking
- ADMET (Absorption, Distribution, Metabolism, Excretion and Toxicology)
- Result and Discussion
- Conclusion
- References
Determination of Plant Extract Yield
The percentage of plant extract yield was determined from the equation provided below:
% yield (g/100g of the dry plant material) = (W1*100)/W2
Here, W1 represents the weight of extract after solvent evaporation And, W2 represents the weight of dry powder of leaves.
- Research Article
- Abstract
- Introduction
- Material and Method
- Plant Extraction
- Isolation of Myricetin from the Extract
- Determination of Plant Extract Yield
- Preparation of Moieties
- Molecular Docking
- ADMET (Absorption, Distribution, Metabolism, Excretion and Toxicology)
- Result and Discussion
- Conclusion
- References
Preparation of Moieties
The moieties prepared were based on myricetin or we can say that the moieties prepared have myricetin as their base, the compounds prepared undergo CADD or Computer Aided drug design Software to check their affinity with their respective receptors [4].
- Research Article
- Abstract
- Introduction
- Material and Method
- Plant Extraction
- Isolation of Myricetin from the Extract
- Determination of Plant Extract Yield
- Preparation of Moieties
- Molecular Docking
- ADMET (Absorption, Distribution, Metabolism, Excretion and Toxicology)
- Result and Discussion
- Conclusion
- References
Molecular Docking
Molecular docking holds a vital role in both structural molecular biology and computer-aided drug design. Its primary function is to predict where a ligand binds to a known protein structure in three dimensions. This prediction is crucial for understanding how molecules interact and for developing potential drugs. Effective docking methods explore high-dimensional spaces and utilize a scoring system to rank potential binding positions accurately. Docking plays a key role in virtual screening of large compound libraries, ranking results, and proposing structural hypotheses about how ligands hinder their target. This process is invaluable in lead optimization, enabling the design of better drugs. The setup of input structures for docking is as significant as the docking process itself. Sometimes, interpreting the results of stochastic search techniques can be challenging. Typically, molecular docking involves a small molecule binding with a larger one, known as ligand-protein docking. However, there is a growing interest in exploring protein-protein docking scenarios. This expanding field showcases the evolving complexity and importance of molecular docking in the realm of scientific research and drug development, bridging the gap between theoretical predictions and practical applications. The entire process plays a crucial role in advancing our understanding of molecular interactions and propelling drug discovery efforts. The docking of the pyridazine derivatives has been performed by Auto Dock Vina software which is available free on site https://vina.scripps.edu/. The working of Auto Dock vina is found on https://vina.scripps.edu/tutorial/
He helps to introduce proper working of Auto Dock vina. For work on Auto Dock vina these three tools are important: Auto Dock MGL Tool (https://ccsb.scripps.edu/mgltools/ downloads/.), Auto Dock Vina (https://vina.scripps.edu/.), Pymol visualization tool (https://pymol.org/2/.). These tools are freely available on their respected sites [5].
- Research Article
- Abstract
- Introduction
- Material and Method
- Plant Extraction
- Isolation of Myricetin from the Extract
- Determination of Plant Extract Yield
- Preparation of Moieties
- Molecular Docking
- ADMET (Absorption, Distribution, Metabolism, Excretion and Toxicology)
- Result and Discussion
- Conclusion
- References
ADMET (Absorption, Distribution, Metabolism, Excretion and Toxicology)
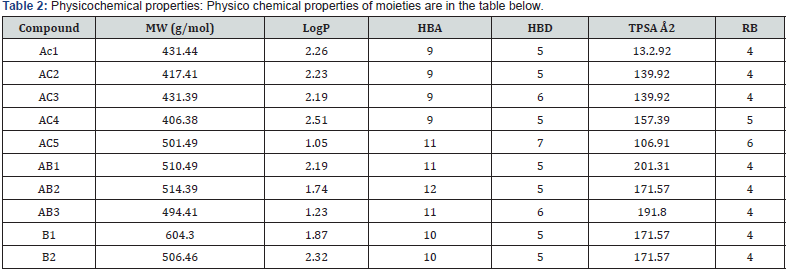
The primary purpose of calculating ADMET properties is to provide an initial estimation of compounds projected to their pharmacological behaviours to become a drug. The derivatives employed in this research study had their absorption, distribution, metabolism, excretion, and toxicological properties (ADMET) calculated. The Swiss ADME website was utilized to compute various physicochemical properties, including molecular weight (MW), hydrogen bond acceptor (HBA), hydrogen bond donor (HBD), octanol/water partition coefficient (LogP), topological polar surface area (TPSA), and the count of rotatable bonds (RB). Physicochemical properties like molecular weight (MW), hydrogen bond acceptor (HBA), hydrogen bond donor (HBD), octanol/ water partition coefficient (LogP), topological polar surface area (TPSA), and rotatable bond count (RB) were computed with the help of Swiss ADME website. The physiochemical properties of the compounds were analyzed taking consideration into the Lipinski, Veber and Pfizer toxicity empirical rules.
