Chiral Derivatization Method for the Separation of Enantiomers of LProlinamide by Reverse Phase HPLC
Krishna Kumar Yadav1*, Abhishek. Sharma1, A Mohit Kumar1, A Yuvaraj Zunjarrao1 and Sumit S Chourasiya2
1Analytical Research and Development, IOL Chemicals and Pharmaceuticals Ltd, Barnala – 148101, Punjab, India
2Department of Process Research and Development, IOL Chemicals and Pharmaceuticals Ltd, Barnala – 148101, Punjab, India
Submission:September 01, 2023;Published:September 12, 2023
*Corresponding author:Krishna Kumar Yadav, Analytical Research and Development, IOL Chemicals and Pharmaceuticals Ltd, Barnala – 148101,Punjab, India, Email: krishnakumar@iolcp.com
How to cite this article: Krishna Kumar Y, Abhishek S, Mohit K, Yuvraj Z, Srinivas M. Chiral Derivatization Method for the Separation of Enantiomers of L- Prolinamide by Reverse Phase HPLC. Glob J Pharmaceu Sci. 2023; 11(1): 555801. DOI: 10.19080/GJPPS.2023.11.555801.
Abstract
A highly accurate and sensitive method has been developed for assessing the enantiomeric purity of L-prolinamide hydrochloride using reverse-phase HPLC. The method involves utilizing Marfey’s reagent to derivatize DL-prolinamide, followed by pre-column derivatization into a diastereomer, and subsequent RP-HPLC analysis using Hypersil BDS C18 (4.6 x 250mm, 5.0m). The method allows for excellent separation of the two enantiomers, with a resolution of over 3 and a tailing factor of less than 1.5. Notably, the concentration range for D-prolinamide is 0.15 to 0.75%, with exceptional linearity. Moreover, the limit of detection and limit of quantitation for D-prolinamide are 0.075% and 0.15%, respectively. Overall, this method is highly specific and simple, providing a reliable approach for assessing enantiomeric purity in L-prolinamide hydrochloride.
Keywords: DL-Prolinamide hydrochloride; Enantiomeric purity; Reversed-phase HPLC; Diastereomer; Marfey’s reagent
Introduction
L-prolinamide hydrochloride plays a crucial role in various applications as an important intermediate [1,2]. Its derivatives are commonly used to treat hepatitis C virus infection [3-6], while L-prolinamide itself is utilized as a primary feedstock for creating Vildagliptin, a novel oral antidiabetic medication [7,8]. In scheme 1, the process of synthesizing Vildagliptin from L-prolinamide is illustrated [9-11]. L-prolinamide (3) reacts with chloroacetyl chloride (5) to form an intermediate amide, which is then hydrolyzed to produce 1-Chloroacetyl-2-cyanopyrrolidine (5). Finally, Vildagliptin (VLDA) is generated by the reaction of 1-Chloroacetyl-2-cyanopyrrolidine (5) and aminoadmantanol. This synthesis pathway highlights the significance of L-prolinamide as the fundamental starting material for Vildagliptin production.
L-prolinamide hydrochloride is an easily obtainable raw material with a purity range of 95-98% that can be sourced from various suppliers. L-prolinamide can also be synthesized from L-Proline by reaction with methanolic ammonia [12,13]. However, it is worth noting that this substance can sometimes be contaminated with an undesired isomer, which can pose a problem when it comes to using L-Prolinamide as a starting material for synthesizing Vildagliptin. This is because there is a risk of creating the undesired isomer of Vildagliptin during the synthesis process, which is not ideal.
To address this issue, the Vildagliptin monograph specifies a limit of NMT 0.5% for the undesired isomer. [14] Therefore, it is crucial to control the levels of D-prolinamide, which is the undesired isomer, in L-prolinamide hydrochloride during the synthesis of Vildagliptin to mitigate this risk. This control can be achieved through an analytical method that demonstrates the levels of D-prolinamide present, ensuring that they are kept within the specified limit.
