- Review Article
- Abstract
- Introduction
- Sample Preparation Techniques
- QuEChERS (Quick, Easy, Cheap, Effective, Rugged, Safe)
- Alternative Extraction Procedures for Future Testing in Calliphoridae Specimens
- Dilute-and-Shoot
- Conclusion and Future Perspectives
- Acknowledgement
- Conflict of Interest
- References
Forensic Entomotoxicology: A Brief Look at Sample Preparation Techniques – A Review
Gustavo Andrade Ugalde1, Lara Celestina Santos1, Nayomi de Andrade Chimendes1, Rachel Santos1, Victoria Gomes da Rosa1, Leonardo Cardoso Correa1, Jéssica da Silva Dias1, Fernanda Ziegler Reginato1, Silvia Gonzalez Monteiro2, Daniel Roulim Stainki2 and André Valle de Bairros1*
1Nucleus Applied to Toxicology, Center of Health Sciences, Federal University of Santa Maria, Brazil
2Department of Parasitology and Microbiology, Center of Health Sciences, Federal University of Santa Maria, Brazil
Submission: August 28, 2022; Published: October 18, 2022
*Corresponding author: André Valle de Bairros, Nucleus Applied to Toxicology, Center of Health Sciences, Federal University of Santa Maria, Santa Maria, Brazil
How to cite this article:Ugalde GA, Santos LC, Chimendes NA, Santos R, Rosa VG, et al. Forensic Entomotoxicology: A Brief Look at Sample Preparation Techniques - A Review. Glob J Pharmaceu Sci. 2022; 10(2): 555784. DOI: 10.19080/GJPPS.2022.10.555784.
- Review Article
- Abstract
- Introduction
- Sample Preparation Techniques
- QuEChERS (Quick, Easy, Cheap, Effective, Rugged, Safe)
- Alternative Extraction Procedures for Future Testing in Calliphoridae Specimens
- Dilute-and-Shoot
- Conclusion and Future Perspectives
- Acknowledgement
- Conflict of Interest
- References
Abstract
Forensic entomotoxicology is a relatively new science whose applications aims the detection of toxic substances through a matrix of necrophagous insects in a crime scene. In addition, it aims the investigation of the impacts of the xenobiotics on these insects, generating impacts in the measurement of the post-mortem interval (PMI). Calliphoridae larvae, the most important insect for forensic entomology, has an average amount of 25% of crude fat and 53% of crude protein, being a complex matrix that requires some sample preparation methodology before inserting the sample in some equipment to perform the chemical instrumental analysis. Sample preparation methods should be applied in order to increase the protection of analytical equipment from impurities present in the arthropod matrix, as well as increase the detectability of the test analyte by the analytical instrumentation of choice. Thus, the objective of the present work is to compile sample preparation techniques since traditional procedures until new approaches found in the scientific literature applied for entomotoxicological analysis in Calliphoridae specimens and respective analytes. It was observed the lack of a complete standardization for entomotoxicological approach, mainly for traditional extractions preparations. In this sense, microextraction-based techniques become an even greater challenge for arthropod–like matrix, especially in specimens from Calliphoridae family for xenobiotics determination. So, the development of sample preparation techniques with more sustainable approaches, such as reduced use of solvents, added to the increase of the powerfulness of analytical instrumental techniques should be encourage in order to improve the forensic entomotoxicology approach.
Keywords: Forensic entomotoxicology; Calliphoridae; Blow flie; Sample preparation; LLE; SPE; QuEChERS
- Review Article
- Abstract
- Introduction
- Sample Preparation Techniques
- QuEChERS (Quick, Easy, Cheap, Effective, Rugged, Safe)
- Alternative Extraction Procedures for Future Testing in Calliphoridae Specimens
- Dilute-and-Shoot
- Conclusion and Future Perspectives
- Acknowledgement
- Conflict of Interest
- References
Introduction
Forensic entomotoxicology is consider a branch of the forensic entomology, which studies the potential use of arthropods with scavenger or necrophagous habits for the detection of possible toxicants in cases where usual biological matrices are unavailable for toxicological analysis. These cases can occur in highly decomposed bodies, skeletonized, mummified or burnt remains, with a lack of biological tissue [1,2]. Sarcophagidae and Coleoptera family are studied for forensic entomotoxicology, however the most important species is the blowflies from Calliphoridae family [3].
Detecting potentially fatal toxicants blindly is the great challenge for toxicologists, given the matrix complexity of arthropods [4]. Thus, it is known that the most widely used techniques for analysis in conventional biological matrices, such as human tissues and biological fluids, have been well applied for analytical investigations in matrices of these arthropods [2,4,5] Considering the complexity of the matrices involved in entomotoxicological analyses, it is necessary to perform the sample preparation techniques before the instrumental determination, mainly liquid chromatography (LC) and gas chromatography (GC) [6]. So, the objective of this review is compilate sample preparation techniques found in the scientific literature applied for entomotoxicological analysis in Calliphoridae specimens, together with the toxic substance analyzed and, finally, gather with methodology validation parameters obtained by the authors reviewed when available.
Calliphoridae specimens
Calliphoridae family belongs to Order Diptera and Classe Insecta, being a cosmopolitan group of flies that has more than 150 genera and 1000 species recognized [7]. Because of its ecological diversity, the flies are adapted to different habitats [8]. These flies are small to medium-sized Diptera, generally metallic in shades of blue, violet, green and cuprine. They are popularly called blowflies [9,10] It is composed of 12 subfamilies: Auchmeromyinae, Bengaliinae, Calliphorinae, Chrycomyinae, Helicoboscinae, Luciliinae, Melanomyinae, Mesembrinellinae, Phumosiinae, Poleniinae, Rhiniinae and Toxotarsinae [11], among which 29 genera and 99 species occur in the Neotropical region, grouped in the following subfamilies: Chrysomyinae, Calliphorinae, Lucilliinae, Mesembrillinae, Polleniinae, Rhiniinae and Toxotarsinae [12]. Adults have a thoracic calyptera and meron with well-developed bristles, post-scutellum absent or poorly developed, abdominal segments without distal bristles or, if present, poorly developed, two bristles on the notopleura and ptylineal suture [13]. The larvae can be biontophagous, scavengers or necrobiontophagous, and can cause primary and secondary myiasis, being important as decomposers and also for use in animal and human health [14]. The principal genera recognized in the Neotropics are Cochliomyia Tonwsend, 1915, Compsomyiosps Townsend, 1918, Lucilia Robineau-Desvoidy, 1830 (including Phaenicia Robineau-Desvoidy), Calliphora Robineau-Desvoidy, 1830, and Chrysomya Robineau-Desvoidy, 1830 [12,15].
