Orally Disintegrating Tablet: A Review
Shashank Khailkhura, Bhavana Singh*, Deepika Joshi and Nidhi Semwal
School of Pharmaceutical Science, SGRR University, Dehradun, India.
Submission: September 20, 2022; Published: November 01, 2022
*Corresponding author: Dr. Bhavana Singh, School of Pharmaceutical Science, SGRR University, Patel Nagar, Dehradun, Uttarakhand, 248001, India
How to cite this article: Shashank K, Bhavana S, Deepika J, Nidhi S. Orally Disintegrating Tablet: A Review. Glob J Pharmaceu Sci. 2022; 10(2): 555785. DOI: 10.19080/GJPPS.2022.10.555785.
Abstract
Orally dispersive tablets are solid dosage forms that dissolve in the mouth in within 10 to 30 seconds, enabling waterless ingestion. The tablet dissolves quickly due to its fast breakdown, which also causes the effects to start acting quickly. ODTs can help patients with a variety of conditions, including paediatrics, geriatrics, psychosis, dysphagia, bedridden discomfort, comatose patients, young patients with undeveloped muscular and nervous systems, patients with hand tremors, and patients who travel often. It provides high stability, precise dosage, efficient manufacture, and smaller packing; self-administration is allowed on long journeys because water is not required. ODTs are a cost-effective way to distribute drugs. When a medicine is absorbed through the buccal cavity, ODTs constitute a critical drug delivery method. Spray drying, sublimation, and other scientific procedures like freeze drying, moulding, and direct compression. The availability of ODTs as over-the-counter drugs for the treatment of a range of illnesses is increasing. This article’s objective is to go over the benefits, drawbacks, formulation difficulties, manufacturing methods, patented technologies, commercially available formulations, and evaluation checks of ODT. The word “Oro dispersible tablets” was created by the European Pharmacopoeia. This is an uncoated tablet that dissolves easily in the mouth for 3 minutes before being swallowed.
Keywords: ODTs pills; Sublimation; Moulding; Direct compression; Improved bioavailability
Introduction
Because of its ease of swallowing, discomfort avoidance, adaptability, and, most importantly, patient compliance, oral medication administration is preferred. Many patients find tablets and capsules difficult to swallow and many no longer take their medications as recommended. It is estimated that 50% of the population is affected by these issues, which leads to a higher risk of noncompliance and less effective treatment. For these reasons, tablets that may collapse in the oral cavity, have attracted big attention [1]. Solids dosage forms as oral tablets have the hugest place some of the entire pharmaceutical formulations [2]. Taste covering is a vital steps withinside the formulation of an acceptable fast dissolving/disintegrating tablet (FDDT). Traditional tablet formulation generally does not solve the issue related to taste masking, because it is supposed that the dosage from will not disintegrate until it passes through the oral cavity. The put off the bitterness the pill can be organized through sugar coating on the tablets. Many FDDT Technologies combine of taste masking as well [3-5]. ODTs technology which make drugs dissolve or disintegrate in the oral hallow space without any additional water intake has drawn an extraordinary deal of attention. ODTs are a solid dosage shape that provides the speedy disintegration or dissolution of solid to offer as suspension or answer shape even when placed in the mouth under restricted bio-fluids [6-7]. Orally disintegrating capsules unknown through diverse call consisting of or dispersible pills, shorts disintegrating pill speedy disintegrating pills, rapid or fast dissolving pills, porous drugs mouth dissolving pills, porous drugs mouth dissolving pills and rapimelts [8-10]. European pharmacopoeia has used the term Oro dispersible tablets. This can be described as uncoated pill meant to be positioned in mouth wherein, they disperse with no trouble inside 3min earlier than swallowing [11]. Despite of extraordinary development in pills delivery, the oral direction stays the right direction stays the precise direction for the managements of marketers due to low price of therapy, ease of administration, correct dosage, self- medication, ache avoidance, versatility, main to high levels of affected person compliance. Pills and tablets are the maximum famous dosages shape. however, one crucial downside of such dosage shape is dysphasia or problems in swallowing. This is visible to stricken almost 35% of the overall population. this disease is likewise related to a no of situation.