It is essential to exercise caution when using L-prolinamide hydrochloride as a starting material for synthesizing Vildagliptin, given its potential for contamination with the undesired isomer. However, by taking the necessary precautions and using an analytical method to monitor levels of D-prolinamide, it is possible to avoid creating the undesired isomer of Vildagliptin and ensure that the final product meets the required specifications.

We conducted an extensive literature review and found only one patent report that outlines a specific technique for determining D-prolinamide and L-prolinamide through derivatization using FMOC-Cl [15]. The resulting derivatives, or Diastereomers, are then analyzed using normal phase chromatography employing a Diacel chiralpak IC Chiral column. However, due to poor UV absorption, conventional HPLC methods with UV detectors posed a challenge in detecting the content of L-Prolinamide and D-Prolinamide. To overcome this obstacle, we developed an innovative and costeffective RP-HPLC method for separating the D-prolinamide isomer in L-prolinamide hydrochloride through derivatization (Scheme 2). In this article, we describe the development and validation of this analytical method, which is highly sensitive and accurate in detecting and quantifying trace levels of D-prolinamide in L-prolinamide hydrochloride. It’s worth noting that the identification of D-prolinamide isomer in L-prolinamide hydrochloride through reverse-phase chromatography has yet to be reported.

Materials and Methods
L-prolinamide hydrochloride standard was prepared inhouse with 97.5% purity. D-prolinamide standard was procured from R Scientific with 99.24% purity. Marfey’s Reagent [16] was purchased from Zeta Scientific Analytical grade sodium hydroxide, HPLC grade acetonitrile, and Sodium-1-octansulphonate were purchased from Merck Specialties Pvt. Ltd, India.
Instrumentation
The analysis is performed by using the Waters HPLC system (software: Empower, e2695 separation module, UV and PDA detector). Mettler Toledo electronic balance (XS225 dual range) was used for weighing purposes. Ultra Sonicator (Shivani Scientific Pvt Ltd., Mumbai) was used to prepare and degassing samples and mobile phase. The chromatographic column used was a Hypersil BDS C18 250 x4.6 mm with 5μm particles.
Derivatization procedure (Preparation of diastereomers)
Weighed 20mg of L-Prolinamide hydrochloride standard or sample into a 10mL volumetric flask, added 2mL Sodium bicarbonate solution (2.0mg/mL), and then diluted up to the mark with Sodium bicarbonate solution. Transfer 500μL of this solution, added 500μL derivatizing reagent solution (5mg/mL in Acetonitrile) in a vial, seal the vial properly, and heat at 65°C for one hour. Then cool the sample at room temperature and added 250μL 1M HCl solution, mixed well. Further 300μL of this solution and added 700μL of diluent (Water: ACN, 50:50 v/v) in vial. Mix well and inject. Completion of reaction was monitored by HPLC.
Preparation of solutions and chromatographic conditions
Mobile phase consists of a mixture of buffer and acetonitrile in the proportion of 78:22. Buffer solution was prepared by dissolving 3.0mL of Triethyl amine in 1000ml of water (pH of buffer 6.0 with o-phosphoric Acid). The flow rate of mobile phase was 0.7mL/min. The column oven temperature was maintained at 30°C and column eluent was monitored at 335 nm for 55 min. The injection volume was 10μL. Weighed and transferred 50mg of Marfey’s Reagent into 10 mL volumetric flask dissolved in 2mL acetonitrile diluted up to mark with acetonitrile.
System suitability solution preparation
Weighed and transferred 20mg of L-Prolinamide hydrochloride standard into 10 mL volumetric flask, added 2 mL Sodium bicarbonate solution (2.0mg/mL) and 1mL D-prolinamide stock solution (0.075mg/mL) then diluted up to the mark with sodium bicarbonate solution. Transfer 500μL of this solution, added 500μL derivatizing reagent solution (5mg/mL in Acetonitrile) in a vial, seal the vial properly and heat at 65°C for one hour then cool the sample at room temperature and added 250μL 1M HCl solution, mixed well. Further, 300μL of this solution and added 700μL of diluent in the vial. Mix well and inject.