Biological cycle of calliphoridae specimens
The diptera cycle is holometabolous (complete metamorphosis), composed for stages of egg, larvae (L1, L2, L3), pupae and adult flies. The Calliphoridae adults emerge from the pupae using the ptilinum, which is a membranous structure located between the eyes. This structure presses the pupae, forming a circular slit through which the adult emerges. The cycle is completed in 20 days depending on the environmental temperature. At average temperatures of 22 °C, the life cycle occurs in approximately 24 days. Females of this family lay their eggs on decomposing organic material, with exception of the Cochlyomyia hominivorax that lay their eggs on edges of live animal lesions. After 12 to 24 hours, the Calliphoridae larvae hatch and begin to feed [16]. Each female fly lays an average of 200 eggs per day, totaling up to 3000 eggs in her life [17].
Collected material of insects for studies
Secretions, hemolymph or body parts of L3 larvae and adult insects are used for forensics, biological, immune response and other studies. The insects are sterilized by immersion in a 0.5% sodium hypochlorite solution for 5 minutes and then rinsed with sterile distilled water. Only after these procedures the collection should be done [18,19].
Calliphoridae specimens for forensic entomotoxicology
Calliphoridae larvae are commonly found in decomposed bodies, and it can be used as a tool in investigation for the elucidation of crimes. Estimation of post-mortem intervals (PMI), isolation of human DNA from digestive tract fly larvae for individual identification and alternative matrix for xenobiotic analysis in humans and animals are real possibilities for forensic cases [3,20,21]. Neverthless, xenobiotics determination from Calliphoridae larvae requires a samples preparation before instrumentation application because of the matrix complexity.
A few authors investigating the potential of blow flies as animal feed ingredients, have depicted the contents of crude fat and crude proteins of a few species. According to them, larvae of Calliphora vicina reared in pork liver showed the amount of crude fat and crude protein of 20.1% and 48.3% respectively. Larvae L3 of Chrysomya megacephalla reared in minced pork meat showed 27 % of crude fat and 61.8% of crude protein, while the pupal stage of the same species reared in the same rearing substrate presented 16.5% of crude fat and 46.8% of crude protein. Larvae L3 of Lucilia sericata reared in pork liver presented 28.4% of crude fat and 53.5% of crude protein, and the pupal stage reared also in a pork liver media, presented 26.6% of crude fat and 59% of crude protein. In addition, they reared Photophormia terraenovae in a meat waste media, obtaining for larvae at L3 stage 28.3% of crude fat and 46.3% of crude protein. For the pupal stage of the same species reared in the same media, the amounts of crude fat and crude protein were 23.6% and 56% respectively [22].
- Review Article
- Abstract
- Introduction
- Sample Preparation Techniques
- QuEChERS (Quick, Easy, Cheap, Effective, Rugged, Safe)
- Alternative Extraction Procedures for Future Testing in Calliphoridae Specimens
- Dilute-and-Shoot
- Conclusion and Future Perspectives
- Acknowledgement
- Conflict of Interest
- References
Sample Preparation Techniques
The objective of the sample preparation step is to isolate the components of interest from a sample matrix, because most analytical instruments cannot handle the matrix directly. Sample preparation involves extraction procedures and can also include cleanup procedures for very complex samples. This step must also bring the analytes to a concentration level suitable for detection, and therefore, sample preparation methods typically include enrichment [23].
According to Câmara et al. [24], 30% of the experimental errors and 60% of the time spent on lab are sample preparation related. For these reasons, it is not enough to have high resolution and sensitive equipment at your disposal if the sampling procedure and the sample handling and pretreatment methodologies are not optimized and done effectively. All these variables must be taken into account together to acquire high-quality analytical results with high selectivity and low sensitivity limits and to ensure high accuracy and reproducibility.
Liquid-liquid extraction (LLE)
One of the oldest and most common sample preparation methods in toxicology laboratories is the LLE, is a solvent-based extraction method. LLE is based on the distribution of an analyte between two phases, with the purpose of be extracted from an aqueous sample solution with the help of a water-immiscible organic solvent [25,26]. The driving force for this extraction process is the difference in the solubility of the target analytes between the binary phase formed by the addition of the organic solvent. It is a process that can be used, for example, to increase selectivity by isolating the analyte from interfering species in the matrix, or to increase selectivity by concentrating the analyte from a large volume of sample [6,23].
Beyer et al. [27] were the first authors to report an LLE-based method to detect the qualitative presence of phenobarbital in larval tissue of (Fabricius) in a case of fatal overdose. Since then, several authors have developed methods for the analysis of the most varied types of analytes, being the vast majority in applications larvae or pupae of species of the Calliphoridae family given its importance for cases related to forensic entomology. From the onwards, several studies involving entomotoxicological analysis of Calliphoridae specimens tissues, developed only qualitative studies or even quantitative ones, without referring to any validation of the methodology developed [27-44].
Sadler et al. [45] in research whose purpose was assay and evaluate the bioaccumulation and elimination of four antidepressants in Calliphora vicina larvae, developed an LLEbased method followed by GC-MS determination where it was possible to quantify amitriptyline, temazepam and a combination of trazodone and trimipramine, without a description of a method validation. Nevertheless, the LOD of the method according to the authors were 0.01μg/g larval tissue for all four drugs. Wood et al. [46] described the development of a sensitive extraction method followed by simultaneous LC-MS/MS determination of 10 benzodiazepines (alprazolam, clonazepam, diazepam, flunitrazepam, lorazepam, nordiazepam, oxazepam, prazepam, temazepam and triazolam) in C. vicina larval and puparia tissues. The authors tested four different extractive methodologies, including LLE among them. However, the one that showed the greatest recovery power, both in larval tissue and in pupae, was a simple method based on a simple homogenization followed by precipitation by acetonitrile, whose LOD and LOQ for larval tissue ranged from 1.88 to 5.13pg/mg and between 7.63 and 20.63, respectively. For pupal tissue, LOD and LOQ ranged from 6.28 to 19.03 and 25.23 to 73.93pg/mg, respectively.
Wolff et al. [47] developed and validated an LLE-based method for parathion determination by HPLC-DAD in a pool of arthropods with 29 diptera (24 larvae, 3 pupae, 1 pupa case, 1 adult), 13 coleoptera (adults), 6 hymenoptera (adults), 1 hemiptera (adult), 1 isopod and 3 acarids (adults). It was found that the LOQ for parathion in the respective method was 0.1ppm. Kharbouche et al. [48] developed and validated an LLE-based followed by LC-MS methodology for codeine, norcodeine and morphine determinations in Lucilia sericata (Meigen) larvae, pupae and adults. In all blow flies tissue stages tested, the LOD for codeine, morphine, and norcodeine were 1, 2 and 3ng/g, respectively and the LOQ was fixed at 10ng/g for all matrices. Liu et al. [49] developed and validated an LLE method that determines the concentration of malathion in rabbit tissues and Chysomya megacephala (Fabricius) larvae feeding on those tissues by GCMS. Malathion was found in all muscle and liver tissues assayed on rabbit corpse and in larval tissue as well. They found for this method LOD of 0.1μg/mL and LOQ of 0.3μg/mL value in this method.
