Taguchi Design Study and Formulation, Characterization of Eplerenone Lipid-Based Solid Dispersions Integrated with Gelucire
Mallikarjun Vasam1*, Praneeth Rao Kakullamarri2, Chandrashekar Talluri3, Shanmugarathinam Alagarsamy4 and Balaji Maddiboyina5
1Chaitanya (Deemed to be University)-Pharmacy, India
2Lincoln University college, Malaysia
3Assam Downtown University, India
4Department of Pharmaceutical Technology, Bharathidasan Institute of Technology, Anna University, India
5Tata Consultancy Services Limited, India
Submission: December 8, 2023; Published: December 19, 2023
*Corresponding author: Mallikarjun Vasam, Chaitanya Deemed to be University-Pharmacy, Email: mallikarjunvasam@gmail.com
How to cite this article: Mallikarjun V, Praneeth Rao K, Chandrashekar T, Shanmugarathinam A, Balaji M. Taguchi Design Study and Formulation, Characterization of Eplerenone Lipid-Based Solid Dispersions Integrated with Gelucire. Glob J Pharmaceu Sci. 2023; 11(2): 555808. DOI: 10.19080/GJPPS.2023.11.555808.
Abstract
Eplerenone is a potassium-sparing diuretic of aldosterone antagonist employed to treat chronic heart failure and high blood pressure. Eplerenone is classified as a BSC class II drug since it has poor oral absorption and is difficult to absorb in the body. The solid dispersions approach is the most practical and least expensive method to increase the solubility and dissolution rate of poorly water-soluble eplerenone. Formulating lipid-based solid dispersions employing a mix of hydrophilic lipid carriers has been shown to be quite common. Gelucire 50/13 and Gelucire 44/14 were both in there. These compositions included a range of different concentrations and ratios, including 1:1, 1:3, and 1:5, and their phase solubility properties were tested. A comparison was made between the in vitro dissolving results of our product and those of the market using a 7.4 pH phosphate buffer. Results: FTIR, DSC, and XRD characterised the lipid-based solid dispersions. The crystalline structure of eplerenone was ameliorated. The commercial medicine was 85% dissolved after 2 hours, while F3 dissolved at 99% in the same timeframe. It was discovered that 4 hours after starting the ex vivo permeation study, the permeability of eplerenone was 51%, the penetration of the commercial medication was 76%, and the permeation of formulation F3 was 81%. Taguchi design explored significant factors that contributed responses. Conclusions: Eplerenone is better absorbed and transported with the help of formulation F3. To investigate permeability further, Ex-vivo tests were undertaken with impressive results compared to what was available on the market (Abstract 1).
Keywords: Solid dispersion; Eplerenone; Gelucire; Hydrophilic lipid carriers; Solid dispersion
Background
Oral medicine administration is still preferred by many people. But many new drugs are only moderately soluble Racca Cauda [1]. This drug is administered orally in tablets or capsules, and because of its potency, it influences all factors that affect its solubility, dissolution rate, absorption, and bioavailability Singh et al. [2]. Drugs with limited water solubility and bioavailability have become a problem because of these challenges Makvandi et al. [3]. Before it can be absorbed by the body, an oral active ingredient must first dissolve in the stomach Delfi et al. [4]. The drug may then permeate the intestinal membranes and gain circulatory access throughout the body Maddiboyina et al. [5]. Thus, poor water solubility leads to limited absorption of the drug. Different carriers have been used to form solid dispersions that have various compositions. Drugs like these, which were also divided into crystalline or amorphous forms, showed higher bioavailability. Multiple methods make solid dispersions more soluble, dissolving faster, and bioavailable dos Santos et al. [6]. Mechanisms in better drug dissolution are drug aggregation inhibition, particle size reduction, surface wetting, the use of carriers, and the modification of drugs so they can adapt to the metastable amorphous state Sood et al. [7].
Improved dissolution rates with medication with low aqueous solubility are achieved using phospholipids but in a much lower carrier concentration Vasconcelos et al. [8]. In water, phospholipids spread and organize into round bilayer structures (liposomes) that bind and surround solutes Singh et al. [9]. Entrapped drugs are more easily transported in a bilayer system, which leads to faster and more noticeable absorption. This may be used for releasing medication under control Maddiboyina et al. [10]. Eplerenone is a drug used to treat high blood pressure and heart failure, both of which have various factors contributing to their causes. Eplerenone binds to the mineralocorticoid receptor, prevents aldosterone binding and thus prevents sodium reabsorption and other mechanisms of harmful aldosterone mediation Yadav Maddina et al. [11]. Gelucire is a mixture of triglycerides produced by combining mono-, di-, and triglycerides using PEG esters of fatty acids. They are helpful in oral systems in various ways, such as increased solubility and bioavailability, sustained drug release, masking taste, and keeping active pharmaceutical ingredients (APIs) safe from oxygen, light, and humidity Bertoni et al. [12]. Gelucire, a combination of glycerides and PEG esters, is the primary constituent of sustained-release formulations. Gelucire 50/13 and 44/14 are categorised as hydrophilic grades. Gelucire 50/13 and 44/14 serve identical purposes in serving as controlled release agents and bioavailability extenders. Because of its excellent melting point, its restraints the rate of release of API. In addition, it remains utilized to extend the solubility of poorly soluble medications Arregui et al. [13]. Gelucire 50/13 and 44/14 are emulsifiers with 11 HLB values and are also used as lipids in drug delivery systems Patel et al. [14]. Lipid-based solid dispersions, which are more effective than several other approaches to increase poorly soluble drugs bioavailability, are also helpful (LBSD). It is also said that LBSD has been rendered by utilizing lipid-based hydrophilic carriers (making drugs more soluble and easier to administer) in cases where class II drugs were applied Singh Pai [15].