1. Anti-cancer
a. Bcap-37 = Breast cancer cell.
b. SGC- 7901 = Gastric cancer cell.
c. MDA-MB-231= breast cancer cell.
2. Anti- bacterial
a. Xanthomonas axonopodis pv.citri = Plant bacteria.
b. Pseudomonas syringe pv.actinidiane = Plant bacteria (Table 1).
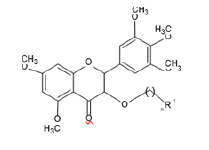
Anti-cancer moiety (AC)
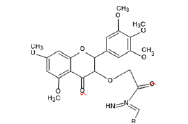
Anti-cancer moiety (AB)
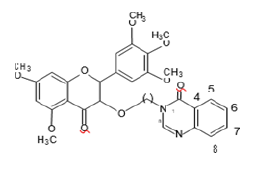
Anti-bacterial moiety
- Research Article
- Abstract
- Introduction
- Material and Method
- Plant Extraction
- Isolation of Myricetin from the Extract
- Determination of Plant Extract Yield
- Preparation of Moieties
- Molecular Docking
- ADMET (Absorption, Distribution, Metabolism, Excretion and Toxicology)
- Result and Discussion
- Conclusion
- References
Result and Discussion
Plant Extraction:- Maceration as a extraction technique was chosen for the extraction of the plant, finely coarse plant leave (250gms) were weighed on a weighing machine and kept in a 1000ml beaker and Acetone (800ml) was poured in the beaker, after stirring the beaker was covered with aluminum foil to ensure no air leak, after that the beaker was kept for 3 days with periodic stirring, once the time period for the maceration was completed i.e., 3 Days, the product was suction filtered, solvent collected after the filtration was evaporated in Rota evaporator, product collected after the evaporation was 26.5gms which is around 10.5% yield. The percentage plant extract yield was collected from the equation written below:
% yield (g/100g of the dry plant material) = (W1*100)/W2
Here, W1 represents the weight of extract after solvent evaporation and, W2 is the weight of dry powder of leaves. So, 26*100/250 =10.4, %yield = 10.4%
Isolation of Myricetin from the Extract
To isolate myricetin, a column chromatography was performed using 10g of acetone leaf extract. The extract was chromatographed over a silica gel column (100-200 mesh) employing solvents of rising polarity. The mixture was then loaded onto the column and elution commenced with 100% hexane, followed by a gradual increase in polarity using chloroform, ethanol, and methanol in ratios of 90:10, 80:20, 70:30, and 50:50, respectively. All collected fractions underwent TLC analysis. Fractions with similar Rf values were combined. TLC development occurred in a twin chamber using various solvent systems, including chloroform and methanol in a 1:1 ratio. Fractions were applied to a silica gel 60 F254 pre-coated aluminium plate with a thickness of 0.2mm. The retardation factor (Rf) was calculated with the help of the formula: Rf = Distance moved by the solute/Distance moved by the solvent. After the Column chromatography multiple fractions were collected and with the help of continuous TLC or Thin layer chromatography the identification of myricetin has done, 3rd fraction was the closest to the Rf of myricetin i.e., 7, So, the 3rd fraction was sent for spectral analysis (Figures 1-3) [6].