i. Children
ii. Parkinsonism
iii. Motion sickness
iv. Elderly patients
v. Mentally disabled persons
vi. Unavailability of water [12]
vii. Unconsciousness (Figure 1)
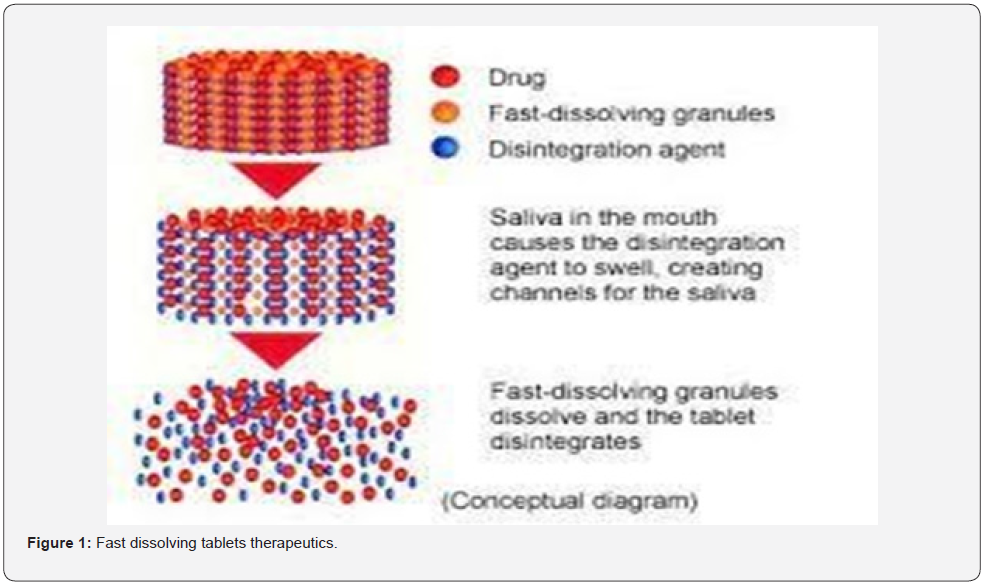
The oral path stays the ideal course for the managements of healing marketers due to the fact the low forged of therapy, production, and simplicity of admiration result in excessive triers of patients. Orally disintegrating tablets (ODTs) swiftly crumble or dissolve with side the oral cavity without the usage of water. The oral course of management is taken into consideration because the maximum broadly popular course because of its comfort of self-managements, compactness, and smooth manufacturing [13,14]. while position withinside the mouth, those dosage paperwork crumble immediately to launch the drugs which dissolve or disperses withinside the saliva. thereafter the drugs might also additionally get absorbed from different sections of git because the saliva journey down in such case bioavailability is considerably extra than that located from conventional dosage bureaucracy [15]. These are beneficial for Paediatrics, geriatric and additionally dysphagic sufferers, main to stepped forward sufferers’ compliance. Nonetheless, oral dosing stays the favored mode of managements for lots of styles of remedy because of its simplicity, versatility convenience and affected person acceptability.in current years, rapid dissolving tablets formula were evolved to triumph over hassle associated with swallowing difficult. While such capsule is positioned in oral cavity, saliva fast penetrates into the pores to purpose drugs disintegration [16].
Ideal Properties of ODT’S
ODTs are being desired as superior dosage paperwork in most times over traditional instant launch dosages bureaucracy for diverse classes of drugs. Its far anticipated to undergo sure first -rate function that make the misdeal. ODTs fall apart or dissolve in mouth inside a completely brief time. They do not require water in administration gift desirable flavor masking residences mouth feel, solid in environmental situations and need to know no longer leave any residue in mouth after oral administration. ODTs now no longer require water on administration, gift applicable flavour covering properties, have to have excessive pills loading capacity attractive mouth feel, strong in environmental situations and have to know no longer leaves any residue in mouth after oral administration [17]. The benefit provided with the aids of using ODTs over instant launch formula might also additionally encompass ease of method designing and manufacturing, unit packaging, smooth to deal with through patients [14,18,19]. No want of water to administer, speedy disintegration of pill outcomes in brief dissolution and speedy absorption which can cause enhanced healing performance because of extended bioavailability [20].