Methods
aterials
Eplerenone was acquired from Aurobindo pharmaceuticals company Hyderabad, India. Gelucire 50/13, Gelucire 44/14 was obtained from Bayer Chemicals, Pune, Maharashtra. Aerosil 380 was obtained from S.D Fine-chem, Pvt. Ltd., Mumbai, India.
Methods
Preparation of standard calibration curves
Calibration curve of eplerenone at 0.1NHCl: Precisely weighed quantity of 10 mg of eplerenone been obtained in a volumetric flask (10ml) and dissolved in 10 ml 0.1N HCl to acquire a concentration of 1000 μg/ml. From this stock solution, 1ml solution was obtained in 10ml of a volumetric flask, and the volume was made up to mark by 0.1N HCl. From this stock solution, 2, 4, 6, 8 and 10 ml remain obtained in 10 ml volumetric flasks, and absorbance values remain indicated at 246 nm Khames [16].
Calibration curve of eplerenone at 7.4 pH phosphate buffer: Precisely weighed extent of 10 mg of eplerenone been obtained in a volumetric flask (10ml) and dissolved in 10 ml 7.4 pH phosphate buffer to get a concentration of 1000 μg/ml. From this stock solution, 1ml solution was obtained in 10ml of a volumetric flask, and the volume was done up to mark by 7.4 pH phosphate buffer. From this stock solution, 2, 4, 6, 8 and 10 ml remain acquired in 10 ml volumetric flasks, and absorbance values were noted at 246 nm.
Saturation solubility studies
This experiment was performed in three different solvents: distilled water, phosphate buffer, and a 0.1 N HCl, with a pH of 6.8, 7.4 respectively. We added an excessive amount of the drug in all three solvents, which were set at 37±0.5°C and continuously agitated for 48 hours Garg et al. [17]. Finally, the obtained cleared solutions of different samples were analyzed at 246 nm using UVspectroscopy.
Phase solubility studies
Solubility examination of gelucire 44/14 & 50/13, phase 3: Gelucire 50/13 and 44/14, with 2, 4, 6, 8, and % concentrations of each drug in distilled water, was developed in a 10 ml container (such as 2, 4, 6, 8, and 10 μg/ml) with the drug present in more concentration dos Santos et al. [6]. The solutions are shaken for 48 hours, filtered, and then UV-spectroscopy is used to estimate the drug concentration at 246 nm.
Drug and excipient compatibility studies
Fourier transform infrared spectroscopy (FT-IR): The KBr pellet practice was used to obtain spectra of the eplerenone- Gelucire 44/14 & 50/13 interaction over the range of 4000-400 cm-1 to learn more about how the interaction occurs Maddiboyina et al. [18].
Differential scanning calorimetry (DSC): The samples have been put through thermal stress to test their reactions. Samples of eplerenone and carrier are kept in a standard pan, and it is heated to 10°C/min up to 300°C with a flow rate of 80 ml/min of dry nitrogen gas used as a carrier Ayyanaar et al. [19].
X-Ray diffraction (X-RD): Analysis is ongoing of the delivery system using Gelucire 50/13, 44/14 and Eplerenone, where samples have been checked through X-RD and diffraction pattern assessment with a step size of 0.017○ and a dwell time of 45 s respectively at 3 and 50, 2θ, at ambient temperature Barlow et al. [20].