Gosselin et al. [50] developed a method for the quantification of methadone and its main metabolite, 2-ethylidene-1,5- dimethyl-3,3-diphenylpyrrolidene (EDDP), in third instar larvae of L. sericata which were reared in substrate containing 4μg/g of methadone. The method comprised a simple LLE, followed by analysis by LC-MS-MS. The method proved to be sensitive enough to identify methadone and EDDP in a single larva, showing limit of quantification of 10pg/mg.
Gosselin et al. [51], in further studies, evaluated the effect of contact of methadone on L. sericata life cycle development and developed and validated an LLE-based methodology followed by a UPLC-MS/MS analytical procedure for determination of methadone and its metabolite EDDP. LOD and LOQ for puparial tissue were set respectively 2pg/mg and 10pg/mg puparial case for both analytes. Also, they inferred that EDDP was not detected in pupae samples, confirming rapid elimination of metabolites by the larvae before pupation.
Bushby et al. [52] developed and validated an LLE method with a recovery power > 80% followed by LC-MS/MS analytical determination of methylphenidate in L. sericata larval matrix using an in vivo rat brain model. The LOD and LOQ obtained for this methodology in the larval matrix were 24 and 80ng/ mL. Magni et al. [53] developed and validated an LLE-based methodology followed by GC-MS determination for nicotine and its predominant metabolite cotinine detection in larval matrix of Calliphora.vomitoria (L.). The method showed for nicotine LOD and LOQ of 0.13 and 0.43ng/mg respectively and 0.38 and 1.2 for cotinine, respectively. Recently, Wang et al. [54] developed an LLE methodology for determination of metamphetamine followed by GC-MS analysis in larval matrix of Aldrichina graham (Aldrich). The calculated LOD was 0.10ng/mg, while LOQ value was 0.33ng/ mg in this method.
Solid-phase extraction (SPE)
SPE method is based to trap the analytes of interest through disposable cartridges, which contain the most various sorbents such as silica, silica bound to polymeric resins or hydrocarbons. These proposed sorbents allows, by chemically and or physical mechanisms, the separation of a wide range of components from the most diverse matrices in their most diverse complexities, avoiding solvents and the matrices interferents per se in the instrument analytical signal, consequently improving the detectability, selectivity and sensitivity of the target compounds [6,55]. SPE allows a more complete extraction of the analyte and a more efficient separation if interferences from samples, demands a reduced usage of solvents, there is no emulsion formation, more convenient manual procedures with particulate removal as part of the methodology, allowing recoveries > 99% in onestep SPE method. On the other hand, there is the possibility of irreversible adsorption of matrix interference, which makes it impossible to reuse the cartridges, making the method relatively expensive [6,55]. As the insect extraction matrix consists of a solid material, the sorbent used will depend on the analyte to be detected, as well as the sample volume to define the appropriate means for extraction [56]. Similar to LLE procedures, the majority of SPE techniques were employed for qualitative purposes to entomotoxicology in Calliphoridae insect [34,57,58].
De Aguiar et al. [59] developed and fully validated a SPEsimilar method followed by liquid chromatography coupled to tandem mass spectrometry (LC-MS/MS) determination. The sample preparation method was consisted by a solid-liquid extraction with low temperature- partitioning (SLE-LTP) and was applied in larvae matrix collected from decomposed corpses. The methodology was developed for several drugs and its metabolites. The LOQ of the methodology employed was 3ng/g for amitriptyline, 1ng/g for carbamazepine, 2.5ng/g for bromazepam, 3ng/g for clonazepam, 2.5ng/g for diazepam, 2ng/g for flunitrazepam, 2ng/g for cocaine and its metabolite benzoylecgonine, 2ng/g for the pesticide aldicarb and 6ng/g and 40ng/g for its sulfone and sulfoxide metabolites, respectively.
Lambiase et al. [60] evaluated the potential of ethyl glucuronide (EtG) and ethyl sulfate (EtS) as potencial biomarkers for ethanol intake in larval tissue of Calliphora vicina showing that the maggots, pupae and puparia could be a useful matrix for the evaluation of ante mortem alcohol ingestion. Before that, to ensure the data collection, the authors developed and validated a SPE followed by a LC-MS/MS methodology for the determination of the toxicants. The LOD and LOQ of the method were 20 and 30pg/mg for EtG and 10 and 20pg/mg for EtS.
Headspace (HS) and solid phase microextraction (SPME)
A usual analysis of HS takes place when a liquid, semi-solid or solid sample in a determinated volume/ weight is sealed inside a vial and incubated during a period in certain temperature. With the help of some device that is exposed to this HS saturated with the analytes, the volatile air is collected and injected into the GC system [6]. HS do not require the use of a solvent to obtain a pure volatile extract from the respective sample, which allows the introduction of analytes without problems into a GC system. Traditional HS consider static or dynamic headspace (SHS or DHS), however there is a HS-based technique known Headspace/solidphase microextraction (HS-SPME) [6,61,62]. SPME was developed in the 1990s to address the need for a sample preparation procedure that could be employed in both the laboratory and on-site [23,63]. Succinctly, SPME sampling consists of exposing a thin polymeric coating fiber into the headspace produced by a given sample matrix for a predetermined time. Since the fiber exposition, the transport of the volatile analytes from the headspace to the coating begins immediately and is considered to be complete when the analyte concentration has reached distribution equilibrium between the sample matrix and the fiber coating. After sampled, the volatiles are desorbed into the GC injection port for a predetermined period of time and desorption temperature [64].
Tabor et al. [65] employed traditional static HS to evaluate the effects of Phormia regina (Meigen) larva fed on pork treated with ethanol on its development inferences to assist on PMI estimations. They found significant differences in body length of third-instar larvae fed on ethanol approximately 12-hours longer post-feeding period compared with a control group. Also, ethanol concentrations in the Phormia regina larva matrix found were 0.01% (w/v) for both LOD and LOQ.
Definis-Gojanovic et al. [66] analyzed multiple samples of a suicide case on decomposed human tissue remains, larval blow flies and of the larval flesh in a diversified level of stages which were collected from the corpse and then were analyzed using HSSPME and gas chromatography with flame ionization detector (GCFID) for the presence of ethanol. However, it was not possible to detect the ethanol content in larval matrix and the authors did not validated the employed methodology. A few authors have studied the changes in pattern of volatile compounds daily released of pupae and or larvae of blow flies as a function of decomposition using laboratory colonies and meat baits by HS-SPME and gas chromatography coupled to mass spectrometry (GC-MS) [67-72]. The authors showed that the volatile profile varied qualitatively and semi quantitatively, with the age of the larva/pupa under investigation and concluded that is possible to increase the accuracy of the estimated PMI, through improved estimation of the age of blowflies present on the corpse, suggesting this type of analysis as a new tool to estimate PMI.
