Application of GastroPlusTM Modeling in Product Development: A Case Study for a Low-Dose Poorly Water-Soluble Compound
Jinjiang Li*, Balvinder Vig and Anisha Mendonza
Drug Product Science and Technology, Bristol-Myers Squibb Corporation, USA
Submission: August 01, 2022; Published: August 22, 2022
*Corresponding author: Jinjiang Li, Drug Product Science and Technology, Bristol-Myers Squibb Corporation, New Brunswick, USA
How to cite this article:Jinjiang L, Balvinder V, Anisha M. Residual Application of GastroPlusTM Modeling in Product Development: A Case Study for a 002 Low-Dose Poorly Water-Soluble Compound.Glob J Pharmaceu Sci. 2022; 10(2): 555781. DOI: 10.19080/GJPPS.2022.10.555781.
Abstract
In this paper, GastroPlus™ modelling was applied to the development of a low-dose poorly water-soluble compound. The initial input parameters for simulation of solution were based on solubility, log D, permeability, and the dose together with dose volume of 250 ml. Typical physiology input parameters were used in simulation. Simulated curves were compared with clinical data for solution, and input parameters were optimized. Using the optimized input parameters from solution, simulated AUC curves of tablets of various strength (1mg, 3mg, and 5mg) were obtained and compared with solution AUC curves as well as clinical AUV curves, from which PK parameters were derived. Predictability of absorption of various dose was discussed for both tablet and solution. Furthermore, impact of dose, pH, and food effect on absorption was explored based on the simulated data. Overall, this case study demonstrated the use of GastroPlus™ in the drug development for a low-dose compound.
Keywords: GastroPlus™; Tablet; Absorption; Predictability; Dose effect; pH effect; Food effect
Abbreviations: BCS: Biopharmaceutical Classification System; PK: Pharmacokinetic; PAMPA: Parallel Artificial Membrane Permeability Assay; FIH: First-In-Human; AUC: Area Under Curve; PVDF: Polyvinylidene Fluoride; ASF: Absorption Scaling Factor
Introduction
Pharmacokinetic (PK) modelling plays increasingly important roles in developing drug products [1]. Especially, when physiology-based PK modelling is combined with understanding of disease states (pharmacodynamic), GastroPlusTM modelling can significantly expedite product development and consequently result in considerable saving of development cost [2,3]. For instance, PK modelling has been shown to increase the success rate of drug candidates [4,5]. Specifically, to support and accelerate clinical studies, PK modelling are used to predict bioavailability in human and provide feedback for improving product design and process development; for example, the effect of particle size and thereby dissolution on drug bioavailability can be modelled with GastroPlusTM to greatly accelerate product development [6]. In this paper, we would like to use a case study to exemplify the usage of GastroPlusTM modelling in the development of a pharmaceutical compound (BMS-820836), through which the impact of PK modelling on the product development, correlating the predicted bioavailability with clinical data, will be demonstrated [7]. BMS-820836, a triple-reuptake inhibitor for serotonin, norepinephrine and dopamine, was developed for treating depression [8,9]. Although the development of this compound was discontinued because of market competition from generic Cymbalta, the development experience of using PK modelling to ameliorate product development can provided an interesting lesson for future development of similar compounds.
BMS-820836 is a weak base with pKa values of 7.8 and 4.9. Because BMS-820836 is a weak base, its aqueous solubility is strongly pH dependent: ∼1μg/mL around pH 7.0 at room temperature and increasing significantly in acidic (gastric) medium. Additionally, BMS-820836 is highly permeable, as confirmed by high parallel artificial membrane permeability assay (PAMPA): 1025nm/s at pH 5.5 and 1106nm/s at pH 7.4, along with significant absolute bioavailability from animals (rat, dog, and cyno). Therefore, BMS-820836 is a biopharmaceutical classification system (BCS) I/II compound, within the projected dose range (20μg-5mg) [10]. Since the starting dose for BMS-820836 is very low, a solution formulation was used to support the first-in-human (FIH) study. However, for later clinical trials, tablets developed by spraying solutions of the dissolved BMS-820836 onto a common tableting powder blend were used. Because FIH was conducted using the solution formulation, a bridging study between the solution formulation and tablets was conducted to their bioavailability. Additionally, both the bioavailability of the solution formulation and tablets was simulated using GastroPlusTM simulation was and compared with clinical data [11].
In this paper, the results of GastroPlusTM prediction of the bioavailability, including PK parameters, for BMS-820836 solution and tablets will be presented, along with clinical data. Default physiology-based parameters were used in the initial simulation of BMS-820836 solution formulation, followed by parameter optimization based on the clinical data. The optimized input parameters were used in simulating tablet bioavailability. For all simulations, pH-solubility profile of BMS-820236, and standard permeability and diffusion parameters were utilized. Furthermore, the effect of gastric pH, doses, as well as food on bioavailability was evaluated. Finally, the predicted bioavailability (area under curve (AUC)) by modelling, together with Cmax and Tmax, were compared with clinical data for their consistency.
Experimental
Materials
Drug substance of BMS-820836 was provided by the department of chemical development of Bristol-Myers Squibb (New Brunswick, NJ). Citric acid monohydrate and sodium citrate were purchased from Sigma-Aldrich Corp. (St. Louis, MO). Peppermint flavor was obtained from Virginia Dare (Brooklyn, NY). Sulfobutyl-β-cyclodextrin (Captisol) was purchased from Cydex Pharmaceuticals Inc. (Ter Lenexa, KS). Other excipients for tablet preparation, including microcrystalline cellulose (PH 102), mannitol (Pearlitol 100SD), pregelatinized starch, and Opadry II white (PVA film based), are acquired from FMC Biopolymers (Philadelphia, PA) and Colorcon Inc. (North Wales, PA), respectively. Lubricant, magnesium stearate, was purchased from Tyco Healthcare Group (Mallinckrodt Division) (St. Louis, MO). Furthermore, chemicals for buffer preparation such as hydrochloric acid, phosphoric acid, and sodium phosphate were obtained from Sigma Aldrich Chemicals (St. Louis, MO). Finally, de-ionized water produced by Mili-Q UV Plus (EMD Millipore) (Billerica, MA) was used for all preparations.
Formulation preparation
pH-solubility profile
To construct a pH-solubility profile, the saturation solubilities of BMS-820836 in the media of various pH values were determined using high performance liquid chromatography (HPLC). In general, excess amount of BMS-820836 was added to aqueous solutions of various pH values in 20ml scintillation vials. Targeted pH values of the media for solubility measurement were adjusted and controlled using hydrochloric acid, citric acid and sodium citrate buffer, as well as phosphate buffer. Then, samples of BMS-820836- 03 suspensions in scintillation vials wrapped with aluminum foil were agitated for 24 hours on a Wrist-Action shaker at room temperature. During this process, excess of solid in each vial was maintained to ensure that solubility equilibrium was reached. The pH of each sample was then measured and recorded after 24 hours. Prior to HPLC analysis, all suspension samples were filtered using 0.45μm Polyvinylidene fluoride (PVDF) membrane filters. All filtrates were analyzed by HPLC after suitable dilution with an acetonitrile-water (1:1) mixture.