Preparation of LBSD
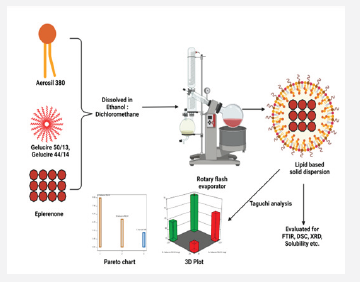
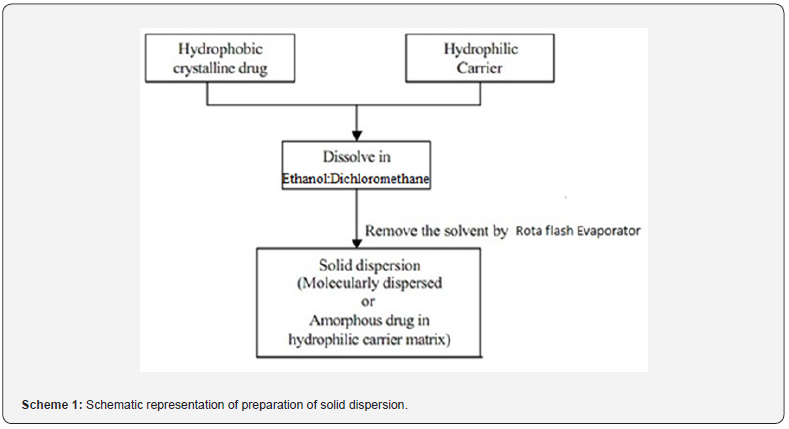
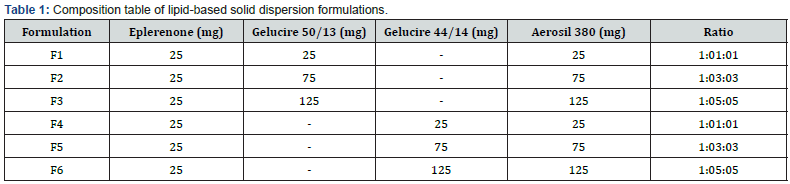
The manufacture of LBSD (with eplerenone) followed the melting of lipid-based hydrophilic carriers on a hot plate (gelucire 44/14 and 50/13), with differing viscosity grades. Table 1 reveals how ratios of 1:1, 1:3, and 1:5 is used. In phase solubility studies, the percentages of carriers are maximized to 6%, boosting the solubility of eplerenone Tran et al. [21]. Aerosil 380 was utilized as inert support in all of the formulas. With Eplerenone (25mg) dissolved in methanol (2ml), along with a lipid-based carrier and aerosil 380, this solution was stored in a dark, dry environment. For one hour, the blend was left to evaporate using a rotary flash evaporator in a round bottom flask with a 60-rpm speed and a 40°C temperature. Once it had dried, the desiccator-collected, solid dispersion was recovered Jhawat et al. [22] (Table 1 & Scheme 1).
Evaluation of LBSD
Micromeritic evaluation
i. Bulk density (BD): A metering cylinder is used to store the sample, and its weight is recorded immediately. The bulk volume is still referred to as the initial volume. The mass-tovolume ratio of the powder is based on this measurement Balaji M et al. [23]. BD(ρ0) = M/Vb Where M=mass of sample taken, Vb=bulk volume of powder.
ii. Tapped density (TD): Tapping the drug sample 50 times in a graduated cylinder using a TD tester at a constant rate filled the cylinder to 50ml Balaji M et al. [23]. TD (ρt) = M/Vf Where M=weight of sample powder taken, Vf = tapped volume.
iii. Carr’s index: This investigation remains necessary to gauge the powder’s BD and TD and the rate at which a crowded location is reached. Carr’s Index (%) = [(TD-BD) x100]/TD.
iv. Hausner’s ratio: Interrelations that contain granular substance and flowability concerning a powder’s value and classifications are classed in table 2. Hausner’s Ratio = TD/BD.
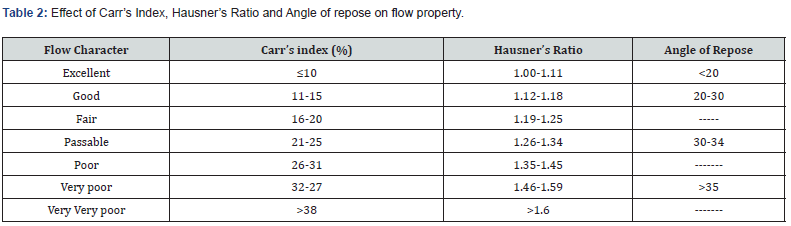
v. Angle of repose: This was completed to determine if any additional ingredients are being added. We know this sample was poured through the funnel and formed a pile of sorts from a side view, as well as the definition of “heap” in this situation Siddam et al. [24]. θ=tan⁻¹(h/r) Where h remains the height of the pile, r remains the radius of the pile.
Drug content
For an hour, the flask was shaken on a rotary shaker with 50ml of methanol and solid eplerenone dispersions. Once the drug’s concentration has been confirmed, the medicine is filtered, diluted, and re-evaluated Maddiboyina et al. [25].
Solid-state characterisation
i. FT-IR Studies: The KBr pellet method was utilized to record the spectrum at a frequency range of 4000–400 cm−1, which would enable the project team to understand better the impact on the preparation’s drug release capabilities.
ii. DSC Studies: Samples of solid dispersions are situated in a pan, and the heating process is a 10°C /min raise to 300°C. They are supported by dry nitrogen gas, used as a carrier at an 80 ml/min flow rate. An aluminium pan used as a reference dish was empty.
iii. X-RD studies: The crystalline/amorphous nature of solid dispersion was analyzed through X-RD. The diffraction pattern remains assessed by a step size of 0.017○ and a dwell time of 45 s at respective step amid 350,2θ at ambient temperature. In addition, the characteristic peaks of the drug obtained in the X-RD pattern of lyophilized solid dispersion were related by the X-RD of the eplerenone to assess the crystalline/amorphous nature.