While these studies have elucidated the chemical composition of larvae samples as a function of decomposition using laboratory colonies and meat baits, Blanar and Pruda-Tiedemann [73] developed a study focusing on blowfly larvae samples collected from an active outdoor cadaveric decomposition model from a pool of larva, also by HS-SPME and GC-MS. A total of 10 molecules from 107 detected were selected as frequently occurring in the larvae matrix. The authors concluded that was feasible to use a larval odor sample to detect previously reported decomposition odor volatiles and through continuous sampling, the odor profile changes as a function of decomposition.
- Review Article
- Abstract
- Introduction
- Sample Preparation Techniques
- QuEChERS (Quick, Easy, Cheap, Effective, Rugged, Safe)
- Alternative Extraction Procedures for Future Testing in Calliphoridae Specimens
- Dilute-and-Shoot
- Conclusion and Future Perspectives
- Acknowledgement
- Conflict of Interest
- References
QuEChERS (Quick, Easy, Cheap, Effective, Rugged, Safe)
QuEChERS is a miniaturized extractive technique which associates a salting-out assisted liquid-liquid extraction initial step with a dispersive solid-phase extraction (d-SPE) with cleanup purposes, which has allowed the extraction a large number of analytes with different physicochemical characteristics with a high degree of enrichment, elimination of interferences from the matrix, robust, low cost, easy and fast handling [25,74]. Furthermore, by the fact of using small amounts of non-toxic solvents and reagents, may be considered laboratory and environmental safe. Another favorable aspect of this procedure is that there is no mandatory apparatus for its application, allowing it to be used in any laboratory [75]. The search for more sustainable extractive methods has been a recurring theme of research in the academic scenario. In this context, the QuEChERS methodology fits for entomotoxicological analysis purposes. Magni et al. [76] were the first authors to employ a QuEChERS-based extraction method in entomotoxicology. They developed and validated a QuEChERS extraction method followed by GC-MS detection of α- and β-endosulfan (organochlorine insecticide and acaricide) in larva, pupa, empty pupa and adult of Calliphora vomitoria L. The insects were reared on bovine liver substracts spiked with endosulfan concentrations related to the concentrations found in body tissues of humans and animals involved in endosulfan poisoning. They demonstrated that the combined QuEChERS extraction and GC-MS approach provided an adequate method to detect both α- and β-endosulfan in blowfly larvae, pupa, empty pupa and adult, showing for α-endosulfan LOD and LOQ of 0.22ng/mg and 0.73ng/mg respectively and, for β-endosulfan LOD and LOQ of 0.21ng/mg and 0.71ng/mg repectively.
Cox [77] developed and validated a method involving extraction of fentanyl and its metabolites by modified QuEChERS followed by LC-MS/MS determination in in larva, pupa, empty pupa and adult of Lucilia sericata and evaluated the effect of these substances on the biological development of the species. The author found for fentanyl LOD and lower limit of quantification (LLOQ) for both, larval and pupal tissue of 0.1μg/kg for 4-NPP, 0.4μg/kg for ß-hydroxyfentanyl, 0.1μg/kg for fentanyl and 0.5μg/ kg for norfentanyl.
Cranston [78] developed and validated a method involving extraction of fentanyl and its metabolites by modified QuEChERS followed by GC-MS determination of ketamine and norketamine in larval tissue of Sarcophaga bullata and evaluated the effect of these substances on the biological development of the species. Analysis of the larval samples proved that both ketamine and norketamine extracted using QuEChERS and analyzed using GCMS could be successfully detected. LOD and LOQ for ketamine were 58.13 and 193.76 ppb respectively and for norketamine were 82.51 and 275.05 ppb respectively. The researchers found employing the QuEChERS methodology for entomotoxicology determinations showed interesting outcomes and further investigations regarding it must be carried out. Following are the main methodologies applied in forensic entomotoxicology to date for the analysis of specimens from Calliphoridae family (Table 1).
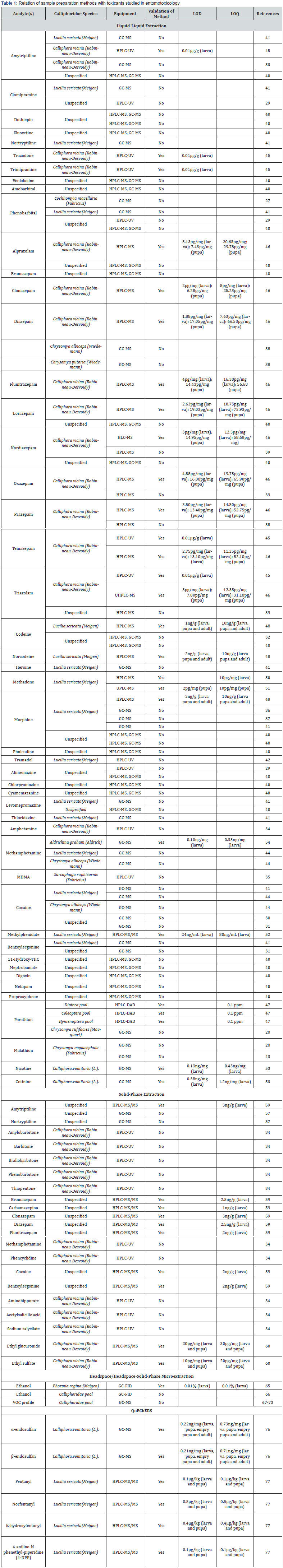
GC-FID, Gas chromatography with flame ionization detector; GC-FID, Gas chromatography coupled to mass spectrometry; HPLC-DAD, high performance liquid chromatography with diode array detector; HPLC-MS, high performance liquid chromatography coupled to mass spectrometry; HPLC-MS/MS, high performance liquid chromatography coupled to tandem mass spectrometry HPLC-UV, high performance liquid chromatography with ultraviolet; detector LOD, limit of detection; LOQ, limit of quantification; UPLC-MS, ultra-performance liquid chromatography coupled to mass spectrometry; VOC, volatile organic compounds.