Solution formulation development
The solution formulation of BMS-820836 (1mg/mL) for FIH was prepared in a compounding site by injecting 20mL of a liquid vehicle into a scintillation vial of 20mg drug. The liquid vehicle consists of 75% (v/v) of 0.1M citric acid/sodium citrate buffer (pH 3) and 25% (v/v) of Simple Syrup solution with peppermint flavor. The simple syrup solution was prepared by mixing 4 parts commercial simple syrup with 1 part of peppermint flavor solution (v/v). To prepare a reconstitution vehicle (0.1M citric acid buffer), 21g of citric acid monohydrate and 45ml of 1N NaOH were first added into a 1000ml volumetric flask followed by addition of water to 1000mL mark. Then, peppermint flavor was incorporated into the reconstitution vehicle by mixing 750ml of 0.1M citric acid and sodium citrate buffer (pH 3) and 250mL of the prepared Simple Syrup solution. Furthermore, in preparing oral solution at the starting dose 0.025mg/mL (25μg/mL), 1mg/ml solution of BMS-820836 in the reconstitution vehicle was diluted 40 times with 0.1M citric acid and sodium citrate buffer (pH 3). Both vehicles were used as placebos for oral solutions (1mg/ml and 0.025mg/mL), respectively.
Preparation of tablets
common blend with a drug loading of 0.5% (w/w) was used to compress tablets of various strength. The common powder blend was prepared by spraying BMS-820836 solution a powder blend. In preparing a solution of BMS-820836, 63g of citric acid monohydrate was first dissolved in 2960 gram of water to prepare 0.1M citric acid solution. Subsequently, 600g of sulfobutyl-β- cyclodextrin was added to above solution to form a sulfo-butyl-β- cyclodextrin and citric acid solution. After, 75g of API was weighed out and dissolved in the sulfobutyl-β-cyclodextrin and citric acid solution to form the solution of BMS-820836. A powder blend, including microcrystalline cellulose, mannitol, and pre-gel starch, was first prepared by mixing these excipients in a 75L granulator (5 minutes at 100rpm). Subsequently, the common blend with 0.5% (w/w) drug loading was made by spraying the solution with dissolved drug to the powder blend (600mL/min). Having finished spaying, the water was then dried off to a loss-on-drying (LOD) value of <2% after further mixing for a few minutes. The dry blend was mixed magnesium stearate followed by compressing into various strength of tablets (0.25mg, 0.5mg and 1.0mg). Finally, the blend was compressed into tablets using a rotary tablet press and followed by film coating (Opadry II White 85F18422) in a Vector or other coater. These tablets (see Table 1 for composition) were used in bioequivalent and other clinical studies.
GastroPlusTM simulation
GastroPlusTM is a mechanistically based simulation software which can simulate the absorption of both solution formulation and tablets through intravenous and oral dosing absorption to generate pharmacokinetic/pharmacodynamic profiles (AUC) in humans and animals, along with PK parameters [12]. Specifically, GastroPlusTM modelling can model bioavailability of either solution formulation and tablets, according to the physiological model (see Table 2 for default input parameters), as well as solubility, log D, and other related criteria (Table 3).

Simulation for solution formulation
To perform GastroPlusTM simulation, the physical-chemical parameters listed Table 3 were used. Physiology-related parameters for fasted human (Table 2), including pH, transit time, absorption scaling factor (ASF) (from stomach to colon), were used. Other parameters listed (Table 3), including body weight (75kg), dosing volume (250mL), permeability and diffusion constants, and blood/plasma concentration ratio (1), together with two compartment parameters (k12=0.05176 1/h; k21=0.11573 1/h), were utilized as well. After the initial simulation, input simulation parameters were optimized based on matching simulated absorption curves with clinical data. The optimized parameters are listed Table 2, where ASFs were changed for duodenum, jejunum 1, and jejunum 2 (Table 2).
Simulation for tablets
For simulation of BMS-820836 tablets, the optimized simulation parameters (Table 2) derived through matching the simulated curves with clinical data were used. Furthermore, physical-chemical parameters listed in Table 3 were also used in the simulation of tablets as well. The simulated curves, together with PK parameters derived, are used for discussion in this paper.
Bioavailability studies
Clinical absorption of both BMS-820836 solution formulation and tablets was obtained from phase I/II clinical trials. The studies were conducted according to typical phase I and II protocols.
Results and Discussion
In this paper, GastroPlusTM simulation was used to simulate the absorption of solution and tablet formulations and predict the effect of doses, gastric pH, and food on the bioavailability of a poorly water-soluble compound-BMS-820836, thus greatly enabling the formulation and process optimization and development. Consequently, bioavailability as predicted by GastroPlusTM, along with the influence of formulation parameters on tablet bioavailability, was able to guide drug product development to support clinical trials. In the following text, application of GastroPlusTM modelling to evaluation of the effect of gastric pH, food, and dose range on absorption will be presented.