In-vitro dissolution studies
Dissolution experiments with type II dissolution apparatus with a 50-rpm paddle speed and a 900 ml dissolution media with a pH of 7.4 and 37±0.5°C have been conducted. At 15, 30, 45, 60, 90, and 120 minutes, a 5-millilitre sample was taken, and the same amount of buffer was added. Next, UV-spectroscopy is applied to the samples drawn Kotla et al. [26].
Ex-vivo permeation studies
The intestinal content and mucus are cleaned out using Krebs-Ringer solution, which washes out everything besides the intestine Maddiboyina et al. [27]. Then, one end of the intestine was tied to the open tube. Then, the drug eplerenone (25mg) was then introduced into the organ bath and warmed to 37ºC using a heated phosphate buffer solution (pH 7.4). Next, a separate receptor compartment was established where 50ml of this solution was kept aerated to maintain the appropriate temperature Londhe & Shirsat [28]. Samples are still being taken and analyzed at fixed intervals, and the drug concentration has been found via UV-spectroscopy, set at a wavelength of 246 nm. The cumulative amount of drug permeated during the study was determined and plotted against the time to calculate the apparent permeability (Papp, cm/s) using the following equation. P (app)=F/ (AC_0) Where F is the flux (F, μg/min) of the drug, A is the surface area of the intestinal sac assumed to be the area of a cylinder (2πrL), C0 is the (μg/ mL) is the initial drug concentration.
Orthognal array taguchi design
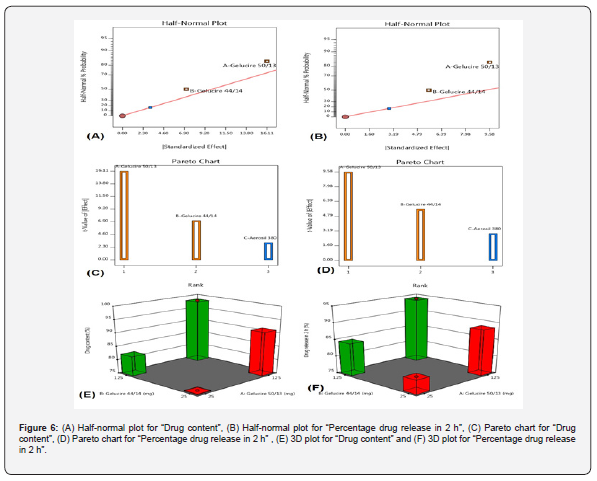
The screening of the prepared Eplerenone lipid based solid dispersion was done by using taguchi design. The main objective of the Taguchi design is to find out the effect of the excipients which are used in the solid dispersion and how it is affecting the effect on the selected responses. The taguchi design was selected in which Gelucire 50/13, Gelucire 44/14, and Aerosil 380 was selected as independent variables and a total four number of runs finalized (Not included in text). The procedure was carried out by statistical software (Design expert V 11.0). The response was monitored such as Drug content and percentage drug release in 2 h. The obtained data were subjected to analysis with the help of statistical modeling; the process order was design model, and model type considered factorial. During the study finding the factors signifying the response was identified by the halfnormal plot, pareto chart and 3D plot in bar diagram showing the responses in changes to variables.
Results and Discussion
Preparation of standard calibration curve
Calibration curve of eplerenone at 0.1 N HCl: Calibration curve of eplerenone has been determined by plotting absorbance V/s concentration at 246 nm, and it follows Beer’s law. The results show that the R2 value is 0.998, and the slope is 0.0452, respectively.
Preparation of calibration curve of eplerenone at7.4 pH phosphate buffer: Calibration curve of eplerenone has been determined by plotting absorbance V/s concentration at 246 nm, and it follows Beer’s law. The results showed the R2 value is 0.9959, and the slope is 0.0452, respectively.
Saturation solubility studies of eplerenone
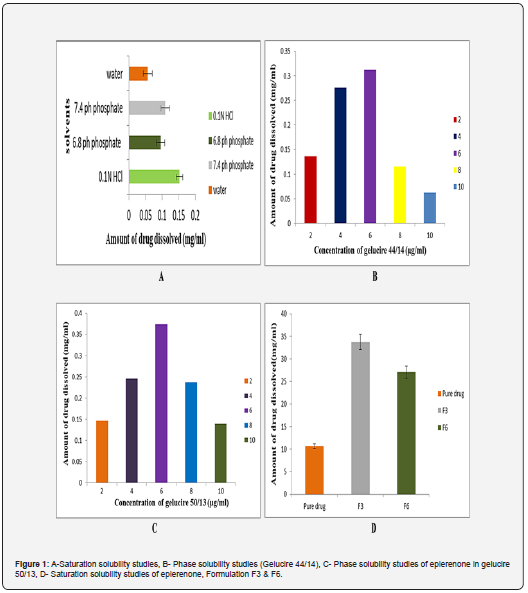
The studies have been performed in multiple solvents to know the solubility of the eplerenone. It is implied that drug solubility was good in the 7.4 pH phosphate buffer, as shown in Figure 1a. This indicates In-Vitro dissolution studies can be performed in 7.4pHphosphate buffer.