- Review Article
- Abstract
- Introduction
- Sample Preparation Techniques
- QuEChERS (Quick, Easy, Cheap, Effective, Rugged, Safe)
- Alternative Extraction Procedures for Future Testing in Calliphoridae Specimens
- Dilute-and-Shoot
- Conclusion and Future Perspectives
- Acknowledgement
- Conflict of Interest
- References
Alternative Extraction Procedures for Future Testing in Calliphoridae Specimens
The most widely used and commonly accepted classical extraction techniques (CETs) are LLE and SPE. The use of CETs as a sample preparation method tends to be slow, laborious and limited to the use of relatively high amounts of environmentally harmful solvents and, sometimes, to present low extractive efficiencies [24]. To overcome the drawbacks of CETs, several novel microextraction techniques with faster, cheaper, and “greener” pretreatment of complex samples has been proposed [24]. Follows a brief mention of some possible methods with this new proposal that could be applied to forensic entomotoxicology by its simplicity and compatibility with the new approach purposes.
Supported liquid extraction (SLE)
SLE is an old technique that has gained prominence and increased use for being simple and providing adequate sample cleaning, resulting in small matrix effect. In this method, the extraction is performed with an appropriate solvent (liquid), but a solid medium is used as a support for the liquid sample. The solid support used does not interact with the analyte, unlike the solid phase used in the SPE, which acts by selectively retaining the intended analyte [82-84] successfully employed SLE to extract analytes of interest from larvae taken from the human body, without generate information about the method validation.
Dispersive liquid-liquid microextraction (DLLME)
Currently, one of the most used techniques for the extraction of analytes in biological material is the DLLME proposed by Razaee and collaborators in 2006 [85]. The principle is based on the mixture of the biological sample with an extractor solvent (organic solvent) that when adding a dispersing solvent (miscible in the solvent extractor), form droplets of the organic solvent wholly dispersed in the aqueous phase, increasing the contact area, thus extracting the analyte, for having more affinity for the apolar solvent [86]. DLLME can also be used for pre-concentration of the desired analyte in cases such as extraction of organic analytes such as pharmaceuticals, amines, phenols and others in aqueous samples and food. To achieve the technique’s maximum yield, some factors such as sample pH, ionic strength, the polarity of the solvent extractor, solvent volume and extraction time must be evaluated. This extractive technique allows the application of environmentally friendly solvents, is considered a Green Chemistry procedure and can be used in any laboratory for forensic analysis of being fast, efficient and low cost [86-88]. However, until this moment, no studies on its use in entomotoxicological analysis with Calliphoridae larvae were found in the scientific literature.
- Review Article
- Abstract
- Introduction
- Sample Preparation Techniques
- QuEChERS (Quick, Easy, Cheap, Effective, Rugged, Safe)
- Alternative Extraction Procedures for Future Testing in Calliphoridae Specimens
- Dilute-and-Shoot
- Conclusion and Future Perspectives
- Acknowledgement
- Conflict of Interest
- References
Dilute-and-Shoot
The technique known as dilute-and-shoot refers to the simple dilution of the sample matrix with a suitable solvent for subsequent instrumental analysis. Depending on the matrix to be analyzed, which may include biological matrices such as urine, sweat and saliva, among others, the development of the technique is possible. However, in more complex matrices, such as serum, milk or plasma, previous steps are required to prepare the sample itself [89].Dilute-and-shoot technique simply aims to reduce the effects of the matrix, not eliminating undesirable co-extractors, qualifying itself as a good alternative because it reduces the total sample preparation time, qualifying as a technique with a “Green” bias, given its reduced use of reagents and supplies and does not require specific instrumentation for its performance. However, its biggest bottleneck occurs in the need for a selective analysis and in samples with trace-level concentrations of the target analyte [90].
Furthermore, according Sulyok et al. [91], in addition to being a technique with sustainable prerogative, the simplicity of execution of the method allows an effective application in routine analyzes with high demand. Hence, has been applied in several types of analytes, such as drugs of abuse, forensics and food safety, and can be used across a range of “omics” studies such as metabolomics. However, there are some difficulties with regard to obtaining reproducibility, accuracy, precision and reproducibility of the technique, especially in multiresidue analyses, which demands further investigations. Until this moment, there are no studies on its use in entomotoxicological analysis with Calliphoridae larvae in the scientific literature.
- Review Article
- Abstract
- Introduction
- Sample Preparation Techniques
- QuEChERS (Quick, Easy, Cheap, Effective, Rugged, Safe)
- Alternative Extraction Procedures for Future Testing in Calliphoridae Specimens
- Dilute-and-Shoot
- Conclusion and Future Perspectives
- Acknowledgement
- Conflict of Interest
- References
Conclusion and Future Perspectives
The development of studies in forensic entomotoxicology has increased over time, they are still viewed with skeptical eyes by some researchers, especially in quantitative determinations, in a context where the correlation between the presence of the toxicant responsible for the death in the necrophagous arthropods has not yet been completely related to the actual concentrations administered. The lack of a complete standardization of the entomotoxicological approach, mainly for CETs, allowing that microextraction-based techniques to become an even greater challenge for arthropod–like matrix, especially in specimens from Calliphoridae family, the first specimen and major constituent of cadaveric fauna. So, the development of sample preparation techniques with more sustainable approaches, such as reduced use of solvents, added to the increase of the powerfulness of analytical instrumental techniques should be encourage in order to improve the forensic entomotoxicology approach.
- Review Article
- Abstract
- Introduction
- Sample Preparation Techniques
- QuEChERS (Quick, Easy, Cheap, Effective, Rugged, Safe)
- Alternative Extraction Procedures for Future Testing in Calliphoridae Specimens
- Dilute-and-Shoot
- Conclusion and Future Perspectives
- Acknowledgement
- Conflict of Interest
- References
Acknowledgement
We would like to acknowledge CAPES for the master’s scholarships and CNPq as well as FAPERGS for scientific initiation scholarships for undergraduate students.
- Review Article
- Abstract
- Introduction
- Sample Preparation Techniques
- QuEChERS (Quick, Easy, Cheap, Effective, Rugged, Safe)
- Alternative Extraction Procedures for Future Testing in Calliphoridae Specimens
- Dilute-and-Shoot
- Conclusion and Future Perspectives
- Acknowledgement
- Conflict of Interest
- References
Conflict of Interest
We declare no conflict of interest.
- Review Article
- Abstract
- Introduction
- Sample Preparation Techniques
- QuEChERS (Quick, Easy, Cheap, Effective, Rugged, Safe)
- Alternative Extraction Procedures for Future Testing in Calliphoridae Specimens
- Dilute-and-Shoot
- Conclusion and Future Perspectives
- Acknowledgement
- Conflict of Interest
- References
References
- Goff ML, Brown WA, Omori AI, Lapointe DA (1994) Preliminary observations of the effects of phencyclidine in decomposing tissues on the development of Parasarcophaga ruficornis (Diptera: Sarcophagidae). J Forensic Sci 39(1): 123-128.
- Introna F, Campobasso CP, Goff ML (2001a) Entomotoxicology. Forensic Sci Int 120(1-2): 42-47.