Limitations of ODTs [21]
a. Most of instances soluble diluents used for formulating ODTs would possibly render hygroscopic dosage which may also result in balance issues.
b. The pill can also additionally depart ugly flavour and /or grittiness in mouth if now no longer formulated properly.
c. Specialized packing is probably required for hygroscopic and mild touchy drugs.
d. Precautions to be taken whilst administrating immediately after removing from pack.
e. Rapid drug therapy intervention.
f. After oral managements they must go away minimum or no residue in mouth.
g. The mouth feel should be pleasant.
Advantage of ODTs [22]
a. Ease of administration to sufferers who refused to swallow a tablet, which included paediatric, geriatric, mentally ill, disable and uncooperative sufferers.
b. Rapid dissolution of drug and absorption may produce rapid onset of action.
c. Ability to provide advantages of liquid medication in the form of solid preparation (Figure 2).
Disadvantage of ODTs [23]
a. Rapid disintegrating pills are hygroscopic in nature a lot be saved at managed surrounding i.e., humidity and temperature.
b. For nicely stabilization and protection of solid products, ODT require unique packaging.
c. Typically have insufficient mechanical electricity hence, cautions dealing with is required.
d. Depart unsightly flavour and /or grittiness in mouth if now no longer formulated properly [24].
Technical Preparation of Oro-Dispersible Tablet
Freezing drying or lyophilization
A manner wherein water is sublimated from wherein water is sublimated from the product after freezing is known as drying. Freeze drying. Freeze dried paperwork provide extra fast dissolution than different to be had strong products after freezing is referred to as freeze drying. Freeze dried bureaucracy provide extra speedy dissolution than different to be had strong products. The lyophilisation procedure imparts sleek amorphous shape to the blocking sellers and now and again to the drugs thereby enhancing the dissolution traits of the formulation. The usage of freeze drying is restrained because of excessive value of the device and processing different most important drawback of the very last dosage bureaucracy consist of loss of bodily resistance in popular blister pack. R.P Scherer patented Zaydis generation with the aid of using freeze drying procedure for the practice of mouth dissolving pills on the premise of patent issued to Gregory et al. Jaccard and leyder additional applied lyophilisation to put together orodispersible drugs of numerous drugs [25,26].
Moulding
Tablets produced with the aids of using Moulding are stable dispersion. Physical shape of the drugs withinside the pill relies upon whether or not and to what quantity it dissolves within sides the molten carrier. The pills can exist discrete debris or micro debris dispersed withinside the matrix disintegrating time, capsules dissolution price and mouth experience will rely on the form of dispersion or dissolution. Moulded pills fall apart extra unexpectedly and provide progressed flavor due to fact the dispersion matrix is in widespread shape water soluble sugars. Moulded drugs standard does now no longer own wonderful mechanical strength. Erosion and breakage of the moulded pills regularly all through dealing with and beginning of blister packs [27,28].
Sublimation
Because of low porosity, compressed pills composed of excipients as pill matrix cloth frequently do now no longer dissolve unexpectedly within side the water porous pill that show off true mechanical power and dissolve speedy were developed. inert strong ingredients (ex, urea, urethane, ammonium carbonate, camphor, naphthalene) have been brought to different pills excipients and the combination turned into compressed into pill. Removal of risky fabric with the aid of using sublimation [29] (Figure 3).

Sublimation generated a porous structure. Compressed pill containing mannitol and camphor had been organized with the aids of using sublimination techniques. the pill dissolve inside 10- 20 second and showcase enough mechanical energy for realistic uses [29].