Phase solubility study of eplerenone in different concentrations of gelucire 44/14, gelucire 50/13
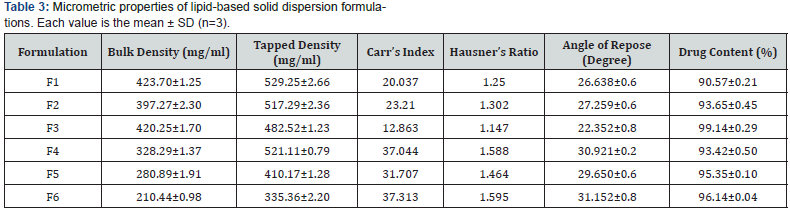
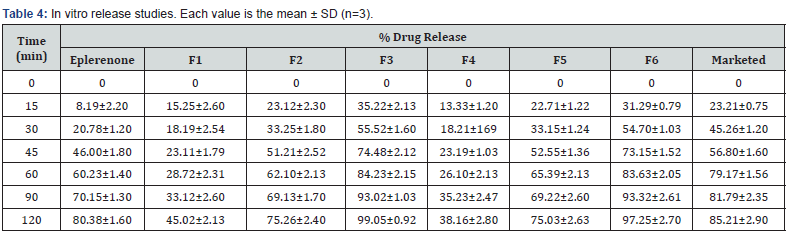
Eplerenone’s initial solubility was shown to be up to 6 μg/ ml of gelucire 44/14 and gelucire 50/13 and then declined are revealed in figure 1b & 1c. The ratios resulting from the studies were 1:1, 1:3, and 1:5 (Figure 1).
Drug and excipient compatibility studies
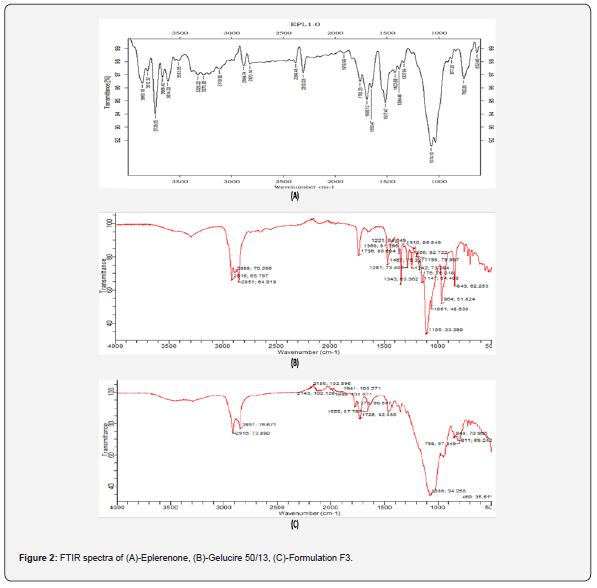
i. FT-IR Studies: The expected peaks of eplerenone and carrier (gelucire 50/13 & 44/13) are present, and nothing has been detected that shouldn’t be there. Eplerenone and gelucire 44/14 & 50/13 have no significant interaction. IR Spectra of pure eplerenone is displayed in figure 2a, and IR Spectra of gelucire 50/13 is shown in figure 2b.
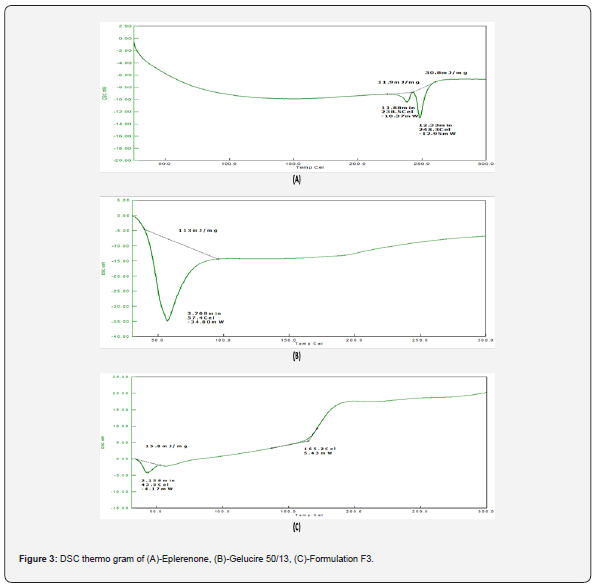
ii. DSC Studies: Thermogram of eplerenone demonstrated a sharp endothermic peak at 248.30C, as depicted in figure 3. The DSC thermogram of gelucire 50/13 showed 57.40C, as illustrated in figure 3b.
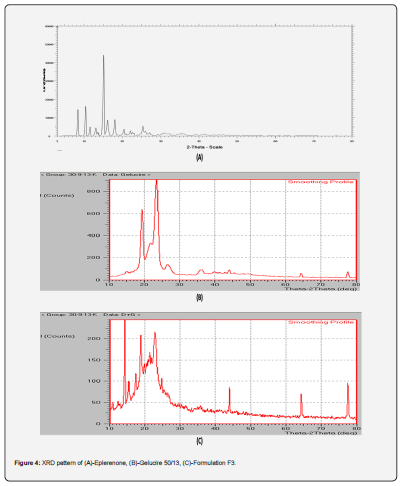
iii. X-RD Studies: The XRD pattern eplerenone figure 4a showed sharp intensity peaks at 10.550, 150(2θ). Sharp, intense peaks may remain owing to the crystalline form of the eplerenone. The diffraction pattern of gelucire 50/13 revealed intensity peaks at 19o and 23.5o, as displayed in figure 4b.