- Campobasso CP, Bugelli V, Carfora A, Borriello R, Villet MH (2019) Advances in entomotoxicology. Weaknesses and strengths, Forensic Entomology: The Utility of Arthropods in Legal Investigations, CRC Press, Boca Raton, pp. 305-330.
- da Silva EIT, Wilhelmi B, Villet, MH (2017) Forensic entomotoxicology revisited-Towards professional standardisation of study designs. Int J Leg Med 131(5): 1399-1412.
- Chophi R, Sharma S, Sharma S, Singh R (2019) Forensic entomotoxicology: Current concepts, trends and challenges. J Forensic Leg Med 67: 28-36.
- Pawliszyn J, Lord HL (2010) Sample preparation handbook. Wiley, USA, p. 504.
- Vargas JE, Wood DM (2010) Calliphoridae, Manual of Central American Diptera, Ontario, NCR Research Press, Canada, pp. 1297-1300.
- Skevington JH, Dang PT (2002) Exploring the diversity of flies (Diptera). Biodiversity 3(4): 3-27.
- Buzzi JZ (1994) Coletânea de nomes populares de insetos do Brasil, edição do autor, Curitiba, Paraná, pp. 230.
- Lenko K, Papavero N (1996) Insetos no Folclore, Manual of Neartic Diptera, Ottawa, Monograph/Agriculture Canada, pp. 657.
- Rognes K (1997) The Calliphoridae (blowflies) (Diptera: Oestridea) are not a monophyletic group. Cladistics. Columbia, US, 13: 27-66.
- Kosmann C, Mello RP, Harterreiten-Souza ES, Pujol-Luz JR (2013) A List of Current Valid Blow Fly Names (Diptera: Calliphoridae) in the Americas South of Mexico with Key to the Brazilian Species. Entomo Brasilis 6(1): 74-85.
- Serra-Freire NM, Mello RP (2006) Entomologia & acarologia na medicina veteriná Rio de Janeiro, p. 200.
- Dillmann JB, Lopes T, da Rosa G, Fracasso M, Tapia Barraza VC, et al. (2022) Safety and efficacy of Lucilia cuprina maggots on treating an induced infected wound in Wistar rats. Experimental parasitology 240: 108337.
- Stevens JR, Wallman JF (2006) The evolution of Myiasis in humans and other animals in the Old and New Worlds (part I): phylogenetic analyses. Trends in parasitology 22(3): 129-136.
- Guimarães JH, Papavero N (1999) Myiasis in man animals in the neotropical region. São Paulo, Pleiade/FAPESP, pp. 15-18.
- Fleischmann W, Grassberger M, Sherman R (2004) Maggot Therapy: A Handbook of Maggot-assisted Wound Healing. Editora Thieme, p. 85.
- Caleffe RRT, Oliveira SR, de Polonio JC, Daquila BV, Ruvolo-Takasusuki MCC, et al. (2021) Bioprospecção de peptídeos antimicrobianos em larvas de Calliphoridae (Diptera): uma revisão sistemática sobre metodologias de extração, purificação e detecçã Saúde (Santa Maria), p. 47.
- Garzon LR, Fracasso M, Viana AR, Giacometi M, Samoel GVA, et al. (2021) Atividade in vitro activity of larval secretions from Lucilia cuprina against Leishmania amazonensis, Trypanosoma cruzi and cell lines. Brazilian Journal of Development 7(8): 82837-82858.
- Wells JD, Paper T, Sperling FAH (2001) DNA-based identification and molecular systematics of forensically important Sarcophagidae (Diptera). J Forensic Sci 46(5): 1098-1102.
- Ghosh S, Ansar W, Banerjee D (2017) Diagnosis of crime reporter flies in forensic entomology: a review. Indian Journal of Entomology, p. 79.
- Prado e Castro C, Ameixa OMCC (2021) Blow flies (Diptera: Calliphoridae) promising candidates as animal feed ingredientes. Journal of Insects as Food and Feed 7(7): 1065-1076.
- Aly AA, Górecki T (2020) Green approaches to sample preparation based on extraction techniques. Molecules 25(7): 1719.
- Câmara JS, Perestrelo R, Berenguer CV, Andrade CV, Gomes TM, et al. (2022) Green Extraction Techniques as Advanced Sample Preparation Approaches in Biological, Food, and Environmental Matrices: A Review. Molecules 27(9): 2953.
- Poole CF (2019) Handbooks in Separation Science: Liquid-phase extraction. Elsevier, p. 796.
- Neves HP, Ferreira GMD, Ferreira GMD, Lemos LR, Rodrigues GD, et al. (2022) Liquid-liquid extraction of rare earth elements using systems that are more environmentally friendly: Advances, challenges and perspectives. Separation and Purification Technology 282: 120064.
- Beyer JC, Enos WF, Stajic M (1980) Drug identification through analysis of maggots. J Forensic Sci 25(2): 411-412.
- Gunatilake K, Goff ML (1989) Detection of organophosphate poisoning in a putrefying body by analyzing arthropod larvae. J Forensic Sci 34(3): 714-716.
- Kintz P, Godelar B, Tracqui A, Mangin P, Lugnier AA, et al. (1990) Fly larvae: A new toxicological method of investigation in forensic medicine. J Forensic Sci 35(1): 204-207.
- Manhoff DT, Hood I, Caputo F, Perry J, Rosen S, et al. (1991) Cocaine in decomposed human remains. J Forensic Sci 36(6): 1732-1735.
- Nolte KB, Pinder RD, Lord WD (1992) Insect larvae used to detect cocaine poisoning in a decomposed body. J Forensic Sci 37: 1179-1185.
- Kintz P, Tracqui A, Mangin P (1994) Analysis of opiates in fly larvae sampled on a putrefied cadaver. J Forensic Sci Soc 34(2): 95-97.
- Sadler DW, Richardson J, Haigh S, Bruce G, Pounder DJ (1997) Amitriptyline accumulation and elimination in Calliphora vicina larvae. Am J Forensic Med Pathol 18(4): 397-403.
- Sadler DW, Robertson L, Brown G, Fuke C, Pounder DJ (1997) Barbiturates and analgesics in Calliphora vicina larvae. J Forensic Sci 42(6): 1214-1215.
- Goff ML, Miller ML, Paulson JL, Lord WD, Richards E, et al. (1997) Effects of 3,4-methylenedioxymethamphetamine in decomposing tissues on the development of Parasarcophaga ruficornis (Diptera: Sarcophagidae) and detection of the drug in postmortem blood, liver tissue, larvae, and puparia. J Forensic Sci 42(2): 276-280.
- Bourel B, Hèdouin V, Martin-Bouyer L, Bècart A, Tournel G, et al. (1999) Effects of morphine in decomposing bodies on the development of Lucilia sericata (Diptera: Calliphoridae). J Forensic Sci 44(2): 354-358.