Spectral analysis of myricetin
For the Spectral analysis of the 3rd fraction the sample was sent to OXIGEN ANALYTICAL LABORATORIES,
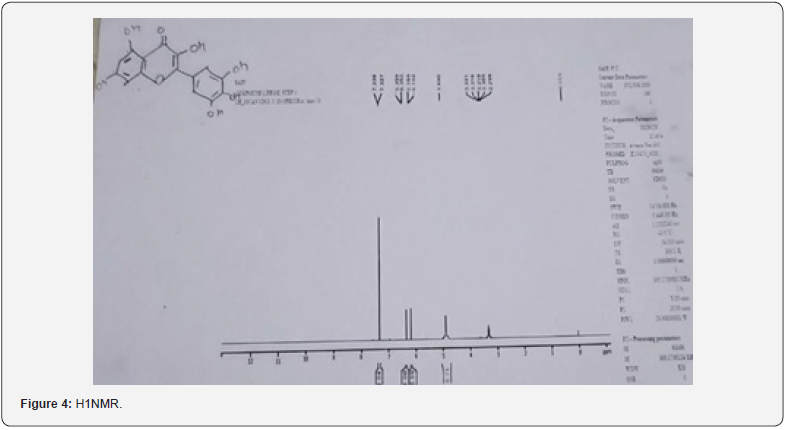
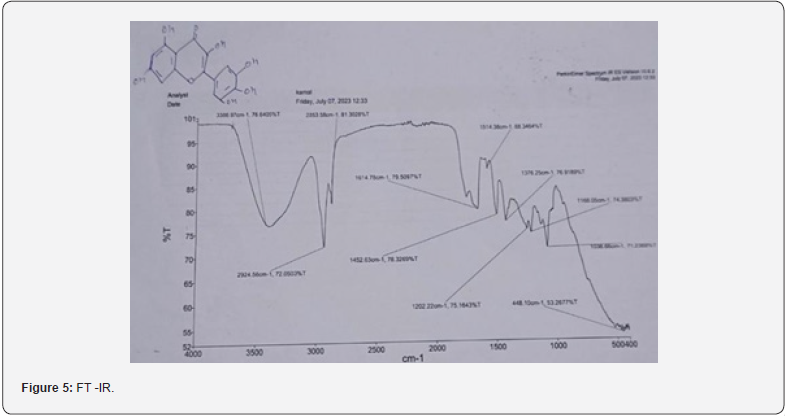
Baddi, Himachal Pradesh, for IR or Infrared Rays Spectroscopy and For H1NMR or Nuclear Magnetic Resonance Spectroscopy the sample was sent to Sophisticated Analytical Instrumentation Facility, CIL and UCIM, Punjab University, Chandigarh. FT-IR (Figure 4) was done on 07/07/2023 and H1NMR (Figure 5) was done on 24/07/2023.
Interpretation of spectra
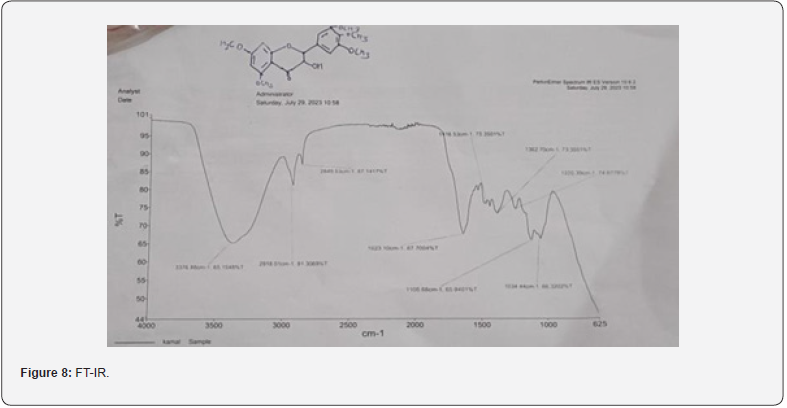
FT-IR is below, the first broad band at 3386.97cm-1 is of hydroxyl group, then the second band at 1614.78 cm-1 is for carbonyl group and the third band at 1514 cm-1 is for aromatic groups present in the moiety. H1NMR, the first peak at 0ppm is of CDCL3, second peak at 6.162ppm is as OH, third peak at 6.356ppm is as OH a bit downfield due to its position in the moiety, fourth peak and the biggest peak at 7.204ppm is as of Aromatic ring. *There were many unidentified peaks and bands in both spectra can be due to impurities [7].
Methylation of myricetin
After the isolation and identification of myricetin, the next step involved methylation. 2.5 grams of myricetin were dissolved in dry acetone (150 c.c./ml). To this solution, 10 grams of anhydrous potassium carbonate and approximately 25 grams of methyl iodide were added. The mixture was refluxed on a water-bath for 60 hours, replenishing the methyl iodide daily to compensate for evaporation loss. After the allotted time, the excess methyl iodide and the solvent were distilled off. Water was added to the residue to dissolve the potassium salts, causing the methyl ether to separate out. This mixture was filtered, washed with a small amount of water, and then recrystallized from dilute alcohol to obtain the final product. Once the reaction was over it was filtered using suction filtration, and the yield was calculated.