System suitability evaluation
Performance of the method was determined by injecting system suitability solution of 2.0mg/mL of L-prolinamide and 0.0075mg/mL of D-prolinamide. Method performance criteria were resolution between two diastereomer peaks should be at least 3.0.
Method validation
To attain reliable and replicable data, performing method validation is of utmost importance. Therefore, in the realm of analytical method development, method validation holds a prominent position, not only during drug and drug product development but also in post-marketing and pharmacokinetic studies. As such, the HPLC (High-Performance Liquid Chromatography) method that was developed underwent thorough validation in accordance with the ICH (International Council for Harmonisation of Technical Requirements for Pharmaceuticals for Human Use) guideline Q2 (R1) on validation of analytical procedures [17]. The parameters considered during the validation process include specificity, quantification limits, detection limit, linearity, precision, and accuracy. By adhering to these parameters, the method validation process ensures high reliability and reproducibility of the results obtained from the HPLC method.
Specificity
Specificity is the ability to assess unequivocally the analyte in the presence of components that may be expected to be present. Typically, these might include impurities, degradants etc [17].
Linearity
Linearity is the ability (within the specified range) to obtain test results directly proportional to the concentration of analyte in the sample. Linearity is evaluated by visual inspection of the signal plot as a function of analyte concentration. If there is a linear relationship test results are calculated by regression line by the method of least squares [17].
Limit of detection (LOD) and limit of quantification (LOQ)
LOD and LOQ of D-prolinamide derivative and L-prolinamide derivative were determined by Signal to noise Ratio (Visual observation) [17]. Solutions of both the enantiomers (diastereomers) were prepared at concentrations 0.0011 mg/mL and 0.0022 mg/mL and injected, respectively.
Precision
The closeness of agreement (degree of scattering) between a series of measurements obtained from multiple sampling of the same homogenous sample under the prescribed conditions. Precision of the method was determined by injecting six different preparations at 100% specification limit and determining % RSD of D-prolinamide content values shown in table 1.
Accuracy
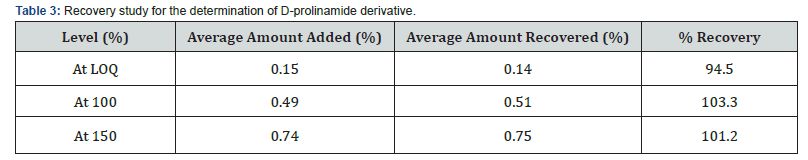
Recovery studies determined the accuracy of the method. D-prolinamide was spiked in the pre-analysed sample of L-prolinamide hydrochloride and its percent recovery was determined.
Results and Discussion
Our primary objective was to develop a precise and costeffective technique to determine the enantiomeric purity of L-prolinamide Hydrochloride, without relying on expensive chiral stationary phases. In order to achieve this, we initially focused on analyzing the physicochemical properties of the analyte.
Our investigations revealed that prolinamide possessed a chiral center and two basic moieties, namely primary amine (-NH2) and secondary amine (-NH), which are part of the amide group and proline ring, respectively. Such structural properties make prolinamide a suitable candidate for forming a derivative with Merfey’s reagent, as depicted in scheme 2.
Having explored several alternatives, we determined that a mobile phase consisting of a 78:22 mixture of buffer and acetonitrile, with a pH of 6, and a flow rate of 0.7 mL/min, was optimal for the analysis. We maintained the column oven temperature at a constant 30°C and monitored the column eluent at a wavelength of 335 nm for 55 minutes, with an injection volume of 10μL, as outlined in table 2. Through our rigorous testing, we have successfully developed a highly accurate and affordable method for determining the enantiomeric purity of L-prolinamide Hydrochloride, which we anticipate will significantly benefit the scientific community.