Preparation of LBSD
The LBSD of eplerenone, which is created by melting the diverse lipid-based hydrophilic carriers gelucire 44/14 and 50/13 of different viscosity grades, possesses numerous unique properties that provide excellent formulation flexibility and used in different ratios1:1, 1:3, 1:5 (Table 1) in glass scintillation vials.
Evaluation of LBSD
Micromeritic evaluation
i. Bulk density: Table 3 reveals that all test samples had BD values ranging from 210.44 to 423.70.
ii. Tapped density: As shown in table 3, the values of TD for all the preparations total 335.36 - 632.52.
iii. Carr’s Index: As table 3 shows, the values were constituted from 2.777 to 37.313. The test gave a carr’s index of 12.863 for Formulation F3, indicating good flow properties.
iv. Hausner’s Ratio: table 3 shows the values that remain unchanged. Among the various designs, F3 possesses good flow properties, meaning it flows as well as 1.147.
v. Angle of repose: Values for each preparation ranged from 31.152 to 22.352. A good result was obtained in the table with formulation F3 (22.352), as shown in Table 3. Micromeritic properties of LBSD were created using different carriers for eplerenone formulations; formulation F3 showed excellent flow characteristics, as seen in table 3.
Drug content
It was conducted for all LBSD formulations that were specified previously. Table 3 shows that the values discovered ranged from 90.57 to 99.14, and formulation F3 had the highest value found.
Solid-state characterisation
i. FT-IR Studies: The specific peaks of eplerenone and carrier (gelucire 50/13) are evident in the lipid-based solid dispersion formulation that was chosen, and no additional peaks were identified. Figure 2c shows IR Spectra of formulation F3, indicating no interaction/incompatibility between the drug and carrier.
ii. DSC studies: F3 had a broad endothermic peak at 43.50C, a high peak at 57.40C, and an even higher peak at 165.20C shown in figure 3c, which all were because of gelucire 50/13 and drug eplerenone. In LBSD, DSC conducted a sharp endothermic peak transfer and reduced melting points compared to eplerenone.
iii. X-RD studies: LBSD’s selected formulation (F3) displayed an XRD pattern with reduced intensities and number of peaks, demonstrating that eplerenone was no longer crystalline, as shown in figure 4c. F3 lipid-based solid dispersions (an amorphous form) also displayed a significant drop in their peaks.
In-vitro release studies
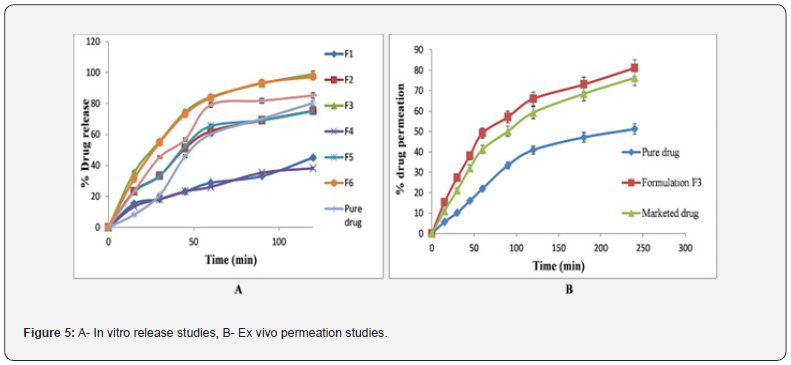
Release studies of the designed LBSD preparation of eplerenone took place in a pH 7.4 phosphate buffer for 2 hours and were documented in table 4, as shown in figure 5a. Research has discovered that the gelucire ratios directly correlate with the drug dissolution rate. The results showed that the formulation from gelucire 50/13 had better drug release than that from gelucire 44/14. It was also discovered that the composition F3 (which is 1:5 with gelucire 50/13) is a superb formulation because 99.05% of the drug was released in only 2 hours. When comparing eplerenone, the market release and the best formulation, the release of the drug found that the ideal formulation led to an 80.38% release, the market release led to an 85.2% release, and the best formulation was released at 99.05%.
Saturation solubility studies comparison
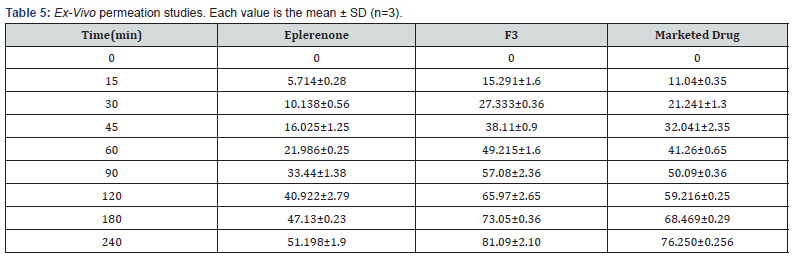
Eplerenone studies, F3 and F6 formulations, and water distilled to be pure were all used in these experiments. Figure 1d illustrates that F3 exhibited better solubility than the eplerenone and F6 formulation.