- Hedouin V, Bourel B, Martin-Bouyer L, Becart A, Tournel G, et al. (1999) Determination of drug levels in larvae of Lucilia sericata (Diptera: Calliphoridae) reared on rabbit carcasses containing morphine. J Forensic Sci 44(2): 351-353.
- Carvalho LML, Linhares AX, Trigo JR (2001) Determination of drug levels and the effect of diazepam on the growth of necrophagous flies of forensic importance in southeastern Brazil. Forensic Sci Int 120(1-2): 140-144.
- Pien K, Laloup M, Pipeleers-Marichal M, Grootaert P, De Boeck G, et al. (2004) Toxicological data and growth characteristics of single postfeeding larvae and puparia of Calliphora vicina (Diptera: Calliphoridae) obtained from a controled nordiazepam study. Int J Legal Med 118(4):190-193.
- Tracqui A, Keyser-Tracqui C, Kintz P, Ludes B (2004) Entomotoxicology for the forensic toxicologist: much ado about nothing? Int J Legal Med 118(4): 194-196.
- Campobasso CP, Gherardi M, Caligara M, Sironi L, Introna F (2004) Drug analysis in blowfly larvae and in human tissues: A comparative study. Int J Legal Med 118(4): 210-214.
- El-Samad LM, El-Moaty ZA, Makemer HM (2011) Effects of tramadol on the development of Lucilia sericata (Diptera: Calliphoridae) and detection of the drug concentration in postmortem rabbit tissues and larvae. J Entomol 8: 353-364.
- Bakr R, Ramadan R, El-Sawy S, Hussien S (2012) Ultrastructure of the midgut of the third larval instar of Chrysomya megacephala (Diptera: Calliphoridae) fed on malathion treated diet. Egypt Acad J Biol Sci 3(1): 13-26.
- Bugelli V, Forni D, Bassi LA, Di Paolo M, Marra D, et al. (2014) Forensic entomology and the estimation of the minimum time since death in indoor cases. J Forensic Sci 60(2): 525-531.
- Sadler DW, Fuke C, Court F, Pounder DJ (1995) Drug accumulation and elimination in Calliphora vicina larvae. Forensic Sci Int 71(3): 191-197.
- Wood M, Laloup M, Pien K, Samyn N, Morris M, et al. (2003) Development of a rapid and sensitive method for the quantification of benzodiazepines in Calliphora vicina larvae and puparia by LC–MS-MS. J Anal Toxicol 27(7): 505-512.
- Wolff M, Builes A, Zapata G, Morales G, Benecke M (2004) Detection of parathion (O, O-diethyl-O-(4-nitrophenyl) phosphorothioate) by HPLC in insects of forensic importance in Medellin, Colombia. Anil Aggrawal’s Internet J Forensic Med Toxicol 5: 6-11.
- Kharbouche H, Augsburger M, Cherix D, Sporkert F, Giroud C, et al. (2008) Codeine accumulation and elimination in larvae, pupae, and imago of the blowfly Lucilia sericata and effects on its development. Int J Legal Med 122(3): 205-211.
- Liu X, Shi Y, Wang H, Zhang R (2009) Determination of Malathion levels and its effect on the development of Chrysomya megacephala (Fabricius) in South China. Forensic Sci Int 192(1-3): 14-18.
- Gosselin M, Ramirez-Fernandez MdelM, Wille SM, Samyn N, De Boeck G, et al. (2010) Quantification of methadone and its metabolite 2-ethylidene-1,5-dimethyl-3,3-diphenylpyrrolidine in third instar larvae of Lucilia sericata (Diptera: Calliphoridae) using liquid chromatography–tandem mass spectrometry. J Anal Toxicol 34(7): 374-380.
- Gosselin M, Di Fazio V, Wille SM, Fernandez MdelM, Samyna N, et al. (2011a) Methadone determination in puparia and its effect on the development of Lucilia sericata (Diptera, Calliphoridae). Forensic Sci Int 209(1-3): 154-159.
- Bushby SK, Thomas N, Priemel PA, Coulter CV, Rades T, et al. (2012) Determination of methylphenidate in Calliphorid larvae by liquid–liquid extraction and liquid chromatography mass spectrometry-Forensic entomotoxicology using an in vivo rat brain model. J Pharm Biom Anal 70: 456-461.
- Magni PA, Pazzi M, Vincenti M, Alladio E, Brandimarte M, et al. (2016) Development and validation of a GC-MS method for nicotine detection in Calliphora vomitoria (L.) (Diptera Calliphoridae). Forensic Sci Int 261: 53-60.
- Wang S, Zhang C, Chen W, Ren L, Ling J, et al. (2020) Effects of Methamphetamine on the Development and Its Determination in Aldrichina grahami (Diptera: Calliphoridae). J Med Entomol 57(3): 691-696.
- Lanças FM (2004) Extração em Fase Sólida (SPE). São Carlos: RiMa, p. 96.
- Robinson BBA (2019) Determination of fentanyl qualification and quantification in Phormia regina (Meigen) (Calliphoridae) using LC-MS/MS. State University of New York at Oswego, USA.
- Miller ML, Lord WD, Goff ML, Donnely B, Mcdonough ET, et al. (1994) Isolation of amitriptyline and nortriptyline from fly puparia (Phoridae) and beetle exuviae (Dermestidae) with mummified human remains. J Forensic Sci 39: 1305-1313.
- Rashid RA, Osman K, Ismail MI, Zuha RM, Hassan RA (2008) Determination of malathion levels and the effect of malathion on the growth of Chrysomya megacephala (Fabricius) in malathion-exposed rat carcass. Trop Biomed 25(3): 184-190.
- de Aguiar FJ, Brandao M, Sodre FF, Caldas ED (2014) Simultaneous determination of prescription drugs, cocaine, aldicarb and metabolites in larvae from decomposed corpses by LC-MS-MS after solid-liquid extraction with low temperature partitioning. Forensic Toxicol 33: 93-103.
- Lambiase S, Groppi A, Gemmellaro D, Morini L (2017) Evaluation of ethyl glucuronide and ethyl sulfate in Calliphora vicina as potential biomarkers for ethanol intake. J Anal Toxicol 41(1): 17-21.
- Wenzl T, Lankmayr EP (2001) Effect of the water content of cardboard on the static headspace extraction of volatile analytes. J Sep Sci 24(10-11): 885-888.
- Cavalli JF, Fernandez X, Lizzani-Cuvelier L, Loiseau A-M (2003) Comparison of static headspace, headspace solid phase microextraction, headspace sorptive extraction, and direct thermal desorption techniques on chemical composition of French olive oils. J Agric Food Chem 51(26): 7709-7716.