% yield (g/100g of the myricetin) = (W1*100)/W2
The weight of filtrate W1 = 1.2gm & Weight of isolated Myricetin W2 = 2.5gm So, 1.2*100 / 2.5 = 48%
%Yield = 48%
Spectral analysis of methylated myricetin
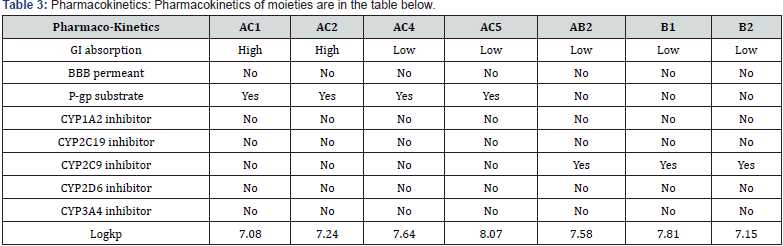
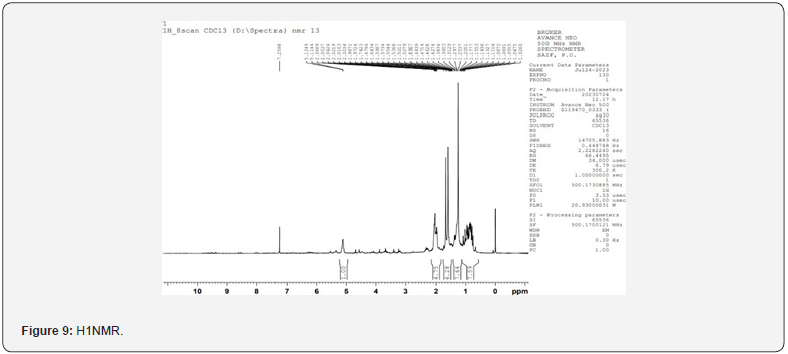
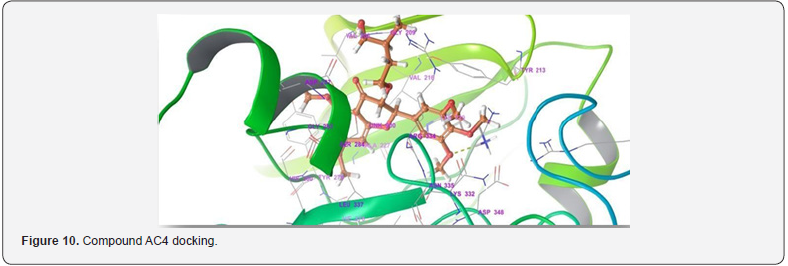
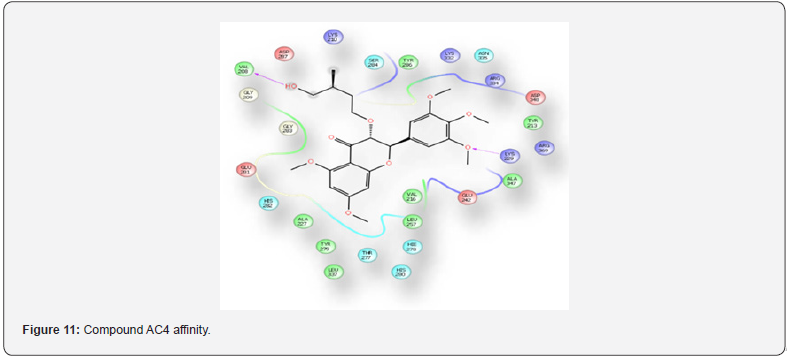
For the Spectral analysis of the sample was sent to OXIGEN ANALYTICAL LABORATORIES, Baddi, Himachal Pradesh, for FTIR or Infrared Rays Spectroscopy and For H1NMR or Nuclear Magnetic Resonance Spectroscopy the sample was sent to Sophisticated Analytical Instrumentation Facility, CIL and UCIM, PU, PUNJAB (Figure 6). FT-IR was done on 29/07/2023. The first band seen on 3376.88cm-1 is for hydroxyl group and the second band at 1623.10cm-1 is for carbonyl group and the third band at 1416cm-1 is sense to be as aromatic groups and the last band at 1362.70cm-1 is for OCH3. H1NMR was done on 29/07/2023 on brukner avance neo 500Mhz with solvent used CDCL3 , the first peakon 0ppm is for CDCL3, the second multiple peaks at 1-2ppm is for R-CH3, the third peak at 3.8ppm is for OCH3 , fourth peak at 5.2ppm is for OH which is a bit up field due to its position in the moiety, the final singlet peak at 7.2ppm is for aromatic ring (Figures 7-9) & (Table 2-4). As shown on the table above compound AC4 shows the best affinity in Drug likeness, ADME screening and Docking among all the moieties, the docking images of compound AC4 has shown figure 10. As seen above the AC4 compound has the highest docking score and good Drug likeness and ADMET score as well so our targeted compound for the theoretical synthesis [8].