Solution simulation
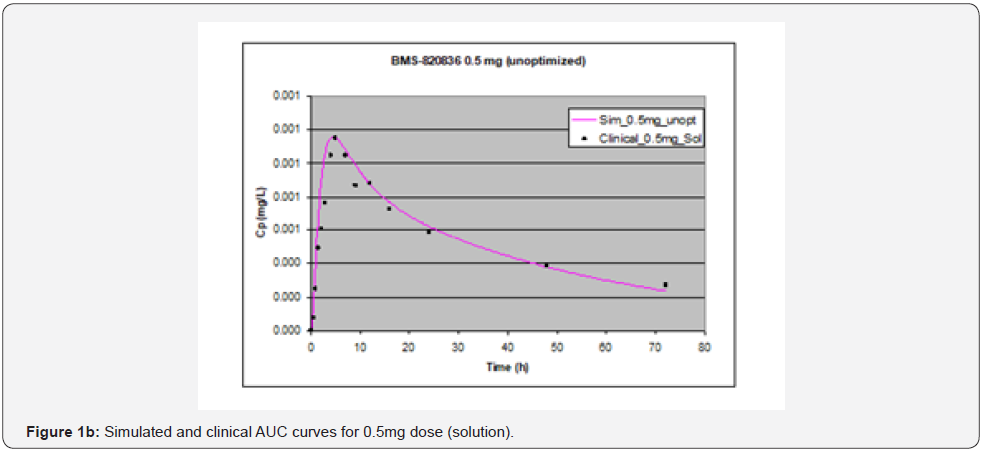
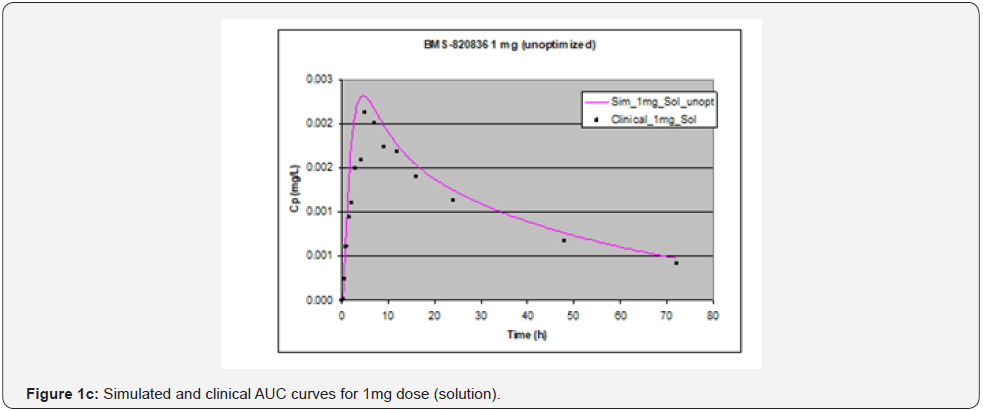
In simulating the bioavailability of the oral solution formulation of BMS-820836 used in FIH, the default physiology-based input parameters (See preoptimized input parameters in Table 2) were used. The simulated plasma concentration versus time curves (PCTC), also known as AUCs, are displayed in Figure 1, where solid curves represent the data from GastroPlusTM prediction, while the scattered lines exhibit the data attained from FIH. In general, the predicted bioavailability curves (AUCs), relative to the clinical data, varies with doses, in which the predicted bioavailability (AUCs) changed from overprediction at doses of 0.1mg to underprediction at doses of 3mg and 5mg, where the predicted curves are higher than those of clinical AUCs at low doses (0.1) and the predicted curves are lower than those of clinical data at high doses (3 and 5mg). So, with the initial default input parameters (preoptimization in Table 2), GastroPlusTM simulation either overpredicted or underpredicted the bioavailability of BMS-820836, depending on doses. To improve the predictability of GastroPlusTM modelling, pH and transit-time of gastrointestinal segments were optimized after matching the simulated curves with clinical AUCs (as shown in Table 2), in which transit time was generally reduced except for that in stomach. To validate the model prediction with the optimized input parameters, GastroPlusTM simulation was performed again, and these simulated curves are shown in Figure 2: Figure 2a (0.1mg), Figure 2b (0.5mg), Figure 2c (1mg), Figure 2d (2mg), Figure 2e (3mg), and Figure 2f (5mg) [13]. These figures display the AUCs of various doses, where clinical data are shown for comparison. As shown in these figures, after