- Arthur CL, Pawliszyn J (1990) Solid phase microextraction with thermal desorption using fused silica optical fibers. Anal Chem 62(19): 2145-2148.
- Risticevic S, Lord H, Górecki T, Arthur CL, Pawliszyn J (2010) Protocol for solid phase microextraction method development. Nature Protocols 5: 122-139.
- Tabor K, Fell RD, Brewster CC, Pelzer K, Behonick GS (2005) Effects of antemortem ingestion of ethanol on insect successional patterns and development of Phormia regina (Diptera: Calliphoridae). J Med Entomol 42: 481-489.
- Definis-Gojanovic M, Sutlovic D, Britvic D, Boze K (2007) Drug analysis in necrophagous flies and human tissues. Arh Hig Rada Toksikol 58(3): 313-316.
- Ye G, Li K, Zhu J, Zhu G, Hu C (2007) Cuticular hydrocarbon composition in pupal exuviae for taxonomic differentiation of six necrophagous flies. J Med Entomol 44(3): 450-456.
- Frederickx C, Dekeirsschieter J, Brostaux Y, Wathelet JP, Verheggen FJ, et al. (2012) Volatile organic compounds released by blowfly larvae and pupae: new perspectives in forensic entomology. Forensic Sci Int 219(1-3): 215-220.
- Zhu G, Yu XJ, Xie LX, Luo H, Wang D, et al. (2013) Time of death revealed by hydrocarbons of empty puparia of Chrysomya megacephala (Fabricius) (Diptera: Calliphoridae): a field experiment. PLoS ONE 8(9): e73043.
- Frere B, Suchaud F, Bernier G, Cottin F, Vincent B, et al. (2014) GC-MS analysis of cuticular lipids in recent and older scavenger insect puparia. An approach to estimate the postmortem interval (PMI). Anal Bioanal Chem 406(4): 1081-1088.
- Zhu GH, Jia ZJ, Yu XJ, Wu KS, Wu LS, et al. (2017) Predictable weathering of puparial hydrocarbons of necrophagous flies for determining the postmortem interval: a field experiment using Chrysomya rufifacies. Int J Legal Med 131(3): 885-894.
- Lunas BM, Paula MC, Michelutti KB, Lima-Junior SE, Antonialli-Junior WF, et al. (2019) Hydrocarbon and fatty acid composition from blowfly eggs represents a potential complementary taxonomic tool of forensic importance, J Forensic Sci 64(6): 1720-1725.
- Blanar K, Prada-Tiedemann PA (2020) Characterization of the volatile odor profile from larval masses in a field decomposition setting. Forens Chem 21: 100288.
- Anastassiades M, Lehota S, Stajnbaher D, Schenck FJJ (2003) Fast and Easy Multiresidue Method Employing Acetonitrile Extraction/Partitioning and “Dispersive Solid-Phase Extraction” for the Determination of Pesticide Residues in Produce. J AOAC Int 86(2): 412-431.
- Musarurwa H, Chimuka L, Pakade VE, Tavengwa NT (2019) Recent developments and applications of QuEChERS based techniques on food samples during pesticide analysis. J Food Compost Anal 84: 103314.
- Magni PA, Pazzi M, Droghi J, Vincenti M, Dadour IR (2018) Development and validation of an HPLC-MS/MS method for the detection of ketamine in Calliphora vomitoria (L.) (Diptera: Calliphoridae). J Forensic Leg Med 58: 64-71.
- Cox JA (2021) Quantitation of Fentanyl and Metabolites from Blow Fly Tissue and Development Effects of Fentanyl on Lucilia sericata. Graduate Theses, Dissertations, and Problem Reports, p. 10276.
- Cranston CM (2020) Bugs and Drugs: Ketamine Detection from Necrophagous Insects using Gas Chromatography-Mass Spectrometry. Graduate Theses.
- Bairros AV, Lanaro R, Almeida RM, Yonamine M (2014) Determination of ketamine, norketamine and dehydronorketamine in urine by hollow-fiber liquid-phase microextraction using an essential oil as supported liquid membrane. Forensic Science International 243: 47-54.
- Wan L, Lin B, Zhu R, Huang C, Pedersen-Bjergaard S, et al. (2019) Liquid-Phase Microextraction or Electromembrane Extraction? Analytical Chemistry 91: 8267-8273.
- Hansen F, Oiestad EL, Pedersen-bjergaard S (2020) Bioanalysis of pharmaceuticals using liquid-phase microextractioncombined with liquid chromatography–mass spectrometry. Journal of Pharmaceutical and Biomedical Analysis 189: 113446.
- Silvestro L, Savu SR (2015) An update on solid phase-supported liquid extraction. Bioanalysis 7(17): 2177-2186.
- Wilson Z, Hubbard S, Pounder DJ (1993) Drug analysis in fly larvae. Am J Forensic Med Pathol 14(2): 118-120.
- Levine B, Golle M, Smialek J (2000) An unusual drug death involving maggots. Am J Forensic Med Pathol 21(1): 59-61.
- Rezaee M, Assadi Y, Hosseini M-RM, Aghaee E, Ahmadi F (2006) Determination of organic compounds in water using dispersive liquid–liquid microextraction. Journal of Chromatography A 1116(1-2):1-9.
- Martins M, Primel E, Caldas S, Prestes O, Adaime M (2012) Microextração Líquido-Líquido Dispersiva (DLLME): fundamentos e aplicações. Scientia Chromatographica 4(1): 35-51.
- Moreira B, Yokoya J, Gaitani C (2014) Microextração líquido-líquido dispersiva (DLLME): fundamentos, inovações e aplicações biológicas. Scientia Chromatographica 6(3): 186-204.
- Ramin M, Khadem M, Omidi F, Pourhosein M, Golbabei F, et al. (2019) Optimization of dispersive liquid-liquid microextraction procedure for detecting chlorpyrifos in human urine samples. Med J Islam Repub Iran 71: 20-33.
- Greer B, Chevallier O, Quinn B, Botana LM, Elliott CT (2021) Redefining dilute and shoot: The evolution of the technique and its application in the analysis of foods and biological matrices by liquid chromatography mass spectrometry. Trends in Analytical Chemistry 141: 116284.
- Malachova A, Sulyok M, Beltran E, Berthiller F, Krska R (2015) Multi-Toxin Determination in Food -The Power of "Dilute and Shoot" Approaches in LC-MS-MS. LC-GC Europe 28: 542-555.
- Sulyok M, Stadler D, Steiner D, Krska R (2020) Validation of an LC-MS/MS-based dilute-and-shoot approach for the quantification of > 500 mycotoxins and other secondary metabolites in food crops: challenges and solutions. Analytical and Bioanalytical Chemistry 412(11): 2607-2620.






