Spray drying
Spray drying may be used to put together hastily dissolving tablets. This approach is primary based totally upon a specific guide matrix that is ready with the aid of using spray drying an aqueous composition containing aid matrix and different components to form a noticeably porous and high -quality powder. This is then blended with lively element and compressed into tablets. Allen and wang have hired spray drying strategies to put together orodispersible tablets [30,31].
Mass extrusion
This generation contain buying the lively combo the use of the fixing combination of water-soluble polythene glycosol the uses of methanol and expulsion of softened mass thru extruder or syringe to get a cylinder of the product into even section the uses of warmth blade to shape tablets. The dried cylinder also can used to coat granules of sour tasting pills and thereby by making their sour taste [32,33].
Direct compression(dc)
Direct compression (dc) is the maximum and simplest and handiest pill production strategies for MDT as they may be fabricated the use of traditional pill production and packaging equipment and additionally because of availability of tabulating excipients with progressed float compressibility and disintegration properties , mainly flavor production and packaging equipment and additionally because of availability of tabulating excipient with stepped forward go with the drift , compressibility and disintegration properties, specially capsules disintegration bubbling agent and sugar primarily based totally excipients any other DC base technology, flash tab includes covered crystal of drugs and micro granules in conjunction with disintegrate [34].
Tablet moulding
Tablets produced through moulding are strong dispersions. Physical shape of the drug withinside the capsules rely upon whether or not and to what extent, it dissolves withinside the molten service. The drug can exist as discrete small debris or micro debris dispersed withinside the matrix. It can dissolve completely withinside the molten provider to provide strong answer or dissolve partly withinside the molten provider and the closing debris live undissolved and dispersed withinside the matrix. Disintegration time, drug dissolution price and mouth experience will rely upon the sort of dispersion or dissolution.
Fast dissolving films
It incorporates a Nano aqueous answer having water- soluble movie forming polymers (pullulan, carboxymethyl cellulose, hydroxyethyl cellulose) a drug and every other taste - overlaying sellers which might be used to increase a movie because the solvent evaporated. Withinside the case of bitter - tasting pills resin adsorbents or covered micro particle of a tablets may be utilized in a film [35].
Manufacturing Process of ODTs [36]
a) Drugs turned into geometrically blended with microcrystalline cellulose, lactose, and sifted via sieve no. 40.
b) This mixture changed into in addition combined with starch and ferric oxide yellow in a fast mixer granulator.
c) Binder answer became organized with the aid of using dissolving hydroxypropyl methyl cellulose beneath neath stirring in purified water.
d) This binder answer turned into introduced to the aggregate withinside the speedy mixer granules.
e) The granules mass was air – dried for 5 to 10min and further dried at 450C-550C to 10min and passed through sieve no 10.
f) Dry granules were sifted through sieve no 30 using vibratory sifted.
g) In a clean dry blender, the dried granules were mixed with hydroxy methylcellulose and magnesium stearate.
h) This tablet was coating with coated pan.
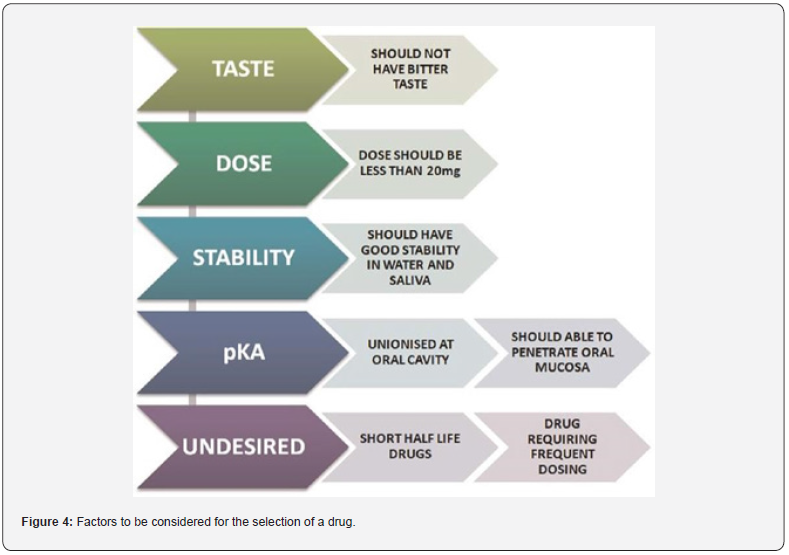
Factors to be Considered for the Selection of the Drug
Factors to be considered for the selection of the drug depend on various factors, which include taste, dose, stability, and pKA. The detailed illustration is shown in Figure 4.