optimization, simulated curves are similar to those from clinical studies, which correctly predict in vivo bioavailability of BMS- 820836. Subsequently, these parameters were used to simulate the bioavailability (AUC) of BMS-820836 in tablets. These results will be discussed in the next section.

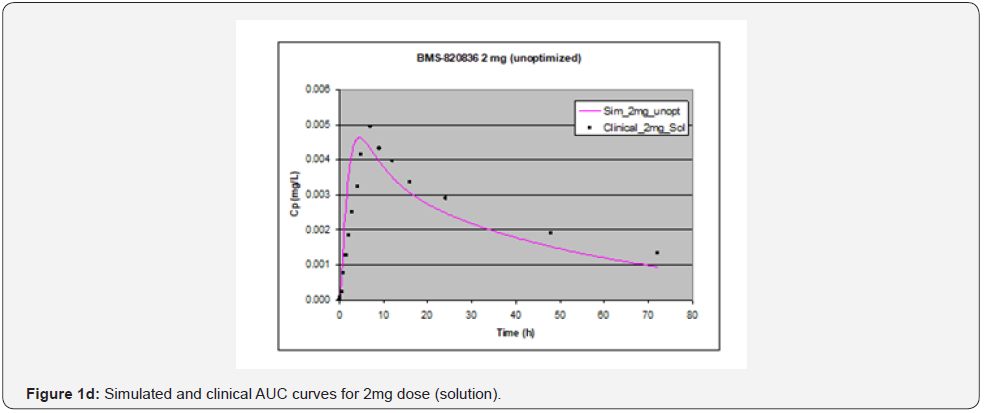

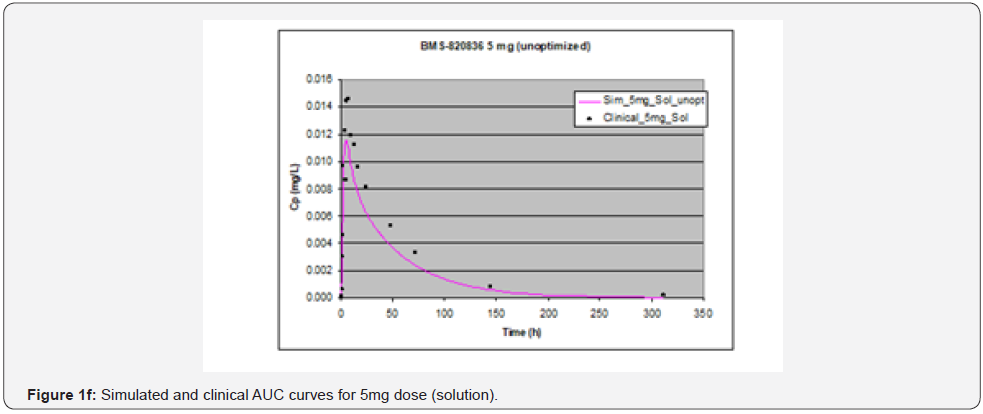
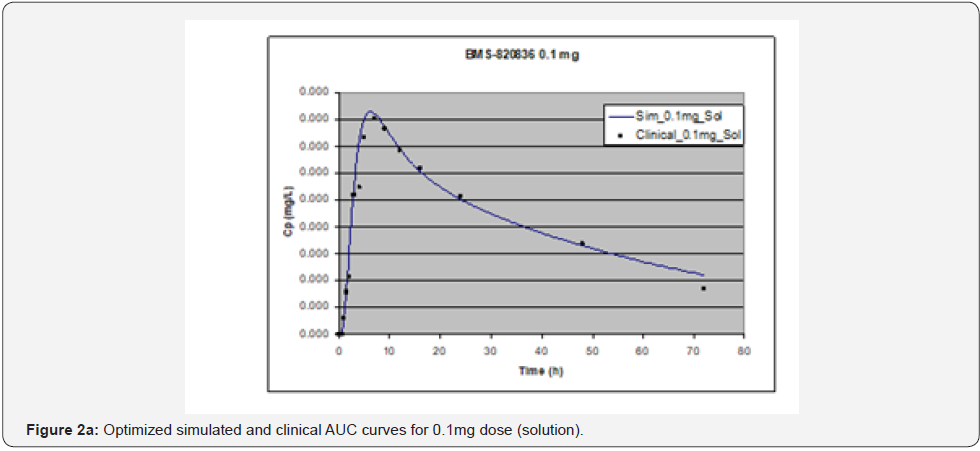
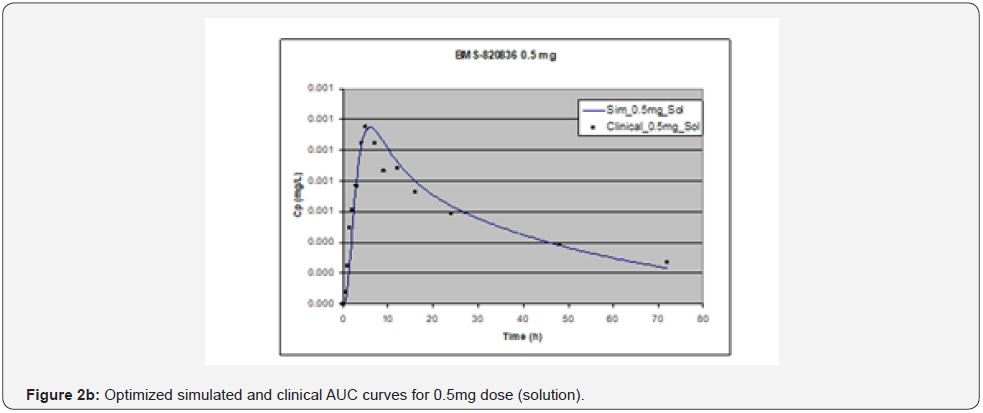
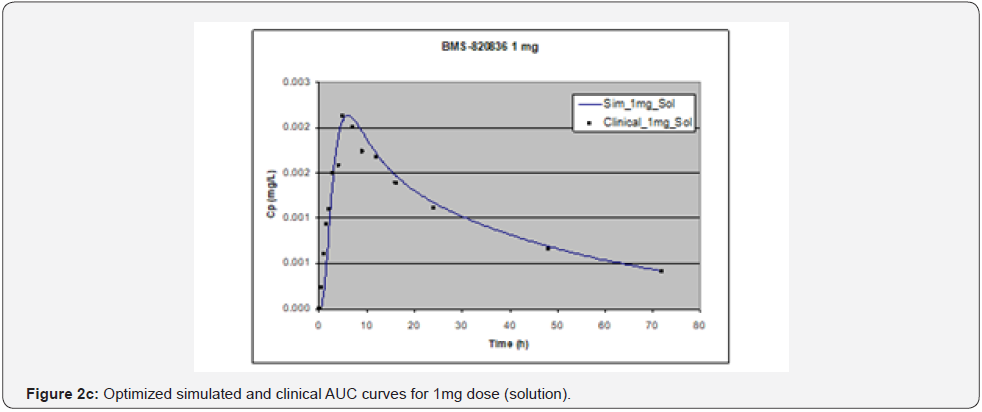
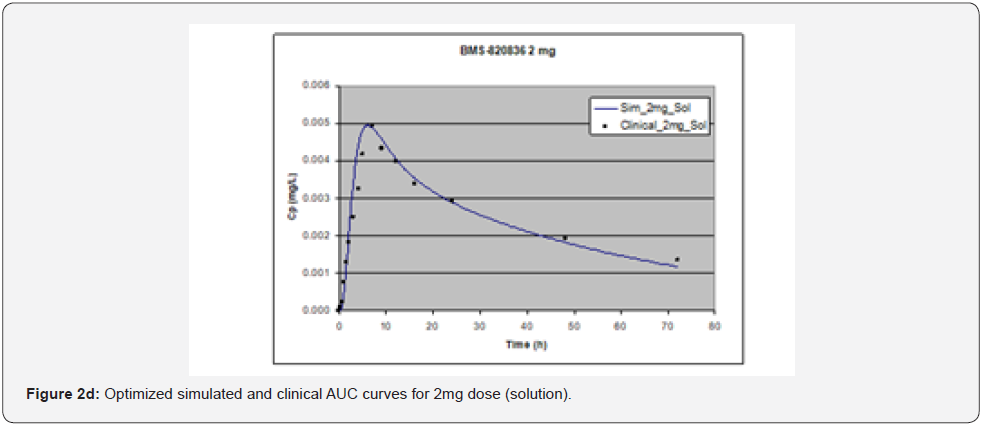
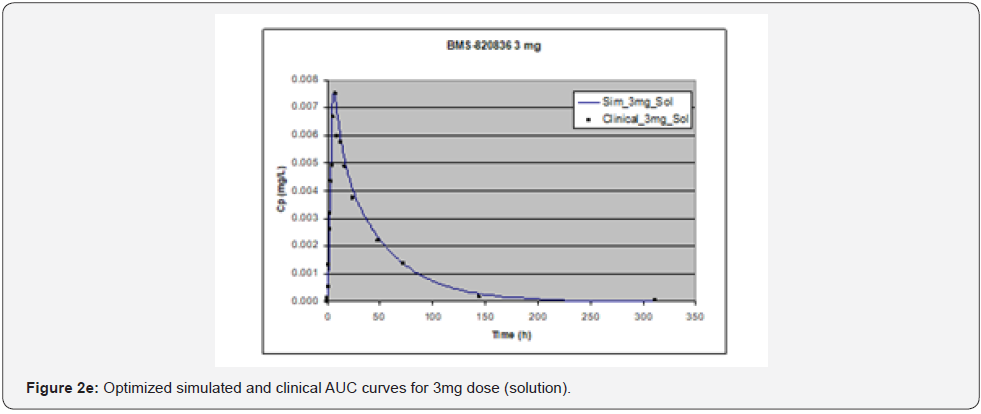
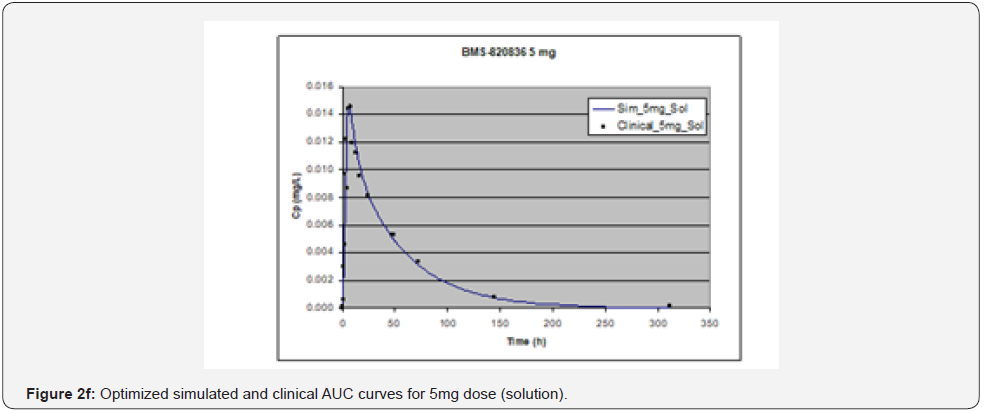
Prediction for tablets
Absorption of BMS-820836 in tablets was simulated using GastroPlusTM, with the optimized input parameters. The simulated AUCs, along with AUCs of the solution formulation and clinical data, are displayed in Figure 3. Figure 3 shows that the simulated curves for 1mg and 3mg tablets, which are similar to those from solution formulations of same doses, match very well with the clinical data while a significant discrepancy was observed for 5mg tablets. The simulated AUC of 5mg tablets is below that obtained clinically as well as that of the simulated AUC for the solution formulation. Furthermore, PK parameters derived from AUCs, both simulated and clinical AUCs, are shown in Table 4. As shown in Table 4, at 1mg dose, PK parameters such as AUC, Cmax, and Tmax are almost equivalent between the solution formulation and tablets, also matching very well with those from clinical analysis. Moreover, ratios of solution over tablet for all three parameters is very close to one. When increasing dose to 3mg, the disparity between tablets and the solution formulation was noted (see Table 4), particularly for Cmax and Tmax. This renders the ratio between solution and tablet for all three parameters