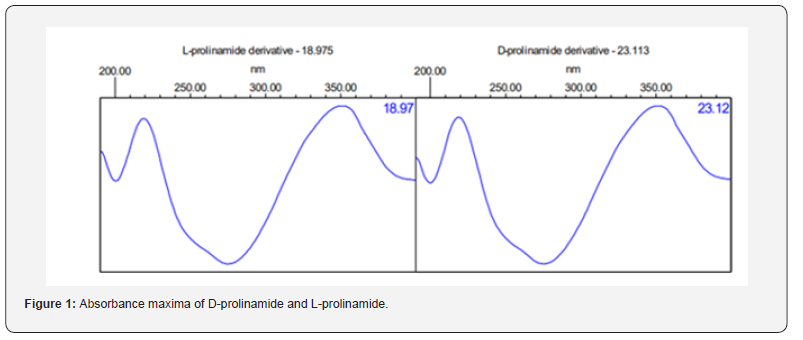
The separation of diastereomers was accomplished with success using reverse phase chromatography, with a resolution value exceeding 4 and a tailing factor below 1.5. A photodiode array detector was utilized on a diluted solution to determine the UV spectrum of the D-prolinamide derivative and L-prolinamide derivative in a suitable diluent. A scan between 190-400 nm led to the discovery of an absorption maximum (λmax) at approximately 335nm for both derivatives. Therefore, this specific wavelength was selected for HPLC analysis, as shown in figure 1.
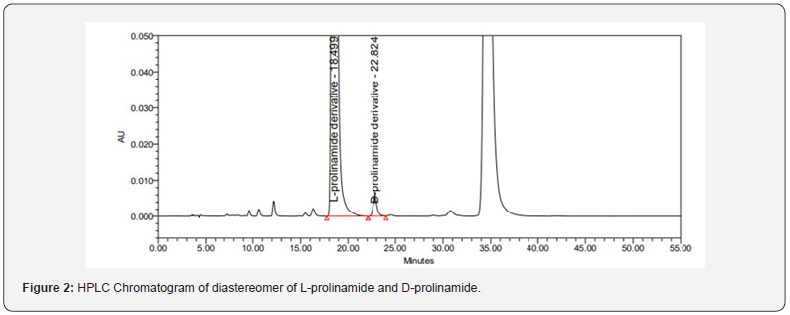
The method employed to validate the estimation of D-prolinamide, an undesired enantiomer, in L-prolinamide hydrochloride has been proven successful. The two enantiomers were efficiently separated employing derivatization with Marfey’s reagent. A representative chromatogram of the prolinamide derivatives’ resolution can be observed in figure 2. A peak at 18.4 min is due to L-Prolinamide diastereomer where a peak at 22.82 min is due to D-Prolinamide. An excellent resolution (R = 5.8) between the two peaks and tailing factor for L-prolinamide derivative and D-Prolinamide derivative 1.3 and 1.3, respectively, were obtained.
Based on the findings presented in figure 3, it has been established that the method utilized for analyzing D-prolinamide was linear. The linear range of the method spans from 0.0022mg/ mL to 0.011mg/mL, which is equivalent to 30% to 150% of the limit of 0.5%. In addition, the correlation coefficient of the method was recorded as 0.999, which indicates a high degree of accuracy. In order to evaluate the precision of the method, six sample solutions were subjected to analysis to determine the % RSD of D-prolinamide area. Upon analyzing the data from the six injections, it was observed that the method is precise, with an SD in RT and % area of D-prolinamide recorded as 0.01 min and 1.62%, respectively (Figure 3 & 4)..
The accuracy of the High-Performance Liquid Chromatography (HPLC) method developed for the analysis of D-prolinamide derivative was assessed through the percentage of recovery. The mean recovery obtained from the analysis was calculated to be 99.66%, with the amount spiked and the amount found presented in table 3. This indicates that the developed HPLC method is highly accurate and reliable for the determination of D-prolinamide derivative.