Ex-Vivo permeation studies
The ex-vivo permeability study was conducted over 4 hours, during which % of the drug permeated the ileum. Table 5 & Figure 5b show that eplerenone has a value of 51.19, the marketed product has a value of 76.25, and the formulation F3 has a value of 81.09. The permeation transport of eplerenone was also seen to be improved via the LBSD formulation. The screening of influential factors as effects were assessed by Taguchi design with randomized subtype considering a total of four numbers of runs was done. The model fitting for the data obtained by the formulations suggested by Taguchi design is validation by factorial type, indicates good fitness for main effect determination. Linear polynomial equations are constructed, and the synergistic and antagonistic factors are evaluated. The model F-value indicates the model is significant and the P-values less than 0.05 indicate model terms are significant. The significant main effects were also identified by the half-normal plot (Figure 6a & 6b). For the responses, “Drug content” is significantly affected by “A”, “B” (Gelucire 50/13, Gelucire 44/14). In case of “Percentage drug release in 2 h again both “A”, “B” appeared to be significant as seen in Figure 6. The data was furthermore ascertained by Pareto chart and exhibited by t-value. The Pareto-chart indicated in both responses, Factor A and B have positive effect than Factor C (Aerosil 380). Similarly, 3D plots were constructed to show the responses with change in variable changes. Figure 6(e) highlighted higher value of drug content for the formulation had maximum of Gelucire 50/13, Gelucire 44/14 (125 mg both) and least value as the amount reduced to 25 mg for both Gelucire. A similar pattern was also observed in (Percentage drug release in 2 h) as seen in figure 6f.
Conclusion
Gelucire 50/13 is used to help develop the lipid-based solid dispersions of eplerenone. The solubility parameters of eplerenone in a lipid-based solid dispersion formulation revealed a rise in solubility. It was found that the dissolution rate of eplerenone remained higher in the lipid-based solid dispersion formulation in dissolution studies. Analysis using FTIR revealed that eplerenone and gelucire 50/13 don’t interact. Eplerenone, a component of lipid-based solid dispersions, had a melting point drop and showed that sharp endothermic peaks had transformed into a broad shape. It was discovered that the lipid-based solid dispersion is amorphous by looking at the XRD pattern, as a reduction in the number and intensity of peaks related to eplerenone was seen. Eplerenone’s lipid-based solid dispersion system was better permeability when tested in permeation studies carried out in the lab. Eplerenone’s solubility and dissolution rate improved after discovering that lipid-based solid dispersions were employed.
Declarations
i. Ethics approval and consent to participate: Not applicable.
ii. Consent for publication: Not applicable
iii. Availability of data and material: Not applicable
iv. Competing interests: The authors have no relevant financial or non-financial interests to disclose.
v. Funding: No Funding was obtained for this study
Acknowledgements
Not Applicable
Authors’ Contributions
MV: Conceptualization, Data curation, Supervision, Writing – review & editing
BM: Supervision, Writing – review & editing
CT: Data curation, Conceptualization
SA: Project administration, Validation, Writing – original draft
BG: Supervision, Validation, Conceptualization
HS: Supervision, Conceptualization
References
- Racca L, Cauda V (2020) Remotely Activated Nanoparticles for Anticancer Therapy. Nano-Micro Lett 13(1): 11.
- Singh S, Kotla NG, Tomar S, Balaji M, Thomas JW, et al. (2015) A nanomedicine-promising approach to provide an appropriate colon-targeted drug delivery system for 5-fluorouracil. Int J Nanomedicine 10: 7175-7182.
- Makvandi P, Ashrafizadeh M, Ghomi M, Masoud N, Hamid Heydari SH, et al (2021) Injectable hyaluronic acid-based antibacterial hydrogel adorned with biogenically synthesized AgNPs-decorated multi-walled carbon nanotubes. Prog Biomater 10(1): 77-89.
- Delfi M, Sartorius R, Ashrafizadeh M, Esmaeel S, Zhang Y, et al. (2021) Self-assembled peptide and protein nanostructures for anti-cancer therapy: Targeted delivery, stimuli-responsive devices, and immunotherapy. Nano Today 38: 101119.
- Maddiboyina B, Desu PK, Vasam M, Veerendra TC, Amareswarapu V Surendra, et al (2021a) An insight of nanogels as novel drug delivery system with potential hybrid nanogel applications. J Biomater Sci Polym Ed 33(2): 262-278.
- Dos Santos C, Buera MP, Mazzobre MF (2011) Phase solubility studies and stability of cholesterol/β-cyclodextrin inclusion complexes. J Sci Food Agric 91(14): 2551-2557.
- Sood S, Maddiboyina B, Rawat P, Ashish Kumar G, Ahmed IF, et al. (2021) Enhancing the solubility of nitazoxanide with solid dispersions technique: formulation, evaluation, and cytotoxicity study. J Biomater Sci Polym Ed 32(4): 477-487.