varied from 1 to 1.18. Although PK parameters for 3mg dose vary between the tablets and clinical data, when compared with 1 mg dose, it can be still considered as equivalent. However, the AUC and Cmax from the GastroPlusTM simulation of 5mg tablets are much below those from clinical data. Concerning Tmax, the Tmax (9.04 hours) from tablet simulation is much longer than that from the solution simulation (6 hours) and the analysis of clinical data (6.17 hours). This suggests that release of BMS-820846 in vivo is much fast than that estimated from tablet simulation (dissolution). In summary, dose can significantly affect simulation curves generated by GastroPlusTM.
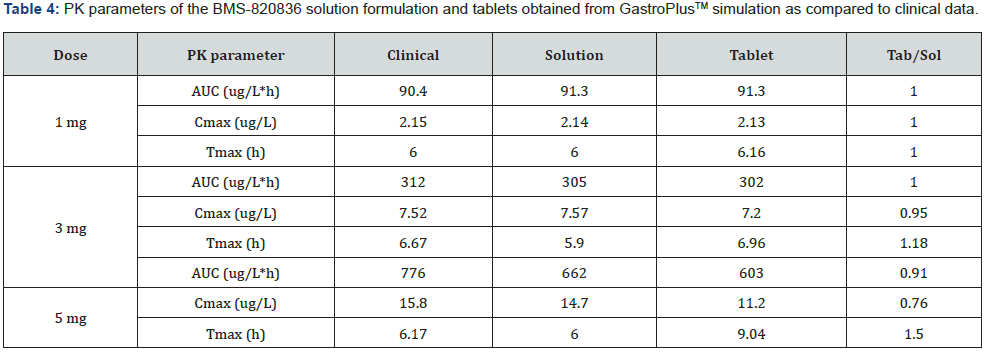
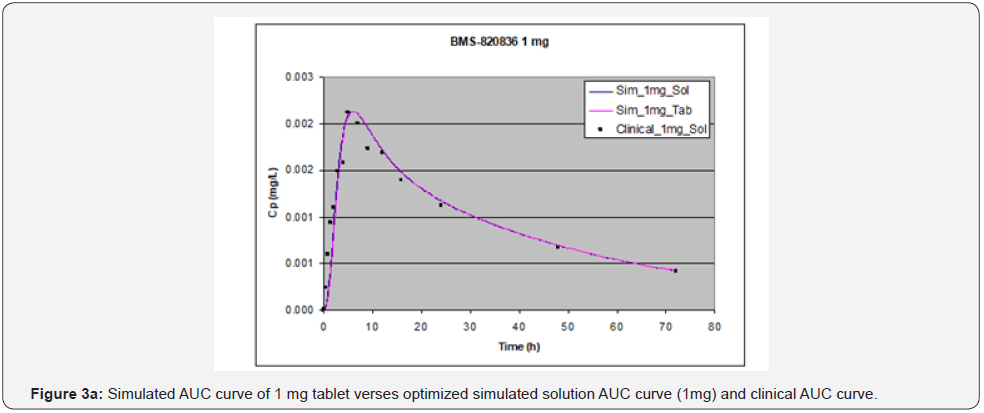
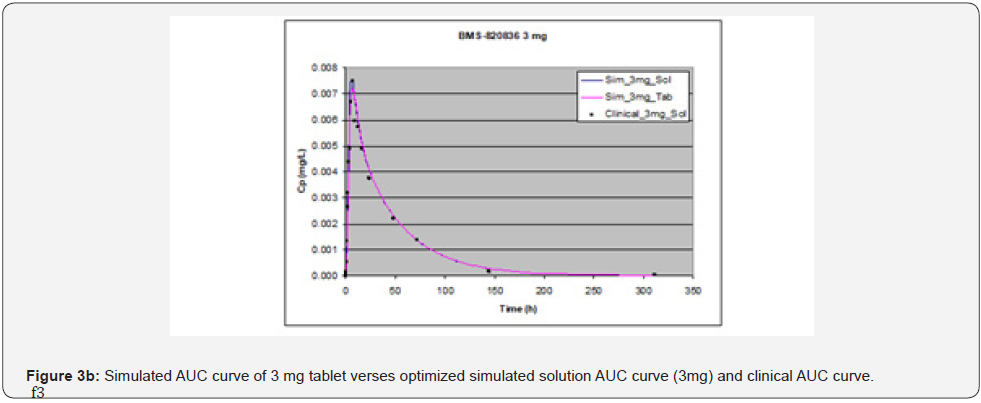
Effect of dose
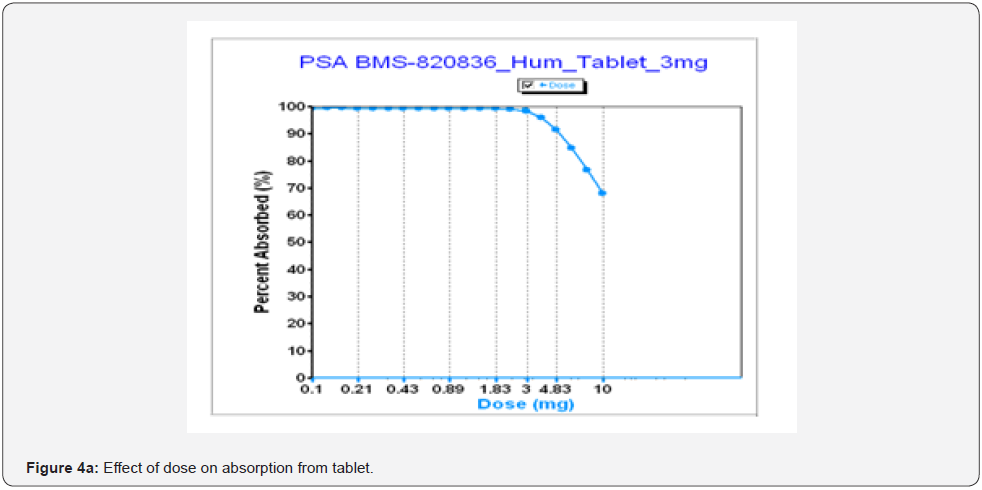
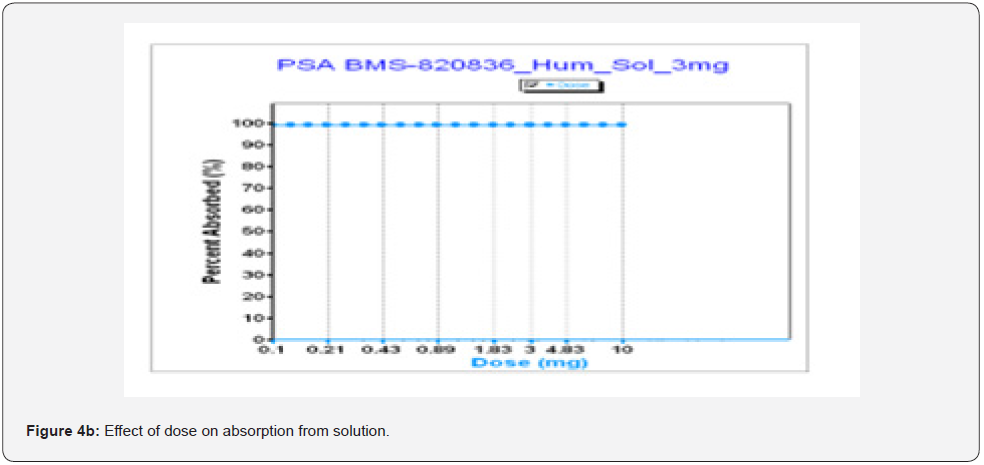
As shown above, dose strength can markedly influence the bioavailability of tablets, according to GastroPlusTM simulation. In developing tablets to support clinical trials, it is important to understand the impact of a dose strength on bioavailability. Figure 4a shows absorption of tablet as a function of dose where absorption drops with increasing dose for tablets. This can be explained as the absorption of BMS-8202536 tablets is limited by dissolution and solubility (see Table 3). As shown in Figure 4a, absorption starts to significantly decrease when a dose (tablet strength) is above 3mg. Once the dose is over 3mg, absorption begin to decrease. Especially, when dose of tablets is increased to 10mg, absorption drops below 70% as the simulation predicted, while the absorption of BMS-820836 from solution formulations remains constant up to 10mg (Figure 4b). This further confirms that absorption of BMS-820836 is limited by dissolution