Future Prospects
These dosage forms may be suitable for the oral delivery of drugs such as protein and peptide-based therapeutics that have limited bioavailability when administered by conventional tablets. These products usually degrade rapidly in the stomach. Should next generation drugs be predominantly protein or peptide based, tablets may no longer be the dominant format for dosing such moieties. Injections generally are not favoured for use by patients unless facilitated by sophisticated auto-injectors. Inhalation is one good alternative system to deliver these drugs, but the increased research into biopharmaceuticals so far has generated predominantly chemical entities with low molecular weights. The developments of enhanced oral protein delivery technology by ODTs which may release these drugs in the oral cavity are very promising for the delivery of high molecular weight protein and peptide.
Conclusion
Orally disintegrating tablets have better patient acceptance and compliance and may offer improved biopharmaceutical properties, improved efficacy, and better safety compared with conventional oral dosage forms. Prescription ODT products initially were developed to overcome the difficulty in swallowing conventional tablets among paediatric, geriatric, and psychiatric patients with dysphagia. Today, ODTs are more widely available as OTC products for the treatment of allergies, cold, and flu symptoms. The target population has expanded to those who want.
References
- Hirani JJ, Rathod DA, Vadalia KR (2009) Orally disintegrating tablets. Tropical Journal of Pharmaceutical Research 8(2): 161-172.
- Olmez SS, Vural I (2009) Advantages and quality control of disintegrating tablets. FABAD Journal of Pharmaceutical Sciences 34(1): 167-172.
- Kawano Y, Ito A, Sasatsu M, Machida Y (2010) Preparation of orally disintegrating tablets with taste masking function: masking effect in granules prepared with correctives using the dry granulation method and evaluation of tablets prepared using the taste-masked granules. Yakugaku Zasshi 130(1): 81-86.
- Panigrahi R, Behera SP, Panda CS (2010) A review on fast dissolving tablets. Web med Central Pharmaceutical Sciences 1(11): 1-16.
- Douroumis DD, Gryczke A, Schminke S (2011) Development and evaluation of cetirizine hcl tastemasked oral disintegrating tablets. American Association of Pharmaceutical Scientists Pharm SciTech 12(1): 141-151.
- Shyamala B, Narmada GY (2002) Rapid dissolving tablets: A novel dosage form. The Indian pharmacist 13(8): 9-12.
- Neeta, Dureja H, Bhagwan H, Dahiya S (2012) Fast dissolving tablet: An overview. Novel Science International Journal of Pharmaceutical Science 1(5): 228-232.
- Konapure SA, Chaudhari PS, Oswal RJ, Kshirsagar SS, Antre RV, et al. (2011) Mouth dissolving tablets an innovative technology. International Journal of Applied Biology and Pharmaceutical Technology 2(1): 496-503.
- Bangale SG, Shinde GV, Rathinaraj BS. New generation of orodispersible tablets: recent advances and future prospects. International Journal of Advances in Pharmaceutical Sciences 2011; 2(3):17-28.
- Jesmeen T, Uddin R (2011) Orodispersible tablets: A short review. Stamford Journal of Pharmaceutical Sciences 4(1): 96-99.
- Fu Y, Yang S, Jeong SH, Kimura S, Park K (2004) Orally fast disintegrating tablets: Developments, technologies, taste masking and clinical studies. Crit Rev Ther Drug Carrier Syst 21(6): 433-476.
- Tejvir Kaur (2001) Review article and Month dissolving tablets. International Journal of Current Pharmaceutical Research 3(1): 58-61.