The signal-to-noise ratio was utilized to determine the Limit of Detection (LOD) and Limit of Quantification (LOQ) for the enantiomer. As a result, the LOD and LOQ for the D-prolinamide derivative were identified to be 0.0011mg/mL and 0.0022mg/ mL, respectively. This shows that the developed HPLC method is highly sensitive and can accurately measure the D-prolinamide derivative even at low concentrations.
Overall, the data obtained from this study demonstrates that the HPLC method developed is highly accurate, precise, and sensitive for the analysis of D-prolinamide derivative. The results obtained can be used for various applications, such as drug development and quality control in the pharmaceutical industry
Conclusions
This report presents a novel RP-HPLC method that has been developed for determining D-prolinamide and L-prolinamide hydrochloride. The method underwent a rigorous evaluation process, which included assessing its specificity, limits of quantification and detection, linearity, precision, and recovery. As a result, the method was found to be simple, sensitive, precise, and accurate, which makes it a valuable tool for the analysis of enantiomeric purity of L-prolinamide hydrochloride and detecting any racemization during the process development of active pharmaceutical ingredients (API). The method can detect D-prolinamide at a level of 0.0011mg/mL, which represents 15% of the limit. This indicates that the method is free from interference, thus providing reliable results. The significance of this finding is that it can be used to identify any impurities and deviations in the production of L-prolinamide hydrochloride. Therefore, this method can be used as an effective quality control tool for ensuring the purity and safety of this crucial pharmaceutical intermediate.
Overall, this report highlights the effectiveness of the RPHPLC method for analyzing D-prolinamide and L-prolinamide hydrochloride. The method’s simplicity, sensitivity, precision, and accuracy make it a valuable tool in the pharmaceutical industry, particularly for analyzing enantiomeric purity and racemization during API process development.
Acknowledgements
Authors thank management of IOL Chemicals and Pharmaceuticals Ltd for granting permission to publish the research work.
References
- Chambers RW, Carpenter FH (1955) On the Preparation and Properties of Some Amino Acid Amides. Journal of the American Chemical Society 77: 1522-1526.
- Palle Acharyulu, CM Raju (2005) Preparation of amino acid amides.
- Mary E Bellizzi, David A Betebenner, Jean-Christophe C Califano (2017) Antiviral compounds.
- Allan C. Krueger, Warren M Kati, William A Carroll, John K Pratt, Douglas K Hutchinson (2012) Anti-viral compounds.
- David A. Degoey, Warren M. Kati (2012) Anti-viral compounds. WO2012051361.
- David A. Degoey, Warren M. Kati (2012) Antiviral compounds.
- Henness S, Keam SJ (2006) Vildagliptin. Drugs 66: 1989-2001.
- Keating GM (2010) Vildagliptin. Drugs 70: 2089-2112
- Castaldi M, Baratella M, Menegotto IG, Castaldi G, Giovenzana C (2017) A concise and efficient synthesis of vildagliptin. Tetrahedron Letters 58(35): 3426-3428.
- Peng J, Feng Y, Tao Z, Chen Y, Hu X (2013) A Cost-Effective Method to Prepare Pure Vildagliptin. Letters in Organic Chemistry 10: 159-163.
- Sagandira CR, Khasipo AZ, Sagandira MB, Watts P (2021) An overview of the synthetic routes to essential oral anti-diabetes drugs. 96: 132378.
- (2012) Preparation method of 2(S)-methylamino-tetrahydropyrrolidine CN102093277 A.
- Hebei Keji Daxue Xuebao (2008) 29: 325-327.
- (2022) Vildagliptin monograph.
- (2015) Synthesis process of Vildagliptin.
- Calabrese M, Stancher B Riccobon P (1995) High‐performance liquid chromatography determination of proline isomers in Italian wines. Journal of the Science of Food and Agriculture 69: 361-366.
- Validation of analytical procedure: text and methodology Q2 (R1).






