and thereby solubility. From the product development point of view, tablets of any dose above 10 mg should be designed with cautious because tablets with above 10 mg strength may encounter absorption issue. Thus, a rational decision for product development was made based on these data.
pH effect
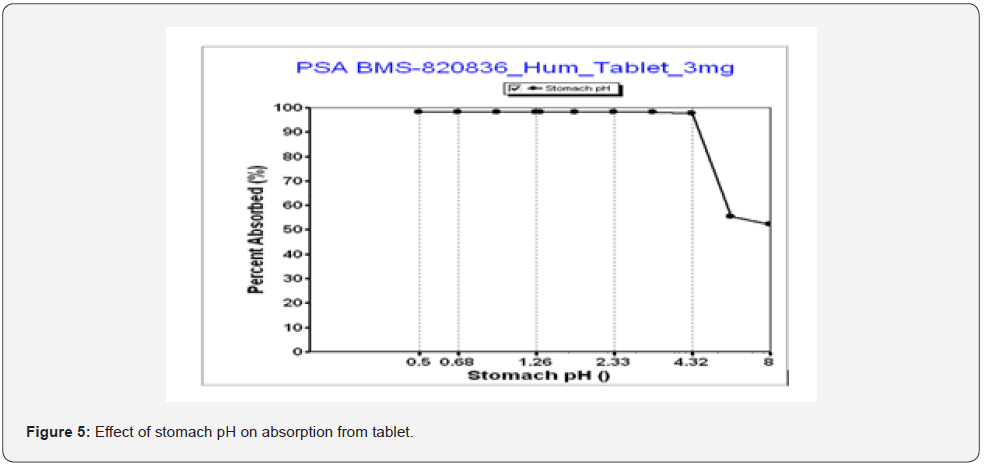
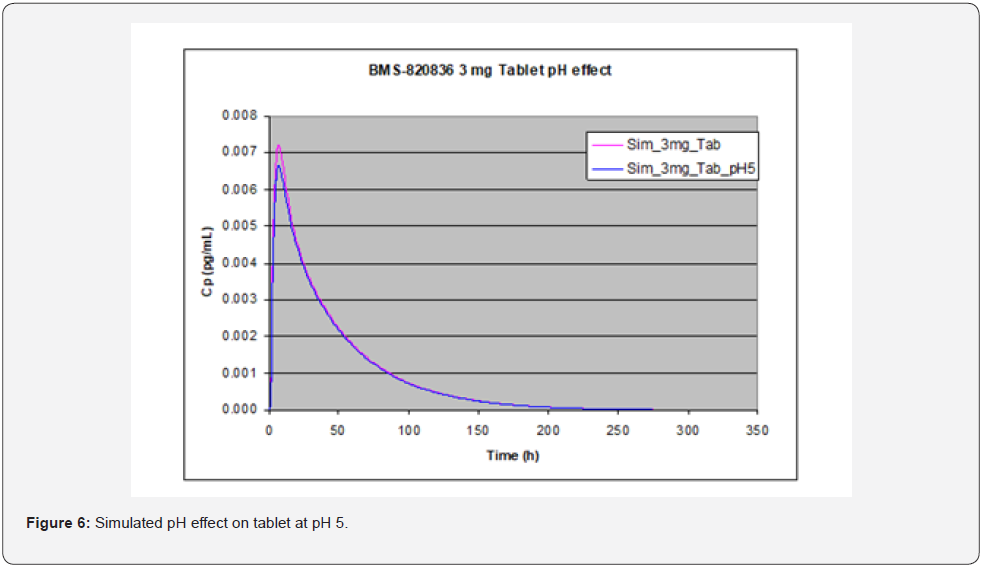
GastroPlusTM simulation can also assist in evaluating the effect of gastric pH on absorption by altering the physiological pH values of stomach [14]. Figure 5 shows an absorption as a function of stomach pH, where absorption of the 3mg tablets of BMS-820826 remained constant until pH reaches 4.32 at which pH the absorption started to decrease. Since BMS-820836 is a weak base, this can be explained as the solubility of BMS-820836 was lowered at pH 4.32 as shown in Table 3. Furthermore, as indicated in Table 3, the solubility of BMS-820836 continues to decrease with pH (Table 3) once beyond pH 4.4, resulting in a significant drop in absorption (Figure 5). To further confirm this, GastroPlusTM the simulations of 3mg tablets at gastric pHs of 1.3 and 5 was carried out with a transit time of 0.25 hour (see Figure 6). As shown in Figure 6, the absorption (AUC) of BMs-820836 at pH 5 was only slightly reduced relative to that at pH 1.3. From Figure 6, AUC, Cmax, and Tmax were derived as listed in Table 5 where the ratio between pH 5 and pH 1.3 for these parameters are close to 1. Overall, GastroPlusTM simulation of gastric pH effect can significantly improve the understanding of the in vivo behavior of BMS-820836, which will facilitate drug product development. Furthermore, food effect on drug absorption is critical for drug development in clinical trials. In the following section, we will focus on evaluating food effect using GastroPlusTM.