- Vasconcelos T, Sarmento B, Costa P (2007) Solid dispersions as strategy to improve oral bioavailability of poor water-soluble drugs. Drug Discov Today 12(23,24): 1068-10075.
- Singh S, Vardhan H, Kotla NG, Balaji M, Dinesh S, et al. (2016) The role of surfactants in the formulation of elastic liposomal gels containing a synthetic opioid analgesic. Int J Nanomedicine 11: 1475-1482.
- Maddiboyina B, Jhawat V, Desu PK, Sivaraman G, Ramya Krishna N, et al. (2021b) Formulation and Evaluation of Thermosensitive Flurbiprofen in Situ Nano Gel for the Ocular Delivery. J Biomater Sci Polym Ed 32(12): 1584-1597.
- Yadav Maddina B, Shilakari Asthana G, Asthana A (2016) Formulation and Development of Polysaccharide Based Mesalamine Nanoparticles 8(7): 676-684.
- Bertoni S, Albertini B, Passerini N (2020) Different BCS class II drug-gelucire solid dispersions prepared by spray congealing: Evaluation of solid-state properties and in vitro Pharmaceutics 12(6): 548.
- Arregui JR, Kovvasu SP, Kunamaneni P, Betageri GV (2019) Carvedilol solid dispersion for enhanced oral bioavailability using rat model. J Appl Pharm Sci 9(12).
- Patel N, Dalrymple DM, Serajuddin ATM (2012) Development of solid SEDDS, III: Application of Acconon® C-50 and Gelucire® 50/13 as both solidifying and emulsifying agents for medium chain triglycerides. J Excipients Food Chem 3(2).
- Singh G, Pai RS (2015) Trans-resveratrol self-nano-emulsifying drug delivery system (SNEDDS) with enhanced bioavailability potential: Optimization, pharmacokinetics and in situ single pass intestinal perfusion (SPIP) studies. Drug Deliv 22(4): 522-530.
- Khames A (2019) Formulation and characterization of eplerenone nano emulsion liquid solids, an oral delivery system with higher release rate and improved bioavailability. Pharmaceutics 11(1): 40.
- Garg AK, Maddiboyina B, Alqarni MHS, Aftab A, Hibab M Aldawsari, et al. (2021) Solubility enhancement, formulation development and antifungal activity of luliconazole niosomal gel-based system. J Biomater Sci Polym Ed 32(8): 1009-1023.
- Maddiboyina B, Jhawat V, Sivaraman G, Sunnapu O, Nakkala RK, et al. (2020b) Formulation Development and Characterization of controlled release core in cup matrix tablets of venlafaxine HCl. Curr Drug ther 15(5): 503-511.
- Ayyanaar S, Kesavan MP, Sivaraman G, et al (2019) A novel curcumin-loaded PLGA micromagnetic composite system for controlled and pH-responsive drug delivery. Colloids Surfaces a Physicochem Eng Asp 573: 188-195.
- Barlow J, Singh D, Bayer S, Curry R (2007) A systematic review of the benefits of home telecare for frail elderly people and those with long-term conditions. J Telemed Telecare 13: 172-179.
- Tran P, Pyo YC, Kim DH, Sang-Eun L, Jin Ki K, et al. (2019) Overview of the manufacturing methods of solid dispersion technology for improving the solubility of poorly water-soluble drugs and application to anticancer drugs. Pharmaceutics 11(3): 32.
- Jhawat V, Gulia M, Maddiboyina B, Rohit D, Gupta S (2020) Fate and Applications of Super porous Hydrogel Systems: A Review. Curr Nanomedicine 10(4): 326-341.
- Balaji M, Ramyakrishna N, Hanumanaik M (2020) Formulation Development and Characterization of Enteric Coated Tablets of Lansoprazole. der Pharm Lett 12: 22-38
- Siddam H, Kotla N, Maddiboyina B, Sima S, Omprakash S, et al (2016) Formulation and evaluation of atenolol floating bioadhesive system using optimized polymer blends. Int J Pharm Investig 6(2): 116-122.
- Maddiboyina B, Hanumanaik M, Nakkala RK, Vikas J, Rawat P, et al. (2020a) Formulation and evaluation of gastro-retentive floating bilayer tablet for the treatment of hypertension. Heliyon 6(11): e05459.
- Kotla NG, Singh S, Maddiboyina B, Omprakash S, Thomas J Webster, et al. (2016) A novel dissolution media for testing drug release from a nanostructured polysaccharide-based colon specific drug delivery system: An approach to alternative colon media. Int J Nanomedicine 11: 1089-1095.
- Maddiboyina B, Asthana A, Asthana GS, Sima S, Ramya M, et al. (2015) Formulation and characterization of polycarbophil coated mucoadhesive microspheres of repaglinide. J Pharm Sci Res 7(11): 972-977.
- Londhe V, Shirsat R (2018) Formulation and Characterization of Fast-Dissolving Sublingual Film of Iloperidone Using Box–Behnken Design for Enhancement of Oral Bioavailability. AAPS Pharm Sci Tech 19(3): 1392-1400.






