Synthetic route of the target compound AC4
To prepare intermediate 1, as previously described, an each. The combined extracts were concentrated, diluted with 20 mL ethanol, stirred, and refluxed for 1 hour. 3mL of concentrated hydrochloric acid were added and refluxed for 2 hours, forming solid precipitate. After cooling, the mixture was filtered, and the product dried at 40°C for 2 hours. Subsequently, (CH2CH2) n and DMF were added and refluxed for 6 hours to obtain intermediate 2. (Figure 11) mixture of myricitrin (2.5g), CH3I (25g), and K2CO3 (10g) was dissolved in dry acetone (150 mL) and stirred at the temperature of 40°C for 60 hours until the reaction was complete, verified by TLC analysis. The filtered mixtures were dissolved in 50 mL water and extracted thrice with 30 mL dichloromethane. Once it reached room temperature, approximately 200 mL H2O was added, and the pH was adjusted to 4-5 using 10% HCl. The mixture was filtered, washed with water, and finally, compound 4 was obtained by re- crystallization from methanol.

For intermediate 3, a mixture of intermediate 3 (0.31g) and anhydrous K2CO3 (0.41g) in DMF (30mL) was stirred at 85°C for 1 hour. Then, DMF (20mL) containing intermediate 2 (0.50g) was slowly added to the mixture and reacted at 105°C for 6 hours [9,10].
- Research Article
- Abstract
- Introduction
- Material and Method
- Plant Extraction
- Isolation of Myricetin from the Extract
- Determination of Plant Extract Yield
- Preparation of Moieties
- Molecular Docking
- ADMET (Absorption, Distribution, Metabolism, Excretion and Toxicology)
- Result and Discussion
- Conclusion
- References
Conclusion
Myrica esculenta was collected and myricetin was isolated from its leaves, and some hypothetical compounds based on myricetin moiety was prepared and Insilco studies was done on it, as all the hypothetical compounds went through in silico studies AC4 or Anti- Cancer 4 showed a good response towards the development of a new drug as it shows good drug likeness and molecular docking score. The synthesis of AC4 is divided into steps i.e., discussed previously, for the initial step myricetin was isolated using column chromatography and TLC and identified using spectral analysis i.e., 1HNMR and FT-IR, then methylation of myricetin was performed mainly using Potassium Permanganate (K2CO3) and Methyl iodide (CH3I) in which K2CO3 was used as a catalyst and methyl iodide was used for methylation, reason behind use of iodine is because of its good group leaving tendency. And the reaction methylated Myricetin was identified by using spectral analysis. So, in conclusion a new drug discovery was targeted and total in silico studies were performed and the best compound among all others was also theoretically synthesized.

- Research Article
- Abstract
- Introduction
- Material and Method
- Plant Extraction
- Isolation of Myricetin from the Extract
- Determination of Plant Extract Yield
- Preparation of Moieties
- Molecular Docking
- ADMET (Absorption, Distribution, Metabolism, Excretion and Toxicology)
- Result and Discussion
- Conclusion
- References
References
- Nicolaou CA, Brown N (2013) Multi-objective optimization methods in drug design. Drug Discov Today Technol 10(3): 427-435.
- Jalhan S, Jindal A, Gupta A, Hemraj (2012) Synthesis, biological activities and chemistry of thiadiazole derivatives and schiff bases. Asian J Pharm Clin Res 5(3): 199-208.
- Zhou SF, Zhong WZ (2017) Drug Design and Discovery: Principles and Applications 22 (2): 279.
- Vesna EH, Radan S (2017) Discovery and Development of Novel Drugs. Prog Mol Subcell Biol 55: 91-104.
- Sliwoski G, Kothiwale S, Meiler J, Lowe EW (2013) Computational Methods in Drug Discovery. Pharmacol Rev 66(1): 334-395.
- Kalyaanamoorthy S, Chen YP (2011) Structure-based drug design to augment hit discovery. Drug Discov Today 16: 831-839.
- Jorgensen WL (2010) Drug discovery: Pulled from a protein’s embrace. Nature 466(7302): 42-43.
- Leelananda SP, Lindert S (2016) Computational methods in drug discovery. Beilstein J Org Chem 12: 2694-2718.
- Morris GM, Lim-Wilby M (2008) Molecular Docking. In: Kukol A (Eds.), Molecular Modeling of Proteins. Methods Mol Biol 443: 365-382.
- Daina A, Michielin O, Zoete V (2017) Swiss ADME: a free web tool to evaluate pharmacokinetics, drug- likeness and medicinal chemistry friendliness of small molecules. Sci Rep 7: 42717.






