Food effect
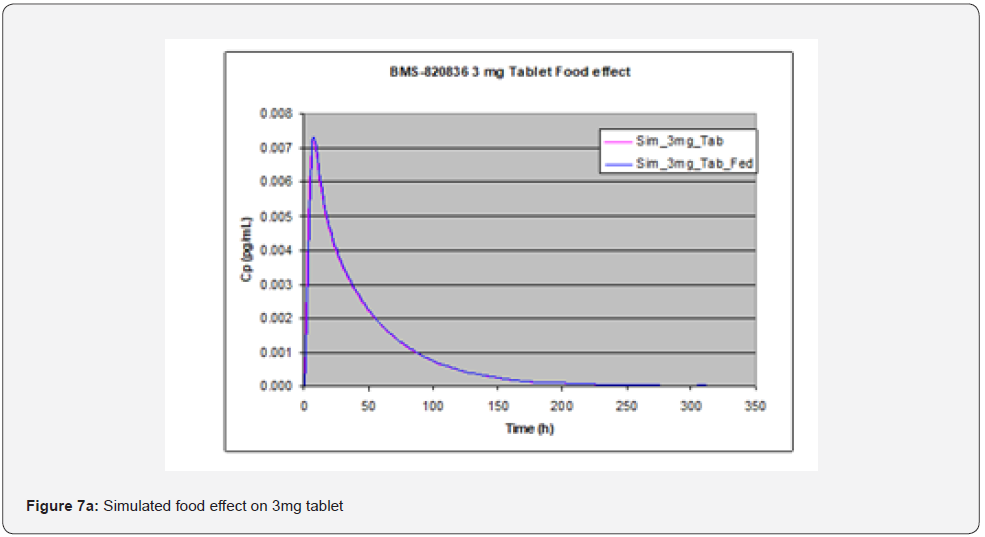
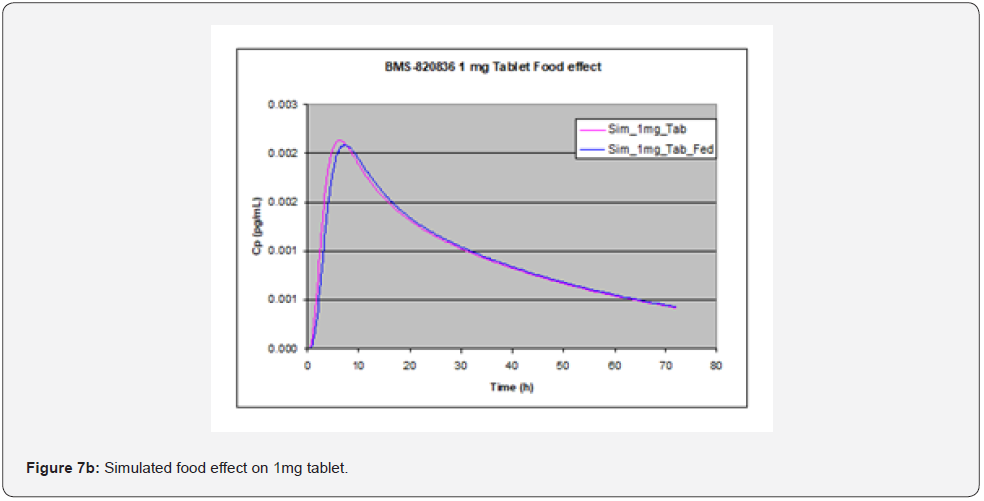
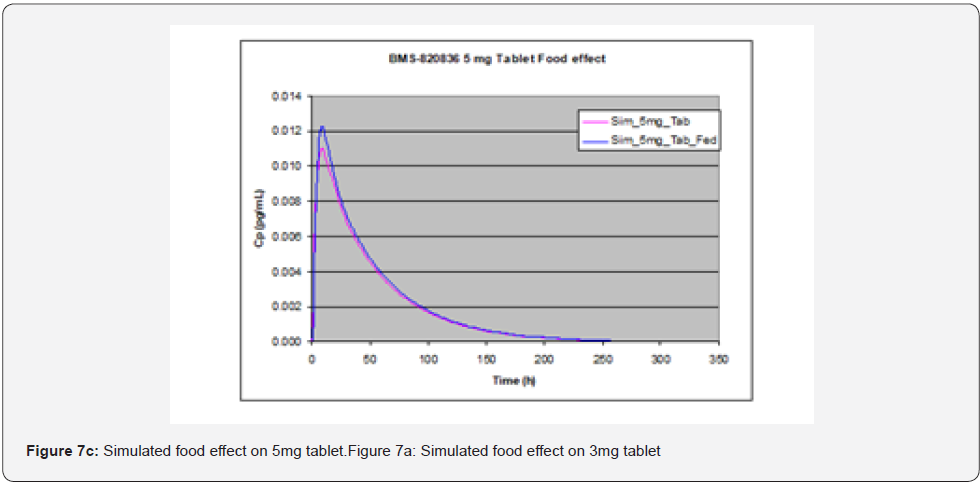
Evaluation of food effect on drug absorption based on GastroPlusTM simulation, achieved by adjusting simulation input parameters including physiological pH and transit time, as well as hepatic flow, can provide significant benefit in assessing the developability of drug candidates [15]. In stomach, pH and transit time in the fasted state greatly from those in the fed state. To perform simulation of the effect of food (fed state), pH and transit time are changed from 1.3 and 0.10h in the fasted state to 4.0 and 1.0h in fed state. pH and transit time in other parts of GI such as duodenum and jejunum are also changed; in the fasted state, pH values in duodenum and jejunum are pH = 6.0 and 6.20, along with transit times of 0.26hr and 0.94hr, while in the fed state pHs are 5.40 and 5.40 in duodenum and jejunum respectively, together with transit times of 0.26hr and 0.94hr. Additionally, hepatic flow rate was increased from 1.5 l/min to 2L/min when switching from the fasted state to the fed state. As shown in Figure 7, GastroPlusTM simulation indicated that no significant food effect was observed for doses below 3 mg (Figure 7a (1mg) and Figure 7b (3mg)) except for slight delay in Tmax in the case of 1 mg tablet whereas a minor enhanced absorption due to food effect was observed for 5mg tablets (Figure 7c) [16]. As shown in Table 3, solubility of BMS-820836 increases with decreasing of pH. However, solubility enhancement of BMS-820836 in biorelevant media is not determined which may explain why absorption of the compound is increased in the fed state as indicated by the simulation [17].