- Sastry SV, Nyshdham JR, Fix JA (2000) Recent technological advances in oral drug delivery: A review. Pharmaceutical Science and Technology Today 3(4): 138-145.
- Seager H (1998) Drug-delivery products and the Zydis fast dissolving dosage form. Journal of Pharmacy and Pharmacology 50(4): 375-382.
- Fu Y, Yang S, Jeong SH, Kimura S, Parket K (2004) Orally fast disintegrating tablets: developments, technologies, taste masking and clinical studies. Critical Reviews in Therapeutic Drug Carrier Systems 21(6): 433-475.
- Patel A, Patel JK, Patel KN, Patel RR (2010) Studies on formulation and in vitro evaluation of fast dissolving tablets of domperidone. Indian J Pharm Sci 2(1): 470-476.
- Chackol AJ, Josel S, Babul N, Michelle M (2010) Design and Development of Orodispersible tablets of Promethazine Theoclate Using Coprocessor Super disintegrants and Subliming Materials. International Journal of Innovative Pharmaceutical Research 1(2): 53-56.
- Brown D (2001) Orally disintegrating tablets: Taste over speed. Drug Delivery Technol 3: 58-61.
- Dobetti L (2003) Fast disintegrating tablets. US Patent, US.
- Behnke K, Sogaard J, Martin S, Bauml J, Ravindran AV, et al. (2003) Mirtazapine orally disintegrating tablet versus sertraline, A prospective onset of action study. J Clin Psychopharmacology 23(4): 358-364.
- Kumar VD, Sharma I, Sharma V (2011) A comprehensive review on fast dissolving tablet technology. Journal of Applied Pharmaceutical Science 01(05): 50-58.
- Saxena V, Khinchi MP, Gupta MK, Agarwal D, Sharma N (2010) Orally Disintegrating Tablets: Friendly Dosage Form. International Journal of Research in Ayurveda & Pharmacy 1: 399-407.
- Kumari S, Visht S, Sharma PK, Yadav RK (2010) Fast Dissolving Drug Delivery System: Review Article. Journal of Pharmacy Research 3(6): 1444-1449.
- Bhowmik D, Chranjib B, Pankaj K, Chandira RM (2009) Fast Dissolving Tablet: An Overview. Journal of Chemical and Pharmaceutical Research 1(1): 163-177.
- Gregory GKE, Ho D (1981) Pharmaceutical dosage form package. US patent 4: 305-502.
- Blank RG, Mainitho Y (1990) Fast dissolving dosage forms. US patent.
- Vanscoik KG (1992) Solid pharmaceutical dosage in tablet triturate form and method of producing same. US patent,
- Pebley WS (1994) Rapidly disintegrating tablet. US patent.
- Koizumi K, Watanabe Y, Morita K, Utoguchi N, Matsumoto M (1997) New method of preparing high porosity rapidly saliva soluble compressed tablets using mannitol with camphor, a subliming material. Int J Pharm 152(1): 127-131.
- Makino T (1998) Fast dissolving tablet and its production. US patent.
- Allen LV, Wang BM (1996) Process for making a particulate support matrix for making rapidly dissolving tablet. US patent.
- Allen LV (2000) Method for producing a rapidly dissolving dosage form. US patent.
- Ishikawa T, Mukai B, Shiraishi S, Naoki U, Makiko F, et al. (1999) Preparation and evaluation of tablets rapidly disintegrating in saliva containing bitter taste-masked granules by the compression method. Chem Pharm Bull 47(10): 1451-1454.
- Bhaskaran S, Narmada GV (2002) Indian Pharmacist 1(2): 9-12.
- Dor JM, Fix JA, Johnson MI (1999) A new in vitro method to measure the disintegration time of a fast disintegration tablet. Proc Int Symp Control Rel Bioact Mater 26: 939-940.
- K Ostrander (2003) Advances in Fast Dispersing Technologies-Zydis. Paper presented at the annual meeting of the AAPS, Salt Lake City, UT.






