Conclusion
In this paper, application of GastroPlusTM simulation to drug product development has been demonstrated. GastroPlusTM simulation using the optimized parameters, derived from the solution formulation of BMS-820836, was applied to evaluating the bioavailability of tablets. Results match well with clinical data when a dose is below 3mg. Additionally, dose-dependent absorption was obtained from the simulation due to solubility limitation. Regarding pH effect, PK simulation can effectively predict the impact of stomach pH on absorption until pH 4.3 for tablets up to 5mg, and these results will be verified by in vivo data. A low absorption is predicted once stomach pH is higher than pH 4.3. Furthermore, effect of food on absorption was simulated based on physiological parameters (pH, transit time, liver flow) of fast and fed states. Based on the simulation with default GastroPlusTM parameters, food does not impact BA for doses below 5 mg. However, slightly high absorption at fed state for 5mg tablets is shown through simulation. Overall, GastroPlusTM simulation can facilitate drug product development of BMS-820836, especially for solid dosage form.
References
- Ette EI, Williams PJ (2007) Pharmacometrics: the science of quantitative pharmacology. American Journal of Pharmaceutical Education 71(4): 75.
- Kimko HHC, Peck CC (2011) Clinical trial simulations: applications and trends. AAPS Press/Springer, New York, US.
- Shargel L, Wu-Pong S, Yu A (2005) Applied biopharmaceutics & pharmacokinetics. (5th edn), McGraw-Hill, New York, US.
- Rajman I (2008) PK/PD modelling and simulations: utility in drug development. Drug Discov Today 13(7-8): 341-346.
- Smara E, Granneman R (1997) Role of population pharmacokinetics in drug development. A pharmaceutical industry perspective. Clin Pharmacokinet 32(4): 294-312.
- Willmann S, Thelen K, Becker C, Dressman JB, Lippert J (2010) Mechanism-based prediction of particle size-dependent dissolution and absorption: Cilostazol pharmacokinetics in dogs. Euro J Pharm Biopharm 76(1): 83-94.
- Zheng M, Appel L, Luo F, Lane R, Burt D, et al. (2015) Safety, pharmacokinetic, and positron emission tomography evaluation of serotonin and dopamine transporter occupancy following multiple-dose administration of the triple monoamine reuptake inhibitor BMS-820836. Psychopharmacology (Berl) 232(3): 529-540.
- Zheng M, Burt D, Chan W, Hawthorne D, Gasior M, et al. (2015) Comparison of different QT interval correction methods for heart rate and QT beat-to-beat method in a thorough QT study of triple monoamine reuptake inhibitor BMS-820836. J Clin Pharmacol 55(10): 1137-1146.
- Bhagwagar Z, Torbeyns A, Hennicken D, Zheng M, Dunlop BW, et al. (2015) Assessment of the efficacy and safety of BMS-820836 in patients with treatment-resistant major depression: results from 2 randomized, double-blind studies. J Clin Psychopharmacol 35(4): 454-459.
- Charalabidis A, Sfouni M, Bergström C, Macheras P (2019) The Biopharmaceutics Classification System (BCS) and the Biopharmaceutics Drug Disposition Classification System (BDDCS): Beyond guidelines. Int J Pharm 566: 264-281.
- Gobeau N, Stringer R, De Buck S, Tuntland T, Faller B (2016) Evaluation of the GastroPlus™ Advanced Compartmental and Transit (ACAT) Model in Early Discovery. Phar Res 33(9): 2126-2139.
- Heikkinen AT, Baneyx G, Caruso A, Parrott N (2012) Application of PBPK modeling to predict human intestinal metabolism of CYP3A substrates – An evaluation and case study using GastroPlusTM. Eur J Pharm Sci 47(2): 375-386.
- Scherholz ML, Forder J, Androulakis IP (2018) A framework for 2-stage global sensitivity analysis of GastroPlus™ compartmental models. J Pharmacokinet Pharmacodyn 45(2): 309-327.
- Villiger A, Stillhart C, Parroytt N, Kuentz M (2016) Using physologically based on pharmacokinetic (PBPK) modelling to gain insights into the effect of physiological factors on oral absorption in paediatric populations. The AAPS J 18(4): 933-947.
- Parrott NJ, Yu LJ, Takano R, Nakamura M, Morcos PN (2016) Physiologically Based Absorption Modeling to Explore the Impact of Food and Gastric pH Changes on the Pharmacokinetics of Alectinib. The AAPS J 18(6): 1464-1474.
- Li X, Shi L, Tang X, Wang Q, Zhou L, et al. (2017) Mechanistic prediction of food effects for Compound A tablet using PBPK model. Saudi J Bio Sci 24(3): 603-609.
- Radwan A, Jayyousi R, Shraim N, Zaid AN (2019) Evaluation of food effect on the oral absorption of clarithromycin from immediate release tablet using physiological modelling. Biopharmaceutics & Drug Disposition 40(3-4): 121-134